Body Image Relates to Exercise-Induced Antinociception and Mood Changes in Young Adults: A Randomized Longitudinal Exercise Intervention
Abstract
1. Introduction
2. Materials and Methods
