Impact of Freeze–Thaw Cycles on Die-Off of E. coli and Intestinal Enterococci in Deer and Dairy Faeces: Implications for Landscape Contamination of Watercourses
Abstract
1. Introduction
2. Materials and Methods
2.1. Provenance of Faeces Used in All Experiments
2.2. Experiment Design
2.2.1. Faecal Mesocosms
2.2.2. Water Mesocosms
2.3. FIO Enumeration
2.4. Statistical Analysis
2.4.1. Faecal Samples
2.4.2. Water Samples
2.4.3. Interpreting Parameter Values of Log—Linear and Exponential Models
3. Results
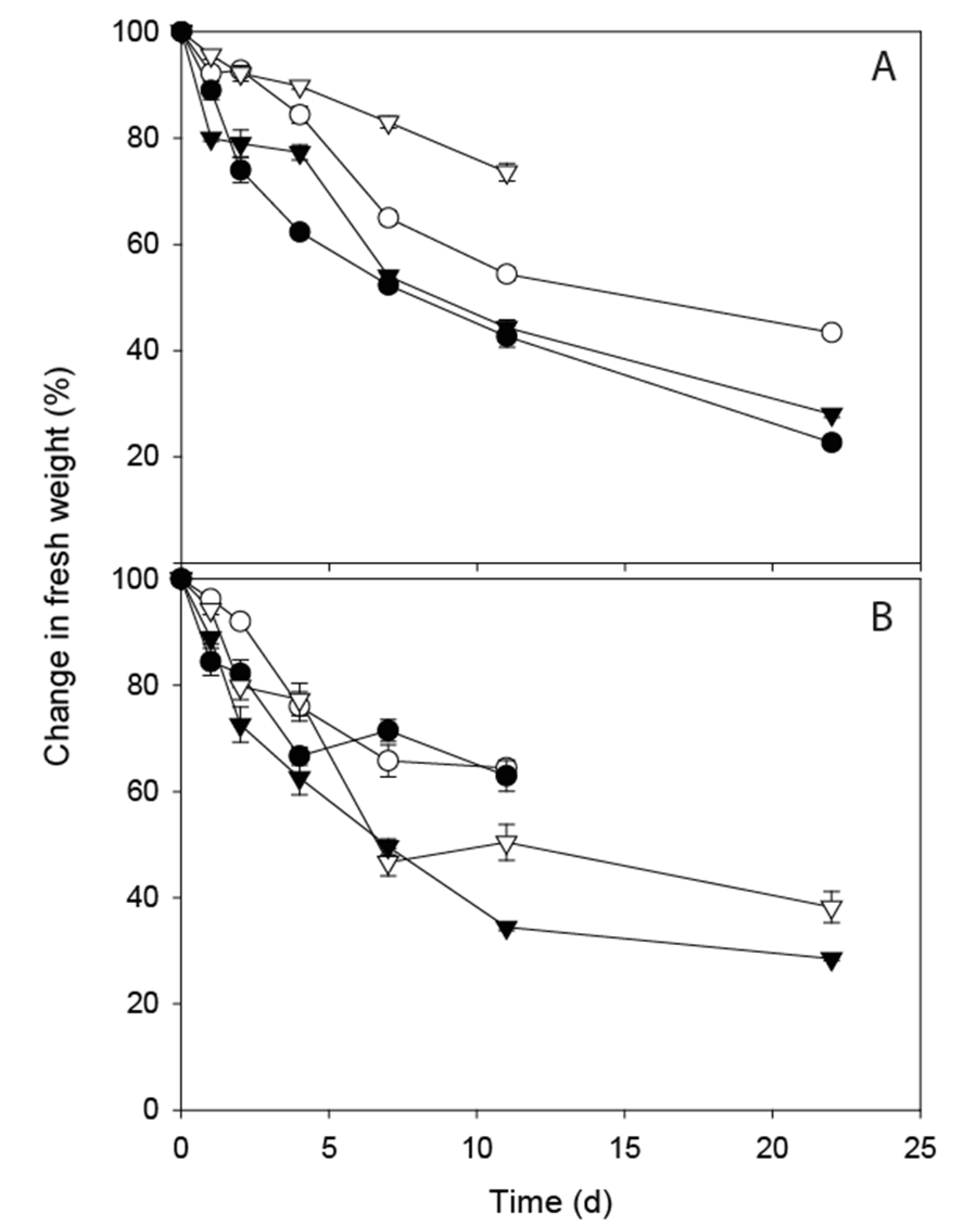
3.1. FIO Die-Off in Faeces
3.2. FIO Die-Off in Water
3.2.1. E. coli
3.2.2. Intestinal Enterococci
3.3. Temperature Fluctuations Within the Faeces
3.4. Changes in Moisture Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliver, D.M.; Bartie, P.J.; Heathwaite, A.L.; Reaney, S.M.; Parnell, J.A.; Quilliam, R.S. A catchment-scale model to predict spatial and temporal burden of E. coli on pasture from grazing livestock. Sci. Total Environ. 2018, 616, 678–687. [Google Scholar] [PubMed]
- Sharma, M.; Millner, P.D.; Hashem, F.; Vinyard, B.T.; East, C.L.; Handy, E.T.; White, K.; Stonebraker, R.; Cotton, C.P. E. coli survival duration in manure-amended soils is affected by spatiotemporal, agricultural, and weather factors: A multi-season, multi-site field study in the Mid-Atlantic US. Appl. Environ. Microbiol. 2019. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Blanch, A.R.; Campos, C.; Jofre, J.; Lucena, F. Pathogens, faecal indicators and human-specific microbial source-tracking markers in sewage. J. Appl. Microbiol. 2019, 126, 701–717. [Google Scholar] [PubMed]
