Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Anthropometric Characteristics and Body Composition
2.4. Blood Pressure
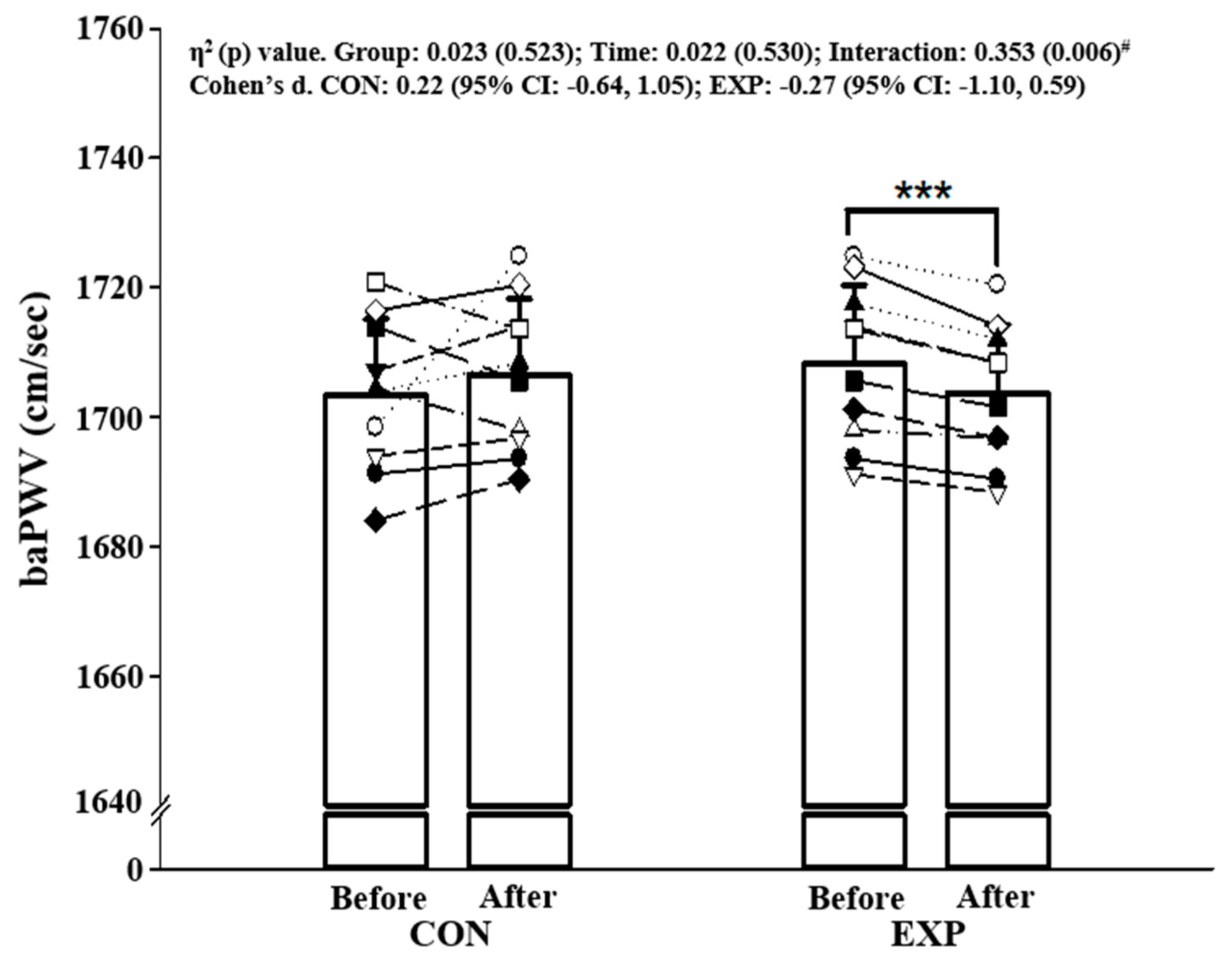
2.5. Arterial Stiffness
2.6. Blood Biochemistry
2.7. Physical Performance
2.8. Statistical Analysis
3. Results
3.1. Body Composition
3.2. BP and Arterial Stiffness
3.3. Cardionascular Risk Factors
3.4. Cardiopulmonary Fitness and Muscular Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, W.; Goodkind, D.; Kowal, P.R. An Aging World: 2015; US Census Bureau: Washington, DC, USA, 2016; p. 95. [Google Scholar]
- Batsis, J.A.; Zagaria, A.B. Addressing obesity in aging patients. Med. Clin. 2018, 102, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and cardiovascular disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matson, T.E.; Renz, A.D.; Takemoto, M.L.; McClure, J.B.; Rosenberg, D.E. Acceptability of a sitting reduction intervention for older adults with obesity. Bmc Public Health 2018, 18, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016, 315, 2284–2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safar, M.E.; Czernichow, S.; Blacher, J. Obesity, arterial stiffness, and cardiovascular risk. J. Am. Soc. Nephrol. 2006, 17, S109–S111. [Google Scholar] [CrossRef]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.; Farooqi, I.S.; Cole, T.J.; O’Rahilly, S.; Fewtrell, M.; Kattenhorn, M.; Lucas, A.; Deanfield, J. Influence of leptin on arterial distensibility: A novel link between obesity and cardiovascular disease? Circulation 2002, 106, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Yu, S.; Xiong, W.; Li, Y.; Li, H.; Li, J.; Li, F. Waist-hip ratio as a predictor of myocardial infarction risk: A systematic review and meta-analysis. Medicine 2018, 97. [Google Scholar] [CrossRef]
- Toto-Moukouo, J.; Achimastos, A.; Asmar, R.; Hugues, C.; Safar, M. Pulse wave velocity in patients with obesity and hypertension. Am. Heart J. 1986, 112, 136–140. [Google Scholar] [CrossRef]
- Miszko, T.A.; Cress, M.E.; Slade, J.M.; Covey, C.J.; Agrawal, S.K.; Doerr, C.E. Effect of strength and power training on physical function in community-dwelling older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M171–M175. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Maloberti, A.; Garatti, L.; Palazzini, M.; Triglione, N.; Occhi, L.; Sioli, S.; Sun, J.; Moreo, A.; Beretta, G.; et al. Funcional improvement after outpatient cardiac rehabilitation in acute coronary syndrome patients is not related to imporvement in left ventiricular ejection fraction. High Blood Press Cardiovasc. Prev. 2020, 27, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.; Kim, J.-S.; Shin, C.-H.; Park, Y.; Kim, J.-H. Associations between skeletal muscle mass, grip strength, and physical and cognitive functions in elderly women: Effect of exercise with resistive theraband. J. Exerc. Nutr. Biochem. 2019, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Shiotsu, Y.; Watanabe, Y.; Tujii, S.; Yanagita, M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp. Gerontol. 