1. Introduction
Microalgae are photosynthetic eukaryotic microorganisms that live in the sea and were one of the first forms of life on Earth [
1]. The number of microalgae species is estimated to range from 45,000 to more than 100,000 [
2], and they have been used as a food source for humans for over a thousand years [
3].
Microalgae can be considered a promising food due to their nutritional characteristics [
4], and their industrial cultivation has increased in recent decades [
5]. They have been used in the production of functional foods [
6], animal feeds, biofuels [
7] and cosmetics [
8].
The effects of microalgae supplementation on humans have been studied previously [
9,
10], and marine bioactive peptides from microalgae have shown therapeutic potential in the treatment or prevention of disease [
11]. The different effects of microalgae supplementation are anti-inflammatory [
12], antioxidant [
13], hypotensive [
14] and hypolipidemic [
15], among others. Supplementation with some microalgae such as spirulina and chlorella has been well studied [
16,
17]; however, the effect of supplementation with
Tetraselmis chuii (TC) is unknown.
TC is green microalgae discovered in the 1950s, and is a unicellular, mobile, 4 to 15 μm size microseaweed, corresponding to the class Prasinophyceae [
18]. It is present in the diet of mussels, oysters, clams, scallops and corals [
19], representing a species of marine microalgae that is easy to grow and safe to eat [
20]. Proportional to its size, TC has a high concentration of amino acids, essential fatty acids, vitamins and minerals (
Table 1) [
21,
22].
The administration of sports supplements in soccer has become a standard procedure, often promoted by team physicians, coaches and even the parents of young players [
23]. However, supplementation with microalgae is not common in sport, although their intake has been gradually introduced into sport due to the bioactive peptides they contain [
24].
Spirulina platensis positively modifies bone marrow production and the cellular immune response, and may be effective as an adjuvant treatment in anaemia or immunodeficiency [
25]. Similarly, other studies observed increases in maximum oxygen consumption (VO
2max) after supplementation with other microalgae, such as spirulina or chlorella [
26,
27]. Nevertheless, the literature about TC supplementation is scarce, and it would be interesting to know the impact of supplementation in humans due to its aforementioned properties. Therefore, this research aimed to evaluate the effects of TC microalgae supplementation during thirty days on ergospirometric parameters in amateur soccer players. In addition, the possible positive or negative effects on haematological and biochemical parameters were investigated.
2. Materials and Methods
2.1. Subjects
Thirty-two male players from a third division Spanish club participated in the study. The subjects were randomised into two groups: control group (CG; n = 16; 22.36 ± 1.36 years; 68.36 ± 3.53 kg; 1.74 ± 0.44 m) and supplemented group (SG; n = 16; 22.23 ± 2.19 years; 69.30 ± 5.56 kg; 1.73 ± 0.35 m). All participants were informed about the purpose of the study and signed a consent form before enrolling. The protocol was reviewed and approved by the Biomedical Ethics Committee of the University of Extremadura (Cáceres, Spain) following the guidelines of the Helsinki declaration of ethics, updated at the World Medical Assembly in Fortaleza (2013), for research involving human subjects (registration code: 99/2016). A code was assigned to each participant for the collection and treatment of the samples in order to maintain their anonymity. To be considered a healthy male and included in the study, participants had to comply with the inclusion criteria: not have haematological problems, not have altered values in the last blood analysis, not have anaemia problems, have four years of minimum training experience, be a nonsmoker, not have taken any supplementation, medication or over-the-counter medication, drug or alcohol in the previous two weeks and not to change their nutritional habits during the study.
At the beginning of the study, the participants completed a physical activity questionnaire (IPAQ) [
28] and had a medical examination to detect any abnormalities. The participants performed 2.9 ± 0.55 metabolic equivalent of task (MET)-hour/day and no case abnormalities were reported.
2.2. Study Design
This research had a double-blind design. SG ingested a 25 mg capsule per day of powdered TC (TetraSOD
®, El Puerto de Santa Maria, Andalucía, Spain) whereas CG ingested a 200 mg placebo tablet containing lactose powder. The nutritional value per day of the placebo pill was 22.6 kcal, 0.4 g water, 5.8 g carbohydrate, 0.1 g protein and 0.09 g lipids. Participants ingested the capsules for thirty days. Both capsules had identical designs to avoid interpretation among subjects. Participants were recommended to ingest all capsules at 10:00 a.m. for homogeneity of results.