- Kay, D.; Crowther, J.; Fewtrell, L.; Francis, C.A.; Hopkins, M.; Kay, C.; McDonald, A.T.; Stapleton, C.M.; Watkins, J.; Wilkinson, J.; et al. Quantification and control of microbial pollution from agriculture: A new policy challenge? Environ. Sci. Policy 2008, 11, 171–184. [Google Scholar]
- Cho, S.; Jackson, C.R.; Frye, J.G. The prevalence and antimicrobial resistance phenotypes of Salmonella, Escherichia coli, and Enterococcus sp. in surface water. Lett. Appl. Microbiol. 2020, 71, 3–25. [Google Scholar] [PubMed]
- Jeong, J.; Wagner, K.; Flores, J.J.; Cawthon, T.; Her, Y.; Osorio, J.; Yen, H. Linking watershed modeling and bacterial source tracking to better assess E. coli sources. Sci. Total Environ. 2019, 648, 164–175. [Google Scholar]
- Cho, K.H.; Pachepsky, Y.A.; Oliver, D.M.; Muirhead, R.W.; Park, Y.; Quilliam, R.S.; Shelton, D.R. Modeling fate and transport of fecally-derived microorganisms at the watershed scale: State of the science and future opportunities. Water Res. 2016, 100, 38–56. [Google Scholar]
- Oliver, D.M.; Bird, C.; Burd, E.; Wyman, M. Quantitative PCR profiling of Escherichia coli in livestock feces reveals increased population resilience relative to culturable counts under temperature extremes. Environ. Sci. Technol. 2016, 50, 9497–9505. [Google Scholar]
- Smith, J.E.; Stocker, M.D.; Hill, R.L.; Pachepsky, Y.A. The effect of temperature oscillations and sediment texture on fecal indicator bacteria survival in sediments. Water Air Soil Pollut. 2019, 230, 270. [Google Scholar]
- Hellberg, R.S.; Chu, E. Effects of climate change on the persistence and dispersal of foodborne bacterial pathogens in the outdoor environment: A review. Crit. Rev. Microbiol. 2016, 42, 548–572. [Google Scholar]