2018, 111, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Bong, Y.; Song, W. The effects of eleastic band exercises and nutritonal education on frailty, strength, and nutritional intake in elderly women. Phys. Act. Nutr. 2020, 24, 1. [Google Scholar] [CrossRef]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrick, K.L.; Hunter, G.R.; Fisher, G.; Glasser, S.P. Changes in vascular hemodynamics in older women following 16 weeks of combined aerobic and resistance training. J. Clin. Hypertens. 2013, 15, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueroa, A.; Park, S.Y.; Seo, D.Y.; Sanchez-Gonzalez, M.A.; Baek, Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011, 18, 980–984. [Google Scholar] [CrossRef]
- Bales, C.W.; Porter Starr, K.N. Obesity interventions for older adults: Diet as a determinant of physical function. Adv. Nutr. 2018, 9, 151–159. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Phadnis, U.; Fowler, K.; Armamento-Villareal, R.; Sun, Z.; Brunetti, L.; Park, J.H.; Kaipparettu, B.A.; Putluri, N. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab. 2019, 30, 261–273.e266. [Google Scholar] [CrossRef]
- Park, H.-Y.; Kim, Y.; Nam, S.-S. Effects of exercise training at lactate threshold and detraining for 12 weeks on body composition, aerobic performance, and stress related variables in obese women. J. Exerc. Nutr. Biochem. 2019, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Sziva, Á.; Mészáros, Z.; Kiss, K.; Mavroudes, M.; Ng, N.; Mészáros, J. Longitudinal differences in running endurance and body mass index—A 25-year comparison. Acta Physiol. Hung. 2009, 96, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Şavkin, R.; Aslan, U.B. The effect of Pilates exercise on body composition in sedentary overweight and obese women. J. Sports Med. Phys. Fit. 2016, 57, 1464–1470. [Google Scholar] [CrossRef]
- Kim, S.-W.; Jung, W.-S.; Park, W.; Park, H.-Y. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16, 5020. [Google Scholar] [CrossRef] [Green Version]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- Weinstein, Y.; Kamerman, T.; Berry, E.; Falk, B. Mechanical efficiency of normal-weight prepubertal boys predisposed to obesity. Med. Sci. Sports Exerc. 2004, 36, 567–573. [Google Scholar] [CrossRef]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. Ser. A: Biol. Sci. Med Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef]
- Collier, S.; Kanaley, J.; Carhart, R.; Frechette, V.; Tobin, M.; Hall, A.; Luckenbaugh, A.; Fernhall, B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre-and stage-1 hypertensives. J. Hum. Hypertens. 2008, 22, 678–686. [Google Scholar] [CrossRef]
- Matsui, M.; Yoshikawa, T.; Mizushima, R.; Tanahashi, K.; Myoenzono, K.; Tanaka, K.; Maeda, S. Association between duration of excessive weight and arterial stiffness in middle-aged and older adults. Clin. Exp. Hypertens. 2020, 42, 213–217. [Google Scholar] [CrossRef]
