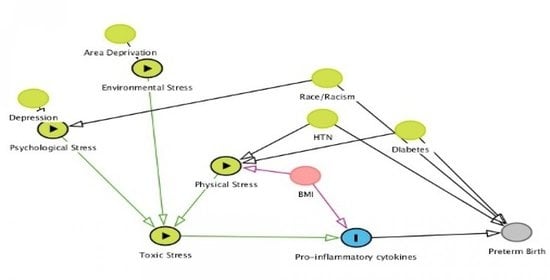
Racial Differences in the Biochemical Effects of Stress in Pregnancy
Abstract
1. Introduction
Environmental Stress Also Contributes to the Disparity
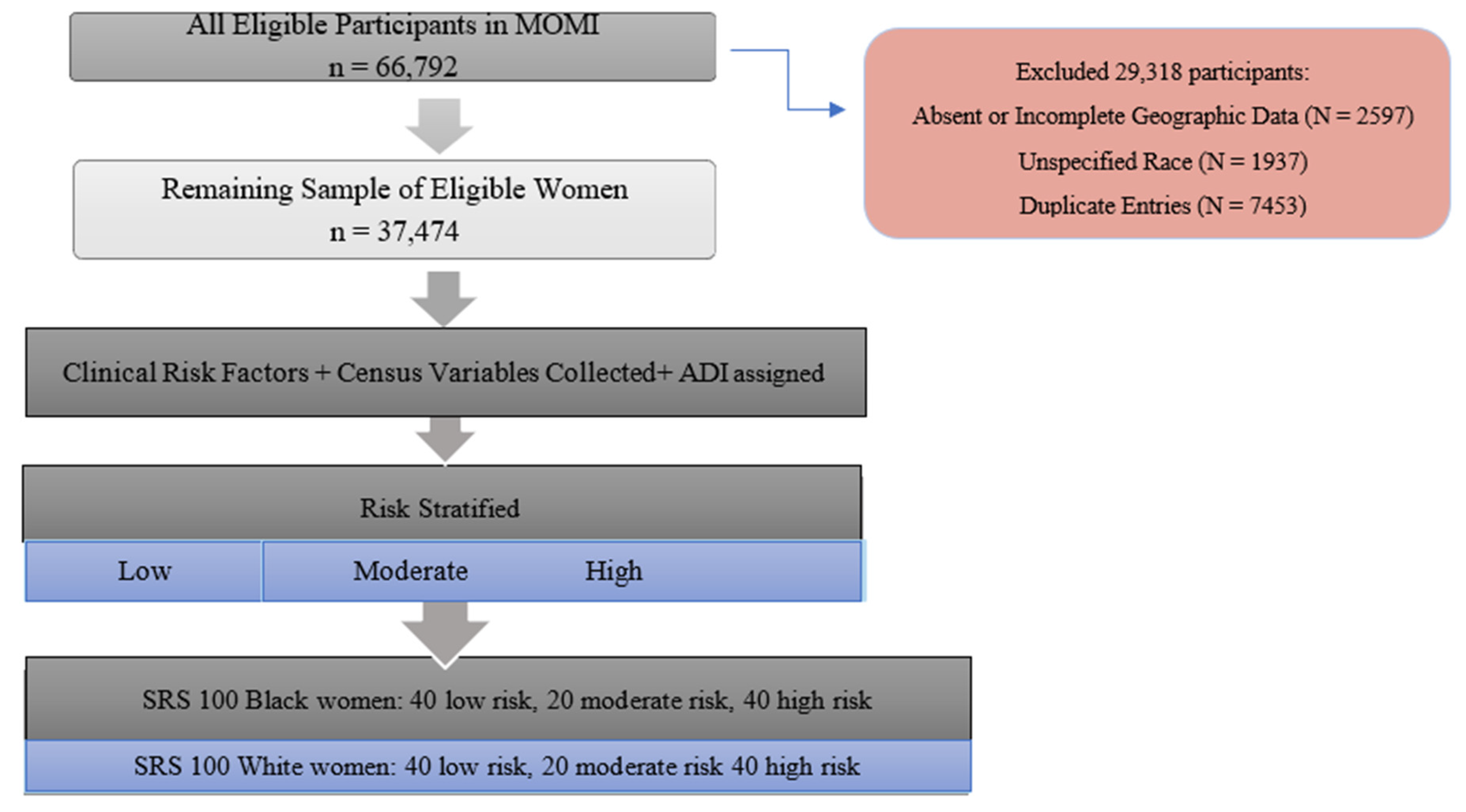
2. Materials and Methods
2.1. Individual-Level Variables
2.2. Census Data
2.3. Area Deprivation Index
2.4. Risk Stratification of Cohort
2.5. Cytokine Analysis
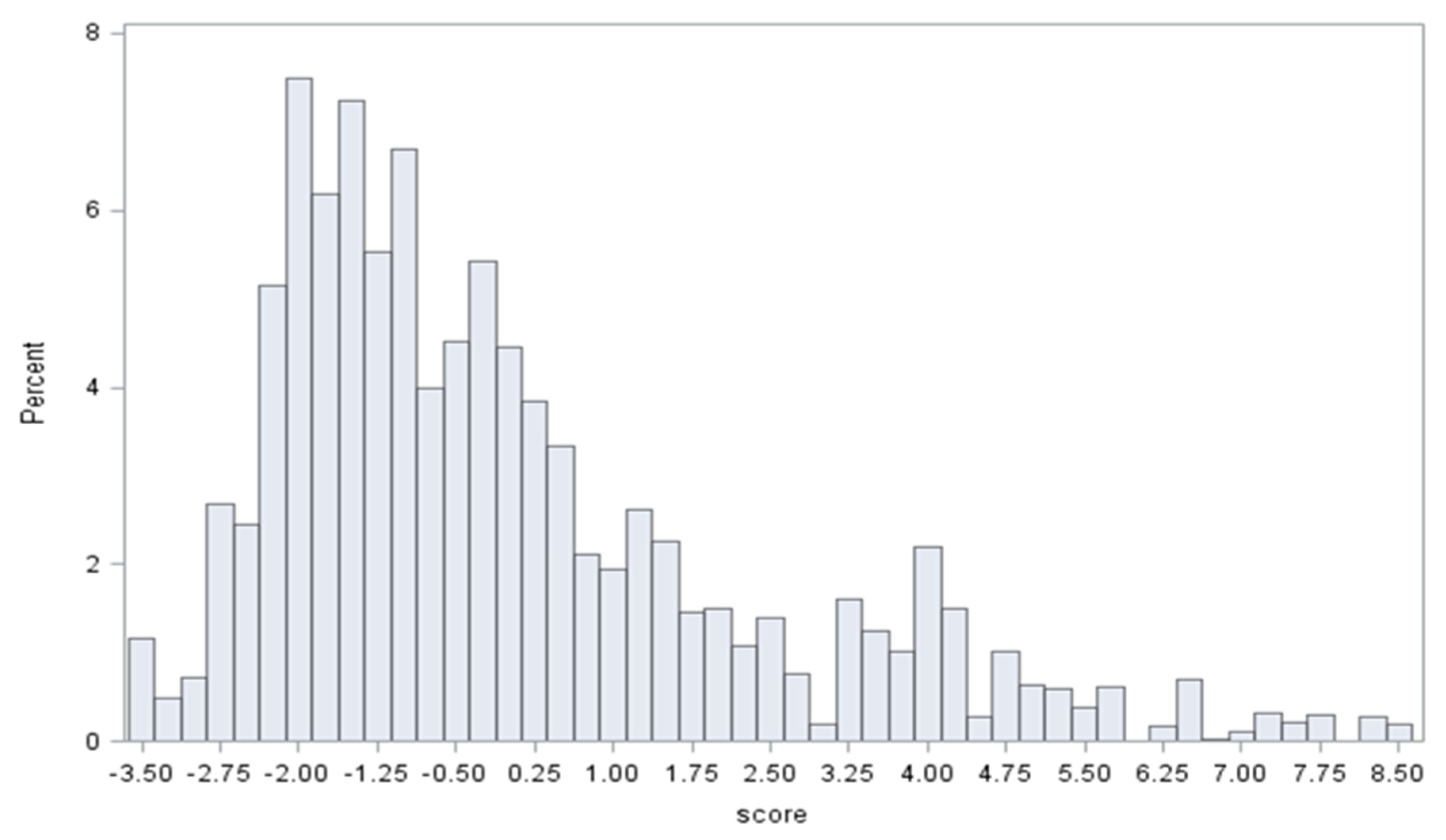
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Approval
References
- Franke, H.A. Toxic Stress: Effects, Prevention and Treatment. Children 2014, 1, 390–402. [Google Scholar] [CrossRef]
- Boyce, W.T.; Ellis, B.J. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005, 17, 271–301. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Sandman, C.A.; Garite, T.J. The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Prog. Brain Res. 2001, 133, 131–142. [Google Scholar] [CrossRef]
- Lannon, S.M.R.; Vanderhoeven, J.P.; Eschenbach, D.A.; Gravett, M.G.; Adams Waldorf, K.M. Synergy and interactions among biological pathways leading to preterm premature rupture of membranes. Reprod. Sci. 2014, 21, 1215–1227. [Google Scholar] [CrossRef]
- Burris, H.H.; Lorch, S.A.; Kirpalani, H.; Pursley, D.M.; Elovitz, M.A.; Clougherty, J.E. Racial disparities in preterm birth in USA: A biosensor of physical and social environmental exposures. Arch. Dis. Child. 2019, 104, 931. [Google Scholar] [CrossRef]
- Kistka, Z.A.; Palomar, L.; Lee, K.A.; Boslaugh, S.E.; Wangler, M.F.; Cole, F.S.; DeBaun, M.R.; Muglia, L.J. Racial disparity in the frequency of recurrence of preterm birth. Am. J. Obstet. Gynecol. 2007, 196, 131.e1–131.e6. [Google Scholar] [CrossRef]
- Samadi, A.R.; Mayberry, R.M. Maternal hypertension and spontaneous preterm births among black women. Obs. Gynecol. 1998, 91, 899–904. [Google Scholar] [CrossRef]
- Katz Eriksen, J.L.; Pilliod, R.A.; Caughey, A.B. 732: Impact of late initiation of prenatal care on pregnancy outcomes among women who use drugs. Am. J. Obstet. Gynecol. 2016, 214, S384. [Google Scholar] [CrossRef]
- Alexander, G.R.; Kogan, M.D.; Nabukera, S. Racial Differences in Prenatal Care Use in the United States: Are Disparities Decreasing? Am. J. Public Health 2002, 92, 1970–1975. [Google Scholar] [CrossRef]