- Porter, K.D.; Quilliam, R.S.; Reaney, S.M.; Oliver, D.M. High resolution characterisation of E. coli proliferation profiles in livestock faeces. Waste Manag. 2019, 87, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.; Chen, W.; Tao, J.; Xu, M.; Guo, P. Effects of fulvic acid and fulvic ions on Escherichia coli survival in river under repeated freeze-thaw cycles. Environ. Pollut. 2019, 247, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Asadishad, B.; Ghoshal, S.; Tufenkji, N. Role of cold climate and freeze–thaw on the survival, transport, and virulence of Yersinia enterocolitica. Environ. Sci. Technol. 2013, 47, 14169–14177. [Google Scholar] [CrossRef] [PubMed]
- Rocard, J.M.; Asadishad, B.; Samonte, P.R.V.; Ghoshal, S.; Tufenkji, N. Natural freeze-thaw cycles may increase the risk associated with Salmonella contamination in surface and groundwater environments. Water Res. X 2018, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.; Barnes, D.L.; Schiewer, S.; White, D.M. Total coliform survival characteristics in frozen soils. J. Environ. Eng. 2007, 133, 1098–1105. [Google Scholar] [CrossRef]
- Oliver, D.M.; Page, T.; Zhang, T.; Heathwaite, A.L.; Beven, K.; Carter, H.; McShane, G.; Keenan, P.O.; Haygarth, P.M. Determining E. coli burden on pasture in a headwater catchment: Combined field and modelling approach. Environ. Int. 2012, 43, 6–12. [Google Scholar] [CrossRef]
- Jeon, D.J.; Ligaray, M.; Kim, M.; Kim, G.; Lee, G.; Pachepsky, Y.A.; Cha, D.H.; Cho, K.H. Evaluating the influence of climate change on the fate and transport of fecal coliform bacteria using the modified SWAT model. Sci. Total Environ. 2019, 658, 753–762. [Google Scholar]