- Shibata, S.; Fujimoto, N.; Hastings, J.L.; Carrick-Ranson, G.; Bhella, P.S.; Hearon Jr, C.M.; Levine, B.D. The effect of lifelong exercise frequency on arterial stiffness. J. Physiol. 2018, 596, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kim, E.D.; Wu, A.; Meyer, M.L.; Cheng, S.; Hoogeveen, R.C.; Ballantyne, C.M.; Tanaka, H.; Heiss, G.; Selvin, E. Central and peripheral pulse wave velocity and subclinical myocardial stress and damage in older adults. PLoS ONE 2019, 14, e0212892. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Combined aerobic and resistance training and vascular function: Effect of aerobic exercise before and after resistance training. J. Appl. Physiol. 2007, 103, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Tanaka, H.; Miyachi, M. Resistance training and arterial compliance: Keeping the benefits while minimizing the stiffening. J. Hypertens. 2006, 24, 1753–1759. [Google Scholar] [CrossRef] [Green Version]
- DeVallance, E.; Fournier, S.; Lemaster, K.; Moore, C.; Asano, S.; Bonner, D.; Donley, D.; Olfert, I.; Chantler, P. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur. J. Appl. Physiol. 2016, 116, 899–910. [Google Scholar] [CrossRef]
- Miyaki, A.; Maeda, S. Arterial stiffness and lifestyle modification. J. Phys. Fit. Sports Med. 2012, 1, 205–210. [Google Scholar] [CrossRef]
- Kondo, T.; Kobayashi, I.; Murakami, M. Effect of exercise on circulating adipokine levels in obese young women. Endocr. J. 2006, 53, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Grund, A.; Krause, H.; Kraus, M.; Siewers, M.; Rieckert, H.; Müller, M. Association between different attributes of physical activity and fat mass in untrained, endurance-and resistance-trained men. Eur. J. Appl. Physiol. 2001, 84, 310–320. [Google Scholar] [CrossRef]
- Weidmann, P.; de Chatel, R.; Schiffmann, A.; Bachmann, E.; Beretta-Piccoli, C.; Reubi, F.C.; Ziegler, W.H.; Vetter, W. Interrelations between age and plasma renin, aldosterone and cortisol, urinary catecholamines, and the body sodium/volume state in normal man. Klin. Wochenschr. 1977, 55, 725–733. [Google Scholar] [CrossRef]
- Sandbakk, S.B.; Nauman, J.; Lavie, C.J.; Wisløff, U.; Stensvold, D. Combined association of cardiorespiratory fitness and body fatness with cardiometabolic risk factors in older Norwegian adults: The generation 100 study. Mayo Clin. Proc. Innov. Qual. Outcomes 2017, 1, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Maiorana, A.; O’Driscoll, G.; Dembo, L.; Cheetham, C.; Goodman, C.; Taylor, R.; Green, D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1999–H2005. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hermoso, A.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Peterson, M.D.; Izquierdo, M.; Prieto-Benavides, D.; Sandoval-Cuellar, C.; González-Ruíz, K.; Ramírez-Vélez, R. Handgrip strength attenuates the adverse effects of overweight on cardiometabolic risk factors among collegiate students but not in individuals with higher fat levels. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variables | CON | EXP | p-Value |