- Rowley, D.L.; Hogan, V. Disparities in infant mortality and effective, equitable care: Are infants suffering from benign neglect? Annu. Rev. Public Health 2012, 33, 75–87. [Google Scholar] [CrossRef]
- Savitz, D.A.; Kaufman, J.S.; Dole, N.; Siega-Riz, A.M.; Thorp, J.M., Jr.; Kaczor, D.T. Poverty, education, race, and pregnancy outcome. Ethn. Dis. 2004, 14, 322–329. [Google Scholar]
- Schoendorf, K.C.; Hogue, C.J.R.; Kleinman, J.C.; Rowley, D. Mortality among Infants of Black as Compared with White College-Educated Parents. N. Engl. J. Med. 1992, 326, 1522–1526. [Google Scholar] [CrossRef]
- James, W.; Collins, J.; David, R.J.; Handler, A.; Wall, S.; Andes, S. Very Low Birthweight in African American Infants: The Role of Maternal Exposure to Interpersonal Racial Discrimination. Am. J. Public Health 2004, 94, 2132–2138. [Google Scholar] [CrossRef]
- Braveman, P.A.; Heck, K.; Egerter, S.; Marchi, K.S.; Dominguez, T.P.; Cubbin, C.; Fingar, K.; Pearson, J.A.; Curtis, M. The Role of Socioeconomic Factors in Black–White Disparities in Preterm Birth. Am. J. Public Health 2015, 105, 694–702. [Google Scholar] [CrossRef]
- Lu, M.C.; Chen, B. Racial and ethnic disparities in preterm birth: The role of stressful life events. Am. J. Obstet. Gynecol. 2004, 191, 691–699. [Google Scholar] [CrossRef]
- Mendez, D.D.; Hogan, V.K.; Culhane, J.F. Institutional racism, neighborhood factors, stress, and preterm birth. Ethn. Health 2014, 19, 479–499. [Google Scholar] [CrossRef]
- Messer, L.C.; Kaufman, J.S.; Dole, N.; Savitz, D.A.; Laraia, B.A. Neighborhood Crime, Deprivation, and Preterm Birth. Ann. Epidemiol. 2006, 16, 455–462. [Google Scholar] [CrossRef]
- Wallace, M.; Harville, E.; Theall, K.; Webber, L.; Chen, W.; Berenson, G. Neighborhood poverty, allostatic load, and birth outcomes in African American and white women: Findings from the Bogalusa Heart Study. Health Place 2013, 24, 260–266. [Google Scholar] [CrossRef]
- Rudnicki, S.R.; Graham, J.L.; Habboushe, D.F.; Ross, R.D. Social support and avoidant coping: Correlates of depressed mood during pregnancy in minority women. Women Health 2001, 34, 19–34. [Google Scholar] [CrossRef]