Table 1 shows the composition of TC [
29].
The measurements were taken on the day previous to the beginning of the supplementation and after thirty days of supplementation. Participants did not intake TC on the day of the assessments. The assessments were performed after two days of inactivity to avoid the influence of training fatigue.
2.3. Blood Extraction and Determination of Haematological and Biochemical Parameters
Participants arrived between 8:00–9:00 a.m. for the extraction of 10 mL of venous blood from the antecubital vein. All extractions were carried out in fasting conditions. The blood sample was collected in a polypropylene tube. A 200 µL sample was taken from each blood tube and precipitated in a ladle and placed in the coulter (Coulter Electronics LTD, Model 6706319; Northwell Drive, Luton, UK) to obtain haematological data. For biochemical parameters, the blood was collected in 5 mL tubes containing ethylenediaminetetraacetic acid (EDTA) as anticoagulants and were centrifuged at 2500 rpm for 10 min. The plasma was separated and the biochemical parameters were determined by spectrophotometric techniques (Coulter Electronics LTD, Model CPA; Northwell Drive, Luton, UK).
2.4. Anthropometry
The anthropometric characteristics were measured in the morning at the same time after an overnight fast, always by the same researcher. A Seca
© 769 (Seca, Hamburg, Germany) scale, with an accuracy of ±100 g; a Seca
© 220 (Seca, Hamburg, Germany) measuring rod, accurate to ±1 mm; a Holtain
© 610ND (Holtain, Crymych, UK) skinfold compass, accurate to ±0.2 mm; a Holtain
© 604 (Holtain, Crymych, UK) bone diameter compass, accurate to ±1 mm; and a Seca
© 201 (Seca, Hamburg, Germany) brand tape measure, accurate to ±1 mm, were used for the anthropometric assessments. The equations of the Spanish Group of Kinanthropometry [
30] were used to calculate the muscle, fat and bone percentage. The anthropometric measurements obtained were height, weight, skinfolds (abdominal, suprailiac, subscapular, tricipital, thigh and leg), bone diameters (bistyloid, humeral biepicondyle and femoral biepicondyle) and muscle perimeters (relaxed arm and leg).
2.5. Nutritional Evaluation
All participants completed a nutritional survey in the first and last week of the study to guarantee that they were following a similar diet. The survey consisted of a 4-day daily nutritional record, of three preassigned week days, and one weekend day. The participants individually indicated the type, frequency and quantity (in grams) of each food consumed each day, and the nutritional composition of their diets was evaluated using different food composition tables [
31].
2.6. Maximum Incremental Test and Threshold Determination
Subjects performed an incremental ergospirometric test on a treadmill (Ergofit Trac Alpin 4000, Germany). The test started with a warm-up at 8 km/h for 10 min and was increased by 1 km/h every two minutes until voluntary exhaustion. The tests were carried out in the laboratory with ambient conditions of 23 ± 2 °C (45–55% relative humidity). Physiological ergospirometric parameters were monitored with a gas analyser (Metamax model no. 762014-102, Cortex, Germany), and heart rate (HR) was monitored with a pulsometer (Polar® “Vantage M”, Kempele, Finland) with sensor band (Polar® H10, Kempele, Finland). All tests were performed from 11:30 a.m. onwards in the same order to avoid the effects of circadian cycles.
After recording the test data, the ventilatory thresholds were determined according to the three-phase model [
32]. The data were obtained at the aerobic threshold (VT1), the anaerobic threshold (VT2), maximum values of the incremental test and after three minutes of recovery.
2.7. Statistical Analysis
Statistical analyses were performed with SPSS 20.0 for Windows (SPSS Inc., Chicago, IL, USA). The normality of the distribution of variables was analysed using the Shapiro–Wilk test and the homogeneity of the variances with the Levene test. A paired samples t-test was used to compare the differences between pre- and post-supplementation and an independent samples t-test was used to compare the differences between CG and SG. A p < 0.05 was considered statistically significant. Results are expressed as means ± standard deviation.