|---|---|---|---|
| Number | 10 | 10 | - |
| Age (years) | 68.5 (0.9) | 69.1 (0.9) | 0.137 |
| Height (cm) | 165.8 (4.8) | 164.1 (3.8) | 0.409 |
| Body weight (kg) | 71.6 (5.0) | 70.7 (3.8) | 0.670 |
| BMI (kg/m2) | 26.0 (0.4) | 26.2 (0.5) | 0.307 |
| Free fat mass (kg) | 45.4 (3.17) | 44.8 (2.4) | 0.671 |
| Body fat (%) | 32.7 (1.8) | 32.4 (1.4) | 0.670 |
| Variables | CON | EXP | η2 (p) Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Cohen’s d (95% CI) | Before | After | p-Value | Cohen’s d (95% CI) | Group | Time | Interaction | |
| Body weight (kg) | 71.6 (5.0) | 72.3 (5.1) | 0.203 | 0.14 (−0.71, 0.97) | 70.7 (3.8) | 69.2 (4.1) | 0.001 ** | −0.37 (−1.20, 0.49) | 0.051 (0.338) | 0.096 (0.183) | 0.442 (0.001) † |
| BMI (kg/m2) | 26.0 (0.4) | 26.3 (0.8) | 0.193 | 0.38 (−0.48, 1.21) | 26.2 (0.5) | 25.7 (0.6) | 0.001 ** | −0.93 (−1.78, −0.01) | 0.037 (0.418) | 0.101 (0.173) | 0.451 (0.001) † |
| Free fat mass (kg) | 45.4 (3.17) | 44.4 (3.1) | 0.013 * | −0.31 −1.15, 0.54 | 44.8 (2.4) | 45.2 (2.7) | 0.064 | 0.15 (−0.70, 0.98) | 0.001 (0.906) | 0.117 (0.140) | 0.437 (0.001) † |
| Body fat (%) | 32.7 (1.8) | 34.3 (1.9) | 0.000 *** | 0.90 (−0.02, 1.74) | 32.4 (1.4) | 30.4 (1.4) | 0.000 *** | −1.39 (−2.27, −0.40) | 0.323 (0.009) † | 0.060 (0.298) | 0.940 (0.000) † |
| Variables | CON | EXP | η2 (p) Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Cohen’s d (95% CI) | Before | After | p-Value | Cohen’s d (95% CI) | Group | Time | Interaction | |
| SBP (mmHg) | 133.0 (3.4) | 134.6 (3.3) | 0.003 ** | 0.45 (−0.42, 1.28) | 136.8 (2.9) | 134.4 (2.5) | 0.000 *** | −0.81 (−1.65, 0.09) | 0.092 (0.193) | 0.170 (0.071) | 0.798 (0.000) † |
| DBP (mmHg) | 88.8 (4.0) | 89.9 (3.2) | 0.159 | 0.28 (−0.57, 1.12) | 92.7 (3.0) | 92.6 (3.3) | 0.880 | −0.01 (−0.85, 0.83) | 0.220 (0.037) † | 0.097 (0.181) | 0.111 (0.152) |
| MAP (mmHg) | 103.6 (3.2) | 104.8 (2.5) | 0.056 | 0.40 (−0.46, 1.23) | 107.4 (2.17) | 106.6 (2.4) | 0.000 *** | −0.29 (−1.12, 0.57) | 0.260 (0.022) † | 0.027 (0.488) | 0.420 (0.002) † |
| PP (mmHg) | 44.2 (4.5) | 44.7 (4.2) | 0.466 | 0.09 (−0.75, 0.93) | 44.1 (4.3) | 41.8 (4.8) | 0.001 ** | −0.56 (−1.39, 0.32) | 0.034 (0.438) | 0.268 (0.019) | 0.429 (0.002) † |
| Variables | CON | EXP | η2 (p) Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Cohen’s d (95% CI) | Before | After | p-Value | Cohen’s d (95% CI) | Group | Time | Interaction | |
| TG (mg/dL) | 115.7 (9.2) | 119.4 (9.0) | 0.019 * | 0.41 (−0.46, 1.24) | 116.8 (9.1) | 111.1 (8.6) | 0.057 | −0.65 (−1.49, 0.24) | 0.050 (0.344) | 0.027 (0.492) | 0.363 (0.005) † |
| TC (mg/dL) | 209.4 (12.2) | 218.1 (11.8) | 0.106 | 0.73 (−0.17, 1.57) | 214.4 (13.9) | 204.2 (12.6) | 0.092 | −0.77 (−1.61, 0.13) | 0.056 (0.314) | 0.002 (0.842) | 0.273 (0.018) † |
| HDL-C (mg/dL) | 40.9 (4.2) | 39.9 (1.2) | 0.384 | −0.28 (−1.11, 0.58) | 40.8 (2.5) | 42.9 (2.8) | 0.092 | 0.77 (−0.13, 1.61) | 0.104 (0.165) | 0.022 (0.536) | 0.176 (0.066) |