- Giurgescu, C.; Sanguanklin, N.; Engeland, C.G.; White-Traut, R.C.; Park, C.; Mathews, H.L.; Janusek, L.W. Relationships among psychosocial factors, biomarkers, preeclampsia, and preterm birth in African American women: A pilot. Appl. Nurs. Res. 2015, 28, e1–e6. [Google Scholar] [CrossRef]
- Dayan, J.; Creveuil, C.; Marks, M.N.; Conroy, S.; Herlicoviez, M.; Dreyfus, M.; Tordjman, S. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosom. Med. 2006, 68, 938–946. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Pickler, R.H. Perinatal stress, fatigue, depressive symptoms, and immune modulation in late pregnancy and one month postpartum. Sci. World J. 2014, 2014, 652630. [Google Scholar] [CrossRef]
- Erickson, K.; Thorsen, P.; Chrousos, G.; Grigoriadis, D.E.; Khongsaly, O.; McGregor, J.; Schulkin, J. Preterm Birth: Associated Neuroendocrine, Medical, and Behavioral Risk Factors1. J. Clin. Endocrinol. Metab. 2001, 86, 2544–2552. [Google Scholar] [CrossRef]
- Premji, S. Perinatal distress in women in low- and middle-income countries: Allostatic load as a framework to examine the effect of perinatal distress on preterm birth and infant health. Matern. Child Health J. 2014, 18, 2393–2407. [Google Scholar] [CrossRef]
- Wallace, M.; Harville, E.; Theall, K.; Webber, L.; Chen, W.; Berenson, G. Preconception biomarkers of allostatic load and racial disparities in adverse birth outcomes: The Bogalusa Heart Study. Paediatr. Perinat. Epidemiol. 2013, 27, 587–597. [Google Scholar] [CrossRef]
- Burris, H.H.; Collins, J.W., Jr.; Wright, R.O. Racial/ethnic disparities in preterm birth: Clues from environmental exposures. Curr. Opin. Pediatr. 2011, 23, 227–232. [Google Scholar] [CrossRef]
- Wright, R.J.; Visness, C.M.; Calatroni, A.; Grayson, M.H.; Gold, D.R.; Sandel, M.T.; Lee-Parritz, A.; Wood, R.A.; Kattan, M.; Bloomberg, G.R.; et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am. J. Respir. Crit. Care Med. 2010, 182, 25–33. [Google Scholar] [CrossRef]
- Coussons-Read, M.E.; Okun, M.L.; Schmitt, M.P.; Giese, S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom. Med. 2005, 67, 625–631. [Google Scholar] [CrossRef]
- Mtali, Y.S.; Lyimo, M.A.; Luzzatto, L.; Massawe, S.N. Hypertensive disorders of pregnancy are associated with an inflammatory state: Evidence from hematological findings and cytokine levels. BMC Pregnancy Childbirth 2019, 19, 237. [Google Scholar] [CrossRef]