3. Results
The results obtained are presented below, before supplementation (pre) and after supplementation (post).
Table 2 shows the anthropometric values in both groups. No significant changes were observed between groups.
Table 3 shows the intake of macronutrients in both groups. There was no significant difference in total macronutrient intake.
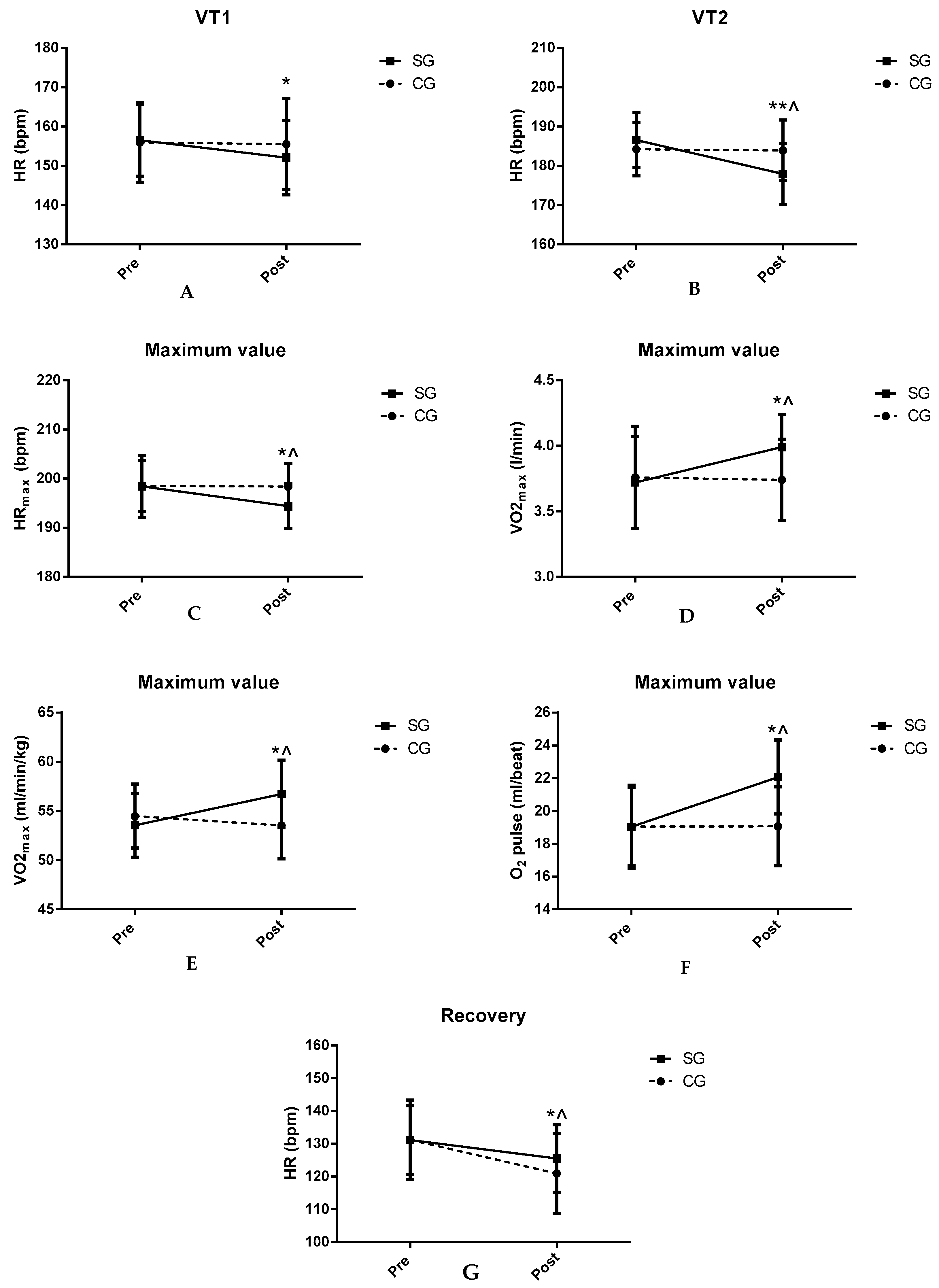
Table 4 shows the ergospirometric data of VT1 in both groups. There was a significant decrease in HR in the SG after TC supplementation (
p < 0.05). There were no significant differences in the other parameters.