- Oliver, D.M.; Porter, K.D.; Pachepsky, Y.A.; Muirhead, R.W.; Reaney, S.M.; Coffey, R.; Kay, D.; Milledge, D.G.; Hong, E.; Anthony, S.G.; et al. Predicting microbial water quality with models: Over-arching questions for managing risk in agricultural catchments. Sci. Total Environ. 2016, 544, 39–47. [Google Scholar]
- Guber, A.K.; Fry, J.; Ives, R.L.; Rose, J.B. Escherichia coli survival in, and release from, white-tailed deer feces. Appl. Environ. Microbiol. 2015, 81, 1168–1176. [Google Scholar]
- Wilkinson, J.M.; Lee, M.R.; Rivero, M.J.; Chamberlain, A.T. Some challenges and opportunities for grazing dairy cows on temperate pastures. Grass Forage Sci. 2020, 75, 1–17. [Google Scholar]
- Russo, G.S.; Eftim, S.E.; Goldstone, A.E.; Dufour, A.P.; Nappier, S.P.; Wade, T.J. Evaluating health risks associated with exposure to ambient surface waters during recreational activities: A systematic review and meta-analysis. Water Res. 2020, 176, 115729. [Google Scholar] [CrossRef] [PubMed]
- Neill, A.J.; Tetzlaff, D.; Strachan, N.J.; Hough, R.L.; Avery, L.M.; Kuppel, S.; Maneta, M.P.; Soulsby, C. An agent-based model that simulates the spatio-temporal dynamics of sources and transfer mechanisms contributing faecal indicator organisms to streams. Part 1: Background and model description. J. Environ. Manag. 2020, 270, 110903. [Google Scholar]
- Neill, A.J.; Tetzlaff, D.; Strachan, N.J.; Soulsby, C. To what extent does hydrological connectivity control dynamics of faecal indicator organisms in streams? Initial hypothesis testing using a tracer-aided model. J. Hydrol. 2019, 570, 423–435. [Google Scholar] [CrossRef]