- LaMarca, B.D.; Ryan, M.J.; Gilbert, J.S.; Murphy, S.R.; Granger, J.P. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr. Hypertens. Rep. 2007, 9, 480–485. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Achtyes, E.; Keaton, S.A.; Smart, L.; Burmeister, A.R.; Heilman, P.L.; Krzyzanowski, S.; Nagalla, M.; Guillemin, G.J.; Escobar Galvis, M.L.; Lim, C.K.; et al. Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav. Immun. 2020, 83, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Franco, A.; Glaser, R.; Iams, J.D. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav. Immun. 2009, 23, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Taneja, V.; Vassallo, R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012, 91, 142–149. [Google Scholar] [CrossRef]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Astiz, M.; Bellini, M.J.; Garcia-Segura, L.M. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav. Immun. 2013, 28, 196–206. [Google Scholar] [CrossRef]
- Messer, L.C.; Laraia, B.A.; Kaufman, J.S.; Eyster, J.; Holzman, C.; Culhane, J.; Elo, I.; Burke, J.G.; O’Campo, P. The development of a standardized neighborhood deprivation index. J. Urban Health 2006, 83, 1041–1062. [Google Scholar] [CrossRef]
- Nurius, P.S.; Green, S.; Logan-Greene, P.; Longhi, D.; Song, C. Stress pathways to health inequalities: Embedding ACEs within social and behavioral contexts. Int. Public Health J. 2016, 8, 241–256. [Google Scholar]
- Ouellet-Morin, I.; Odgers, C.L.; Danese, A.; Bowes, L.; Shakoor, S.; Papadopoulos, A.S.; Caspi, A.; Moffitt, T.E.; Arseneault, L. Blunted Cortisol Responses to Stress Signal Social and Behavioral Problems Among Maltreated/Bullied 12-Year-Old Children. Biol. Psychiatry 2011, 70, 1016–1023. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Carvalho, J.P.; Tyrka, A.R.; Wier, L.M.; Mello, A.F.; Mello, M.F.; Anderson, G.M.; Wilkinson, C.W.; Price, L.H. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol. Psychiatry 2007, 62, 1080–1087. [Google Scholar] [CrossRef]
- Tyrka, A.R.; Wier, L.; Price, L.H.; Ross, N.; Anderson, G.M.; Wilkinson, C.W.; Carpenter, L.L. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol. Psychiatry 2008, 63, 1147–1154. [Google Scholar] [CrossRef]