Table 5 shows the results obtained for both groups at VT2. A significant decrease can be observed in HR in SG after TC supplementation compared to CG and baseline values (
p < 0.01). There were no significant differences in the remaining parameters.
Table 6 shows the maximum values obtained in the incremental test. Decreases in HRmax were observed after TC supplementation in SG compared to baseline (
p < 0.05) and CG (
p < 0.05). In SG, there were significant increases in oxygen pulse, and maximum absolute and relative oxygen consumption (VO
2) values (
p < 0.05).
Table 7 shows the ergospirometric values obtained after 3 min of recovery at the end of the maximum incremental test. Significant decreases in HR in SG were found after TC supplementation compared to CG and baseline values (
p < 0.05). There were no significant differences in the other parameters.
Figure 1 shows the key findings.
Table 8 shows the biochemical parameters studied in both groups. There were increases in glucose (
p < 0.01), uric acid (
p < 0.05) and creatinine (
p < 0.01) values after TC supplementation in SG. In addition, the previous parameters after TC supplementation were higher in SG compared to CG.
Table 9 shows the values obtained for the different haematological parameters studied in both groups. There were increases in haemoglobin (
p < 0.01) and mean corpuscular haemoglobin (MCH) (
p < 0.05) values after TC supplementation in SG compared to CG and baseline values. Haematocrit values and MPV decreased in SG after TC supplementation (
p < 0.05).
4. Discussion
The aim of the present study was to analyse the effect of TC (TetraSOD® El Puerto de Santa Maria, Andalucía, Spain) supplementation on ergospirometric, haematological and biochemical parameters in amateur soccer players. To our knowledge, this is the first study to evaluate the impact of TC supplementation in athletes. TC supplementation during thirty days produced decreases in HR and increased absolute VO2max, relative VO2max, oxygen pulse, haemoglobin and mean corpuscular haemoglobin (MCH) (p < 0.05) In addition, glucose, uric acid and creatinine were higher in SG. No changes were observed in CG, which could indicate that the changes in SG could be due to TC supplementation. It should be noted that there were no differences in nutritional intake. All biochemical parameters were maintained in normal ranges. No negative effects on the body were observed.
TetraSOD
® is a unique commercial product composed of 100% lyophilised TC that is currently marketed for food and nutraceutical applications. In 2017, the European Union gave approval to the company to market its lyophilised TC for use in dietary supplements such as TetraSOD
®, at levels of up to 250 mg/day [
20].
The decrease of HR in SG could be positively related to arterial stiffness [
33,
34]. Previous studies have investigated the effects of the supplementation of other green microalgae, the microalgae chlorella, on arterial stiffness. Otsuki et al. (2013) analysed the effect of 200 mg chlorella supplementation in fourteen men over twelve weeks in a double-blind trial [
35]. They concluded that chlorella supplementation could decrease arterial stiffness due to the nutrients it contains. Another study analysed the supplementation of 200 mg of chlorella in thirty-two subjects, with the authors concluding that multicomponent supplementation derived from chlorella decreased arterial stiffness [
36]. Thus, it could be assumed that differences in HR may be due to changes in arterial stiffness; when artery walls lose their elastic properties and become stiff, they elicit a rise in systolic blood pressure and the heart’s workload [
37].
Some components present in TC may positively affect arterial stiffness; minerals such as potassium could decrease it [
38], as well as unsaturated fatty acids through their anti-inflammatory function [
39]. According to the results obtained, TC could reduce arterial stiffness due to the nutrients it contains and, therefore, decrease HR. The decrease in HR is related to the increase in O
2 pulse [
40]. This could indicate a better metabolic economy of effort by the body.