- Gao, W.; Leung, K.; Hawdon, N. Freezing inactivation of Escherichia coli and Enterococcus faecalis in water: Response of different strains. Water Environ. Res. 2009, 81, 824–830. [Google Scholar]
- Marcellini, M.; Noirjean, C.; Dedovets, D.; Maria, J.; Deville, S. Time-Lapse, in situ imaging of ice crystal growth using confocal microscopy. ACS Omega 2016, 1, 1019–1026. [Google Scholar] [CrossRef]
- Gao, W.; Smith, D.W.; Li, Y. Natural freezing as a wastewater treatment method: E. coli inactivation capacity. Water Res. 2006, 40, 2321–2326. [Google Scholar] [CrossRef]
- Brouwer, A.F.; Eisenberg, M.C.; Remais, J.V.; Collender, P.A.; Meza, R.; Eisenberg, J.N. Modeling biphasic environmental decay of pathogens and implications for risk analysis. Environ. Sci. Technol. 2017, 51, 2186–2196. [Google Scholar] [CrossRef]
- Orruño, M.; Kaberdin, V.R.; Arana, I. Survival strategies of Escherichia coli and Vibrio spp.: Contribution of the viable but nonculturable phenotype to their stress-resistance and persistence in adverse environments. World J. Microbiol. Biotechnol. 2017, 33, 45. [Google Scholar]
- Wei, C.; Zhao, X. Induction of viable but nonculturable Escherichia coli O157: H7 by low temperature and its resuscitation. Front. Microbiol. 2018, 9, 2728. [Google Scholar] [CrossRef]
- Oliver, D.M.; Heathwaite, A.; Haygarth, P.M. A ‘culture’ change in catchment microbiology? Hydrol. Process. 2010, 24, 2973–2976. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, J.Y.; Lim, J.Y.; Kim, S.J.; Song, K.Y.; Yoon, K.S. The fate of cold-stressed or tetracycline-resistant Vibrio spp. in precooked shrimp during frozen storage. J. Food Saf. 2020, 40, e12798. [Google Scholar] [CrossRef]