- Hankin, B.L.; Badanes, L.S.; Abela, J.R.; Watamura, S.E. Hypothalamic-pituitary-adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biol. Psychiatry 2010, 68, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Hellhammer, D.H.; Kirschbaum, C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999, 61, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Mays, V.M.; Coleman, L.M.; Jackson, J.S. Perceived race-based discrimination, employment status, and job stress in a national sample of black women: Implications for health outcomes. J. Occup. Health Psychol. 1996, 1, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.C.; Everett, J.E.; Hamilton-Mason, J. Black Women Talk About Workplace Stress and How They Cope. J. Black Stud. 2012, 43, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R.; Mohammed, S.A. Racism and Health I: Pathways and Scientific Evidence. Am. Behav. Sci. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.C.; Link, B.G.; Tehranifar, P. Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. J. Health Soc. Behav. 2010, 51 (Suppl. S1), S28–S40. [Google Scholar] [CrossRef]
- Hoffman, K.M.; Trawalter, S.; Axt, J.R.; Oliver, M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 2016, 113, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Schulman, K.A.; Berlin, J.A.; Harless, W.; Kerner, J.F.; Sistrunk, S.; Gersh, B.J.; Dube, R.; Taleghani, C.K.; Burke, J.E.; Williams, S.; et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N. Engl. J. Med. 1999, 340, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Dehlendorf, C.; Foster, D.G.; de Bocanegra, H.T.; Brindis, C.; Bradsberry, M.; Darney, P. Race, ethnicity and differences in contraception among low-income women: Methods received by Family PACT Clients, California, 2001–2007. Perspect. Sex. Reprod. Health 2011, 43, 181–187. [Google Scholar] [CrossRef]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Ford, C.L.; Airhihenbuwa, C.O. Critical Race Theory, Race Equity, and Public Health: Toward Antiracism Praxis. Am. J. Public Health 2010, 100 (Suppl. S1), S30–S35. [Google Scholar] [CrossRef]
| White n = 26,232 | Black n = 8994 | p-Value | |
|---|---|---|---|
| Preterm | 2360 (9.0%) | 1097 (12.2%) | <0.0001 |