| LDL-C (mg/dL) | 132.7 (12.5) | 134.9 (7.1) | 0.648 | 0.21 (−0.64, 1.04) | 133.5 (8.2) | 122.5 (7.5) | 0.008 ** | −1.40 (−2.27, −0.40) | 0.174 (0.067) | 0.126 (0.125) | 0.238 (0.029) † |
| FFA (uEq/l) | 471.0 (57.4) | 485.8 (43.5) | 0.471 | 0.29 (−0.57, 1.12) | 469.6 (49.2) | 446.1 (40.8) | 0.094 | −0.51 (−1.34, 0.36) | 0.067 (0.271) | 0.008 (0.715) | 0.130 (0.118) |
| Glucose (mg/dL) | 114.4 (12.0) | 116.5 (6.6) | 0.612 | 0.22 (−0.63, 1.05) | 120.0 (7.8) | 114.3 (7.0) | 0.092 | −0.77 (−1.61, 0.13) | 0.019 (0.559) | 0.026 (0.494) | 0.117 (0.140) |
| EP (pg/mL) | 70.6 (13.1) | 73.2 (10.0) | 0.501 | 0.22 (−0.63, 1.05) | 56.8 (22.9) | 49.2 (19.0) | 0.026 * | −0.33 (−1.16, 0.53) | 0.275 (0.018) † | 0.059 (0.300) | 0.208 (0.043) † |
| NP (pg/mL) | 267.5 (62.0) | 270.8 (60.8) | 0.890 | 0.05 (−0.79, 0.89) | 240.2 (61.8) | 229.2 (71.4) | 0.666 | −0.16 (−1.00, 0.68) | 0.110 (0.154) | 0.003 (0.824) | 0.010 (0.679) |
| Variables | CON | EXP | η2 (p) Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Cohen’s d (95% CI) | Before | After | p-Value | Cohen’s d (95% CI) | Group | Time | Interaction | |
| TNF-α (pg/mL) | 216.2 (38.9) | 234.1 (39.5) | 0.228 | 0.46 (−0.41, 1.29) | 230.9 (35.1) | 214.6 (35.1) | 0.163 | −0.46 (−1.29, 0.41) | 0.002 (0.868) | 0.000 (0.929) | 0.175 (0.066) |
| IL-6 (pg/mL) | 3.6 (0.4) | 3.8 (0.2) | 0.206 | 0.66 (−0.23, 1.49) | 3.8 (0.3) | 3.6 (0.3) | 0.009 ** | −0.20 (−1.03, 0.65) | 0.002 (0.847) | 0.041 (0.391) | 0.155 (0.086) |
| EPO (IU/mL) | 10.9 (3.9) | 11.1 (2.3) | 0.891 | 0.07 (−0.77, 0.91) | 9.5 (2.4) | 11.1 (2.7) | 0.068 | 0.63 (−0.25, 1.47) | 0.029 (0.473) | 0.060 (0.296) | 0.036 (0.421) |
| VEGF (pg/mL) | 33.1 (3.2) | 31.6 (2.7) | 0.041 | −0.50 (−1.33, 0.37) | 32.7 (7.1) | 31.2 (3.7) | 0.271 | −0.14 (−0.97, 0.71) | 0.003 (0.823) | 0.202 (0.047) † | 0.000 (0.956) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, W.; Jung, W.-S.; Hong, K.; Kim, Y.-Y.; Kim, S.-W.; Park, H.-Y. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 7233. https://doi.org/10.3390/ijerph17197233
Park W, Jung W-S, Hong K, Kim Y-Y, Kim S-W, Park H-Y. Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. International Journal of Environmental Research and Public Health. 2020; 17(19):7233. https://doi.org/10.3390/ijerph17197233
Chicago/Turabian StylePark, Wonil, Won-Sang Jung, Kwangseok Hong, Yae-Young Kim, Sung-Woo Kim, and Hun-Young Park. 2020. "Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study" International Journal of Environmental Research and Public Health 17, no. 19: 7233. https://doi.org/10.3390/ijerph17197233
APA StylePark, W., Jung, W.-S., Hong, K., Kim, Y.-Y., Kim, S.-W., & Park, H.-Y. (2020). Effects of Moderate Combined Resistance- and Aerobic-Exercise for 12 Weeks on Body Composition, Cardiometabolic Risk Factors, Blood Pressure, Arterial Stiffness, and Physical Functions, among Obese Older Men: A Pilot Study. International Journal of Environmental Research and Public Health, 17(19), 7233. https://doi.org/10.3390/ijerph17197233