- Akyurt, M.; Zaki, G.; Habeebullah, B. Freezing phenomena in ice–water systems. Energy Convers. Manag. 2002, 43, 1773–1789. [Google Scholar] [CrossRef]
- Sleight, S.C.; Wigginton, N.S.; Lenski, R.E. Increased susceptibility to repeated freeze-thaw cycles in Escherichia coli following long-term evolution in a benign environment. BMC Evol. Biol. 2006, 6, 104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walter, W.D.; Anderson, C.W.; Smith, R.; Vanderklok, M.; Averill, J.J.; Ver Cauteren, K.C. On-farm mitigation of transmission of tuberculosis from white-tailed deer to cattle: Literature review and recommendations. Vet. Med. Int. 2012, 2012, 616318. [Google Scholar] [CrossRef] [PubMed]
- Neill, A.J.; Tetzlaff, D.; Strachan, N.J.; Hough, R.L.; Avery, L.M.; Maneta, M.P.; Soulsby, C. An agent-based model that simulates the spatio-temporal dynamics of sources and transfer mechanisms contributing faecal indicator organisms to streams. Part 2: Application to a small agricultural catchment. J. Environ. Manag. 2020, 270, 110905. [Google Scholar] [CrossRef]
- Muirhead, R.W.; Elliott, A.H.; Monaghan, R.M. A model framework to assess the effect of dairy farms and wild fowl on microbial water quality during base-flow conditions. Water Res. 2011, 45, 2863–2874. [Google Scholar] [CrossRef]
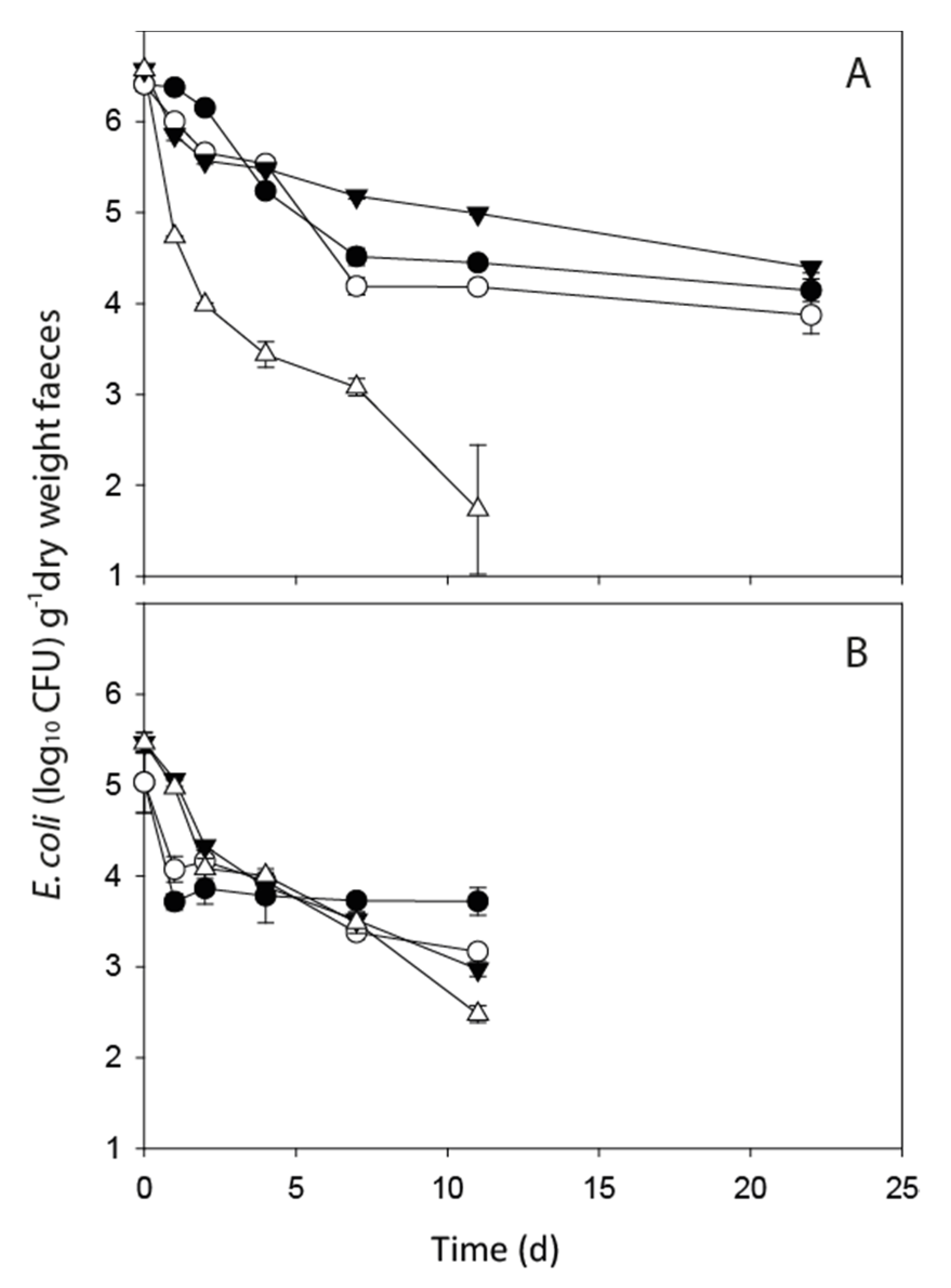
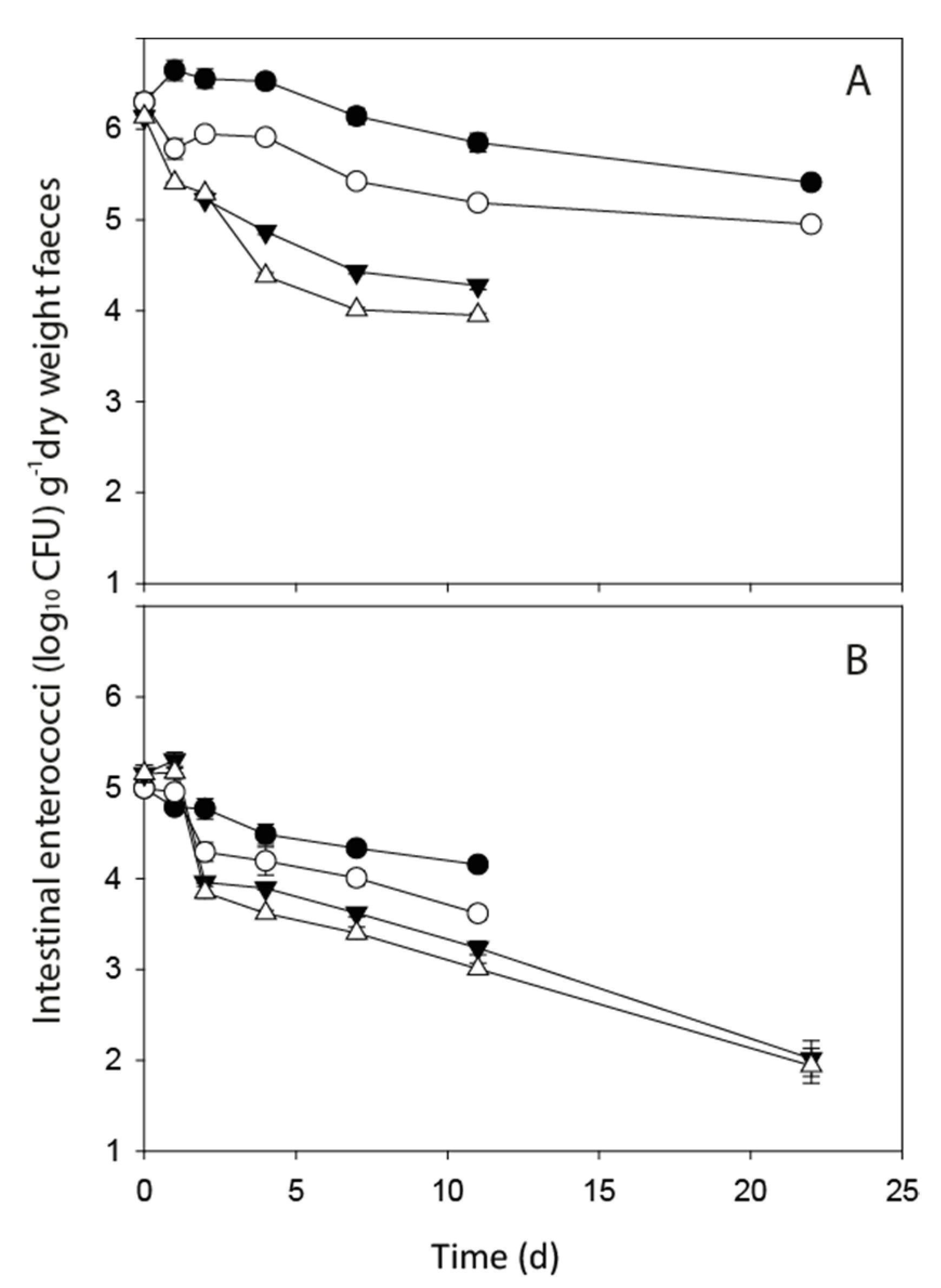
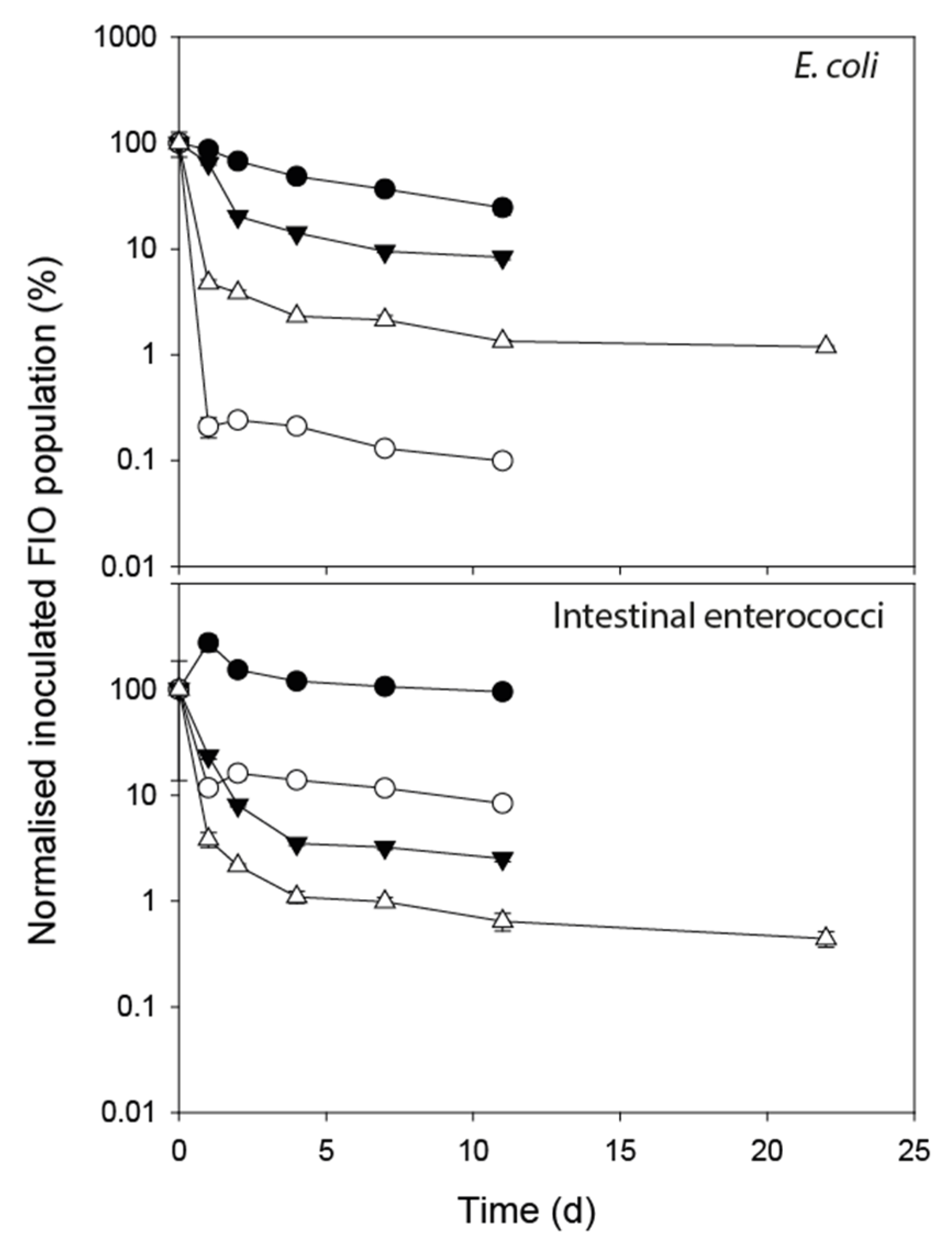
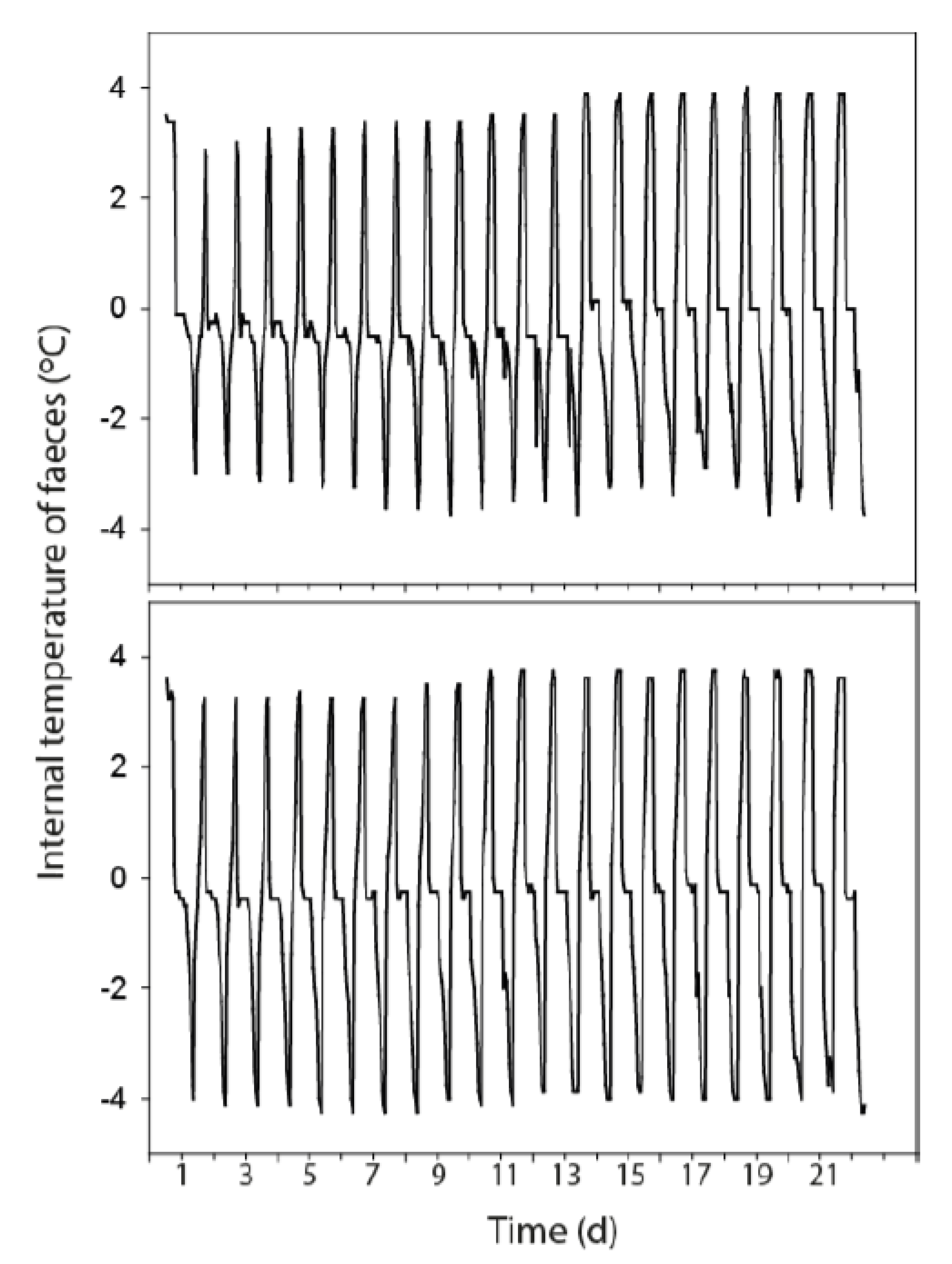
| FIO Concentration in Fresh Faeces (CFU g−1 Dry Weight) | ||||
|---|---|---|---|---|
| Escherichia coli | Intestinal Enterococci | |||
| FIO Source | Mean | SE | Mean | SE |
| Dairy cow | 6.49 | 0.04 | 6.20 | 0.05 |