| Small for Gestational Age(SGA) | 2177 (8.3%) | 1475 (16.4%) | <0.0001 |
| Age | 29.6 (SD 5.4) | 24.8 (SD 5.7) | <0.0001 |
| Nulliparity | 7686 (29.3%) | 3238 (36.0%) | <0.0001 |
| Hypertension | 1679 (6.4%) | 827 (9.2%) | <0.0001 |
| Diabetes | 1653 (6.3%) | 441 (4.9%) | <0.0001 |
| Smoking | 4194 (16.0%) | 1070 (12.3%) | <0.0001 |
| History of Depression | 2308 (8.8%) | 998 (11.1%) | <0.0001 |
| Relationship Status (Committed, Married or Life Partner) | 12,977 (49.5%) | 875 (11.9%) | <0.0001 |
| High School Diploma or less | 9050 (34.5%) | 5726 (63.7%) | <0.0001 |
| Pre-pregnancy weight(kg) | 68.6 (SD 16.8) | 73.7 (20.2) | <0.0001 |
| BMI | 25.4 (SD 5.90) | 27.4 (SD 7.1) | <0.0001 |
| Birth weight(g) | 3349 (SD 580) | 3097 (SD 636) | <0.0001 |
| White (%) | Black (%) | p-Value | |
|---|---|---|---|
| Preterm | 8 | 11 | 0.54 |
| SGA | 6 | 15 | 0.01 * |
| History of Depression | 8 | 12 | 0.39 |
| Hypertension | 11 | 9 | 0.57 |
| Diabetes | 5 | 6 | 0.81 |
| Smoking | 13 | 13 | 0.99 |
| Committed Relationship | 48 | 29 | 0.01 * |
| High School Diploma or less | 42 | 31 | 0.16 |
| BMI > 25 | 6 | 3 | 0.28 |
| Nulliparous | 34 | 29 | 0.43 |
| Age(Years) | 32.4 | 26.8 | 0.03 * |
| Low Risk n = 85 | Moderate Risk n = 39 | High Risk n = 77 | p-Value | |
|---|---|---|---|---|
| BMI > 25 | 3 (3.5%) | 2 (5.1%) | 4 (5.2%) | 0.88 |
| Hx of Diabetes | 2 (2.4%) | 5 (12.8%) | 4 (5.2%) | 0.086 |
| Single | 9 (10.6%) | 25 (64.1%) | 44 (57.1%) | <0.0001 * |
| Hx of Hypertension | 5 (5.9%) | 3 (7.7%) | 12 (15.6%) | <0.01 * |
| ≤HS diploma | 8 (9.4%) | 24 (61.5%) | 42 (54.6%) | <0.0001 * |
| Smoker | 5 (5.9%) | 3 (7.7%) | 23 (29.9%) | <0.0001 * |
| Hx of Depression | 1 (1.2%) | 2(5.1%) | 17 (22.1%) | <0.0001 * |
| Preterm Birth | 6 (7.1%) | 1 (2.6%) | 12 (15.6%) | 0.05 * |
| Nulliparity | 12 (16.5%) | 3 (7.7%) | 17 (22.1%) | 0.07 |
| Mean Age (Years) | 25.3 | 28.8 | 26.6 | 0.79 |
| IL-6 | IL-8 | IL-10 | IL-13 | TNFA | ADI | |
|---|---|---|---|---|---|---|
| IL-6 | 1.00 | 0.38 | 0.58 | 0.83 | 0.12 | −0.16 |
| p-value | -- | <0.001 * | <0.001 * | <0.001 * | 0.10 | 0.03 * |
| IL-8 | 0.38 | 1.00 | 0.13 | 0.30 | 0.18 | 0.01 |
| p-value | <0.001 * | -- | 0.07 | <0.001 * | 0.01 | 0.85 |
| IL-10 | 0.58 | 0.13 | 1.00 | 0.60 | 0.08 | −0.10 |