Concerning ergospirometric values, SG recorded an increase in absolute and relative VO
2max (
p < 0.05). Other research has analysed the effect of supplementation with other microalgae in different groups. For example, Hernández-Lepe et al. (2018) evaluated an exercise programme with and without spirulina, and a nonexercise programme with and without spirulina, in sedentary overweight and obese people [
26]. They observed that supplementation of 4.5 g/day for six weeks with spirulina increased the relative VO
2max in the groups that consumed the microalgae without the exercise programme. Additionally, they reported a decrease in resting HR and an increase in the maximum lactate steady state. Another crossover and double-blind study reported a rise in relative VO
2max after twice-daily supplementation with chlorella (200 mg) for four weeks in ten young people [
41]. In a double-blind trial by Zempo-Miyaki et al. (2017), a multicomponent supplement derived from chlorella (200 mg) for four weeks in thirty-four healthy men increased relative VO
2 max in an incremental maximal test on a cycle ergometer [
27].
Curiously, the maximal speed in the test before and after the supplementation in SG was similar, whereas there was an increase in absolute and relative VO
2max. The rise in VO
2 could be due to an increase in O
2 pulse and haemoglobin. The calcium content of TC could generate an increase in oxygen transport by the haemoglobin. Calcium performs several vital functions in red blood cells that affect oxygen transport and coagulation, and increase the cell half-life [
42,
43]. Increases in intracellular calcium appear to promote the ability of red blood cells to supply oxygen [
43].
TC contains carotenoids and polyphenols [
20] that have antioxidant properties [
44]. Several investigations observed that spirulina supplementation increases some enzymatic antioxidant systems [
45,
46]. The antioxidant properties of TC could explain the performance increase observed in this study.
Haemoglobin and MCH concentrations increased after TC supplementation in SG (
p < 0.01 in haemoglobin;
p < 0.05 in MCH). Microalgae have been used in animals as supplements to improve iron deficiencies [
47]. Nasirian et al. (2017) investigated the effects of
Spirulina platensis (15 and 30 mg/kg body weight) for five weeks on haematological parameters in diabetic rats [
48]. They observed that
Spirulina platensis supplementation of 30 mg/kg body weight improved total red blood cells, haemoglobin, MCH, mean cell volume and whole white blood cells. The authors hypothesised that
Spirulina platensis could stimulate erythropoietin formation. In humans, Selmi et al. (2011) analysed the effect of 500 mg spirulina supplementation for twelve weeks in forty older volunteers [
49]. They reported an increase in MCH in subjects of both sexes, whereas MCV and MCH concentration increased in males. They concluded that more studies are needed to confirm the results in humans. It should be noted that the participants in the previous studies were animals and older people, a different population to those of the present study.
The increases in haemoglobin concentration in SG despite a drop in haematocrit are discordant. This phenomenon could be related to the presence of some haematopoietic factor in the algae. The chlorides present in the microalgae could explain this increase [
9], as cobalt chloride is an erythropoietic factor [
50]. Another mineral, such as iron, could explain this increase in haemoglobin. The effects of iron on erythropoiesis and haemoglobin levels have been previously reported [
51]. Finally, the bioactive peptides present in the microalgae could play a significant role as they have immunomodulation, antihypertensive or anticancer properties [
52]. We believe that during the TC digestion process some bioactive peptide with haematopoietic properties can be obtained. In addition, we believe that the high levels of sodium and chloride present in TC could increase fluid retention and generate hypervolaemia, thereby decreasing haematocrit [
53,
54]. Further research is needed to explain the results obtained. We think that it is a finding of interest for sports performance.
This study has many limitations as it is a preliminary study of algae, which at this moment has not been previously investigated. First, the intensity and volume of training during the study could influence the results. The team’s coach confirmed that the number of training hours and days did not change during the study in both groups. However, the intensity could have been modified. Second, the number of participants in this study was small and micronutrients intake was not considered. Third, there are no studies on these microalgae to compare results, so studies on other algae have been used. Fourth, the state of hydration could not be evaluated to verify the increase in plasma volume.
Current results need to be confirmed with larger sample sizes, and different populations and concentrations of TC to analyse the potential effects of various doses, and establish a dose–response relationship. It would be interesting for future studies to investigate the antioxidant properties of TC as well as the effect on elite athletes, and analyse the intake of micronutrients.