| Red deer | 5.30 | 0.15 | 5.08 | 0.06 |
| Treatment | Exponential Rate Constant λ (Day−1) | Level of Population Stability A (log10 CFU g−1) | Magnitude of Population Decline B (log10 CFU g−1) | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Dairy, Freeze-thaw (4, 0, −4 °C) | 0.166 | 0.028 | 3.757 | 0.164 | 2.692 | 0.172 |
| Dairy, Freeze-thaw (0, −4, −8 °C) | 0.410 | 0.093 | 2.415 | 0.266 | 3.957 | 0.344 |
| Dairy, constant (4 °C) | 0.157 | 0.023 | 3.995 | 0.144 | 2.572 | 0.146 |
| Dairy, constant (0 °C) | 0.166 | 0.034 | 4.504 | 0.144 | 1.877 | 0.149 |
| Deer, Freeze-thaw (4, 0, −4 °C) | 0.215 | 0.089 | 3.035 | 0.274 | 1.696 | 0.249 |
| Deer, Freeze-thaw (0, −4, −8 °C) | 0.122 | 0.042 | 1.658 | 0.701 | 3.661 | 0.653 |
| Deer, constant (4 °C) | n/a | n/a | n/a | n/a | n/a | n/a |
| Deer, constant (0 °C) | 0.075 | 0.019 | 0.688 | 0.615 | 4.630 | 0.580 |
| Treatment | Exponential Rate Constant λ (Day−1) | Level of Population Stability A (log10 CFU g−1) | Magnitude of Population Decline B (log10 CFU g−1) | |||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | |
| Dairy, Freeze-thaw (4, 0, −4 °C) | 0.091 | 0.029 | 4.738 | 0.208 | 1.402 | 0.189 |
| Dairy, Freeze-thaw (0, −4, −8 °C) | 0.314 | 0.067 | 2.993 | 0.165 | 2.316 | 0.181 |
| Dairy, constant (4 °C) | n/a | n/a | n/a | n/a | n/a | n/a |
| Dairy, constant (0 °C) | 0.360 | 0.040 | 3.981 | 0.064 | 2.171 | 0.086 |
| Deer, Freeze-thaw (4, 0, −4 °C) | 0.206 | 0.093 | 3.525 | 0.280 | 1.512 | 0.256 |
| Deer, Freeze-thaw (0, −4, −8 °C) | 0.121 | 0.026 | 1.864 | 0.282 | 3.232 | 0.269 |
| Deer, constant (4 °C) | 0.155 | 0.073 | 3.980 | 0.233 | 1.002 | 0.216 |