| p-value | <0.001 * | 0.07 | -- | <0.001 * | 0.28 | 0.16 |
| IL-13 | 0.83 | 0.30 | 0.60 | 1.00 | 0.09 | −0.10 |
| p-value | <0.001 * | <0.001 * | <0.001 * | -- | 0.20 | 0.17 |
| TNFa | 0.12 | 0.18 | 0.08 | 0.09 | 1.00 | −0.003 |
| p-value | 0.10 | 0.01 | 0.28 | 0.20 | -- | 0.97 |
| Low Risk (0–1 Risk Factors) n = 85 | Medium Risk (2 Risk Factors) n = 39 | High Risk (3+ Risk Factors) n = 77 | p-Value | |
|---|---|---|---|---|
| IL-6 | 10.0 (6.6, 23.3) | 8.9 (6.2, 21.2) | 10.6 (6.5, 23.3) | 0.91 |
| IL-8 | 12.9 (8.7, 30.5) | 13.4 (9.0, 36.2) | 12.2 (7.9, 32.3) | 0.75 |
| IL-10 | 35.9 (25.4, 59.1) | 34.9 (19.3, 51.1) | 34.6 (26.1, 48.6) | 0.63 |
| IL-13 | 7.8 (5.6, 14.1) | 7.6 (5.3, 16.1) | 8.0 (5.3, 14.5) | 0.87 |
| TNF-a | 8.1 (6.1, 10.4) | 7.7 (6.0, 10.8) | 8.1 (6.7, 10.8) | 0.63 |
| Black Women Median (p25, p75) | White Women Median (p25, p75) | p-Value | |
|---|---|---|---|
| IL-6 | 8.8 (5.3, 25.5) | 10.6 (7.5,23.3) | 0.04 * |
| IL-8 | 13.6 (8.0, 40.2) | 12.2 (8.0, 24.6) | 0.43 |
| IL-10 | 36.0 (25.2, 47.7) | 34.9 (23.8, 58.0) | 0.39 |
| IL-13 | 7.7 (5.2, 15.5) | 8.3 (5.6, 14.5) | 0.35 |
| TNF-a | 7.7 (6.1, 10.4) | 8.5 (6.4, 10.8) | 0.39 |
| Low Risk (0–1 Risk Factors) n = 85 | Medium Risk (2 Risk Factors) n = 39 | High Risk (3+ Risk Factors) n = 77 | p-Value 1 | |
|---|---|---|---|---|
| IL-6 | ||||
| White | 10.3 (7.6, 20.5) | 8.2 (6.4, 17.7) | 13.9 (8.6, 26.4) | 0.40 |
| Black | 8.9 (5.7, 25.4) | 8.9 (5.8, 26.9) | 7.9 (4.2, 19.8) | 0.90 |
| p-value 2 | p = 0.24 | p = 0.79 | p = 0.05 * | |
| IL-8 | ||||
| White | 10.2 (7.5, 30.0) | 12.3 (9.0, 46.4) | 12.7 (8.6, 20.5) | 0.45 |
| Black | 15.2 (10.8, 30.5) | 13.6 (10.5, 28.4) | 9.7 (7.0, 59.4) | 0.43 |
| p-value 2 | p = 0.06 | p = 0.57 | p = 0.33 | |
| IL-10 | ||||
| White | 42.3 (28.9, 67.6) | 28.4 (16.3, 58.0) | 32.1 (22.1, 49.4) | 0.11 |
| Black | 31.5 (22.8, 47.0) | 37.7 (20.8, 45.2) | 38.4 (26.9, 48.6) | 0.29 |
| p-value 2 | p = 0.02 * | p = 0.57 | p = 0.33 | |
| IL-13 | ||||
| White | 8.5 (5.8, 14.0) | 7.4 (5.2, 13.5) | 8.3 (6.6, 14.5) | 0.68 |
| Black | 7.40 (5.0, 14.1) | 7.8 (5.7, 16.1) | 7.9 (4.8, 15.8) | 0.98 |
| p-value 2 | p = 0.35 | p = 0.83 | p = 0.51 | |
| TNFa | ||||
| White | 8.5 (6.5, 11.1) | 8.5 (6.0, 10.3) | 8.4 (6.7, 10.4) | 0.96 |
| Black | 7.7 (5.9, 9.0) | 7.4 (6.3, 10.8) | 7.8 (6.7, 11.3) | 0.34 |
| p-value 2 | p = 0.19 | p = 0.78 | p = 0.98 |
| Low Risk (0–1 Risk Factors) n = 85 | Medium Risk (2 Risk Factors) n = 39 | High Risk (3+ Risk Factors) n = 77 | p-Value 1 | |
|---|---|---|---|---|
| IL-6 | ||||
| White | 13.9 (SE 2.2) | 17.2 (SE 7.2) | 16.3 (SE 6.9) | 0.52 |
| Black | 9.6 (SE 1.5) | 16.6 (SE 8.9) | 16.2 (SE 8.1) | 0.35 |
| p-value 2 | p = 0.02 * | p = 0.79 | p = 0.99 | |
| IL-8 | ||||
| White | 11.4 (SE 3.4) | 18.6 (SE 5.8) | 18.9 (SE 2.2) | 0.45 |
| Black | 11.6 (SE 2.7) | 18.0 (SE 5.2) | 12.1 (SE 3.2) | 0.78 |
| p-value 2 | p = 0.75 | p = 0.98 | p = 0.02 * | |
| IL-10 | ||||
| White | 43.0 (SE 16.2) | 44.1 (SE 8.8) | 56.4 (SE 16.7) | 0.82 |
| Black | 62.8 (SE 19.5 ) | 63.8 (SE 8.6) | 74.8 (SE 6.6) | 0.91 |
| p-value 2 | p = 0.20 | p = 0.09 | p = 0.40 | |
| IL-13 | ||||
| White | 6.6 (SE 8.8) | 8.1 (SE 4.7) | 7.3 (SE 3.6) | 0.79 |
| Black | 4.2 (SE 10.7) | 7.8 (SE 4.7) | 7.2 (SE 3.8) | 0.34 |
| p-value 2 | p = 0.26 | p = 0.93 | p = 0.81 | |
| TNFa | ||||
| White | 10.9 (SE 2.2) | 13.2 (SE 1.2) | 10.1 (SE 0.9) | 0.12 |
| Black | 14.3 (SE 2.6) | 15.8 (SE 1.4) | 13.7 (SE 0.8) | 0.84 |
| p-value 2 | p = 0.36 | p = 0.09 | p = 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekeke, P.; Mendez, D.D.; Yanowitz, T.D.; Catov, J.M. Racial Differences in the Biochemical Effects of Stress in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 6941. https://doi.org/10.3390/ijerph17196941
Ekeke P, Mendez DD, Yanowitz TD, Catov JM. Racial Differences in the Biochemical Effects of Stress in Pregnancy. International Journal of Environmental Research and Public Health. 2020; 17(19):6941. https://doi.org/10.3390/ijerph17196941
Chicago/Turabian StyleEkeke, Paris, Dara D. Mendez, Toby D. Yanowitz, and Janet M. Catov. 2020. "Racial Differences in the Biochemical Effects of Stress in Pregnancy" International Journal of Environmental Research and Public Health 17, no. 19: 6941. https://doi.org/10.3390/ijerph17196941
APA StyleEkeke, P., Mendez, D. D., Yanowitz, T. D., & Catov, J. M. (2020). Racial Differences in the Biochemical Effects of Stress in Pregnancy. International Journal of Environmental Research and Public Health, 17(19), 6941. https://doi.org/10.3390/ijerph17196941