| Deer, constant (0 °C) | 0.085 | 0.027 | 1.586 | 0.557 | 3.527 | 0.525 |
| Treatment | Modelled Linear Decline Rate (Day−1) a | D-Values (Days) | R2 |
|---|---|---|---|
| Deer, Freeze-thaw (4, 0, −4 °C) b | 6.209 & 0.086 | 26.8 c | 0.714 c |
| Deer, Freeze-thaw (0, −4, −8 °C) b | 2.957 & 0.06 | 37.2 c | 0.749 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, E.O.; Quilliam, R.S.; Oliver, D.M. Impact of Freeze–Thaw Cycles on Die-Off of E. coli and Intestinal Enterococci in Deer and Dairy Faeces: Implications for Landscape Contamination of Watercourses. Int. J. Environ. Res. Public Health 2020, 17, 6999. https://doi.org/10.3390/ijerph17196999
Afolabi EO, Quilliam RS, Oliver DM. Impact of Freeze–Thaw Cycles on Die-Off of E. coli and Intestinal Enterococci in Deer and Dairy Faeces: Implications for Landscape Contamination of Watercourses. International Journal of Environmental Research and Public Health. 2020; 17(19):6999. https://doi.org/10.3390/ijerph17196999
Chicago/Turabian StyleAfolabi, Emmanuel O., Richard S. Quilliam, and David M. Oliver. 2020. "Impact of Freeze–Thaw Cycles on Die-Off of E. coli and Intestinal Enterococci in Deer and Dairy Faeces: Implications for Landscape Contamination of Watercourses" International Journal of Environmental Research and Public Health 17, no. 19: 6999. https://doi.org/10.3390/ijerph17196999
APA StyleAfolabi, E. O., Quilliam, R. S., & Oliver, D. M. (2020). Impact of Freeze–Thaw Cycles on Die-Off of E. coli and Intestinal Enterococci in Deer and Dairy Faeces: Implications for Landscape Contamination of Watercourses. International Journal of Environmental Research and Public Health, 17(19), 6999. https://doi.org/10.3390/ijerph17196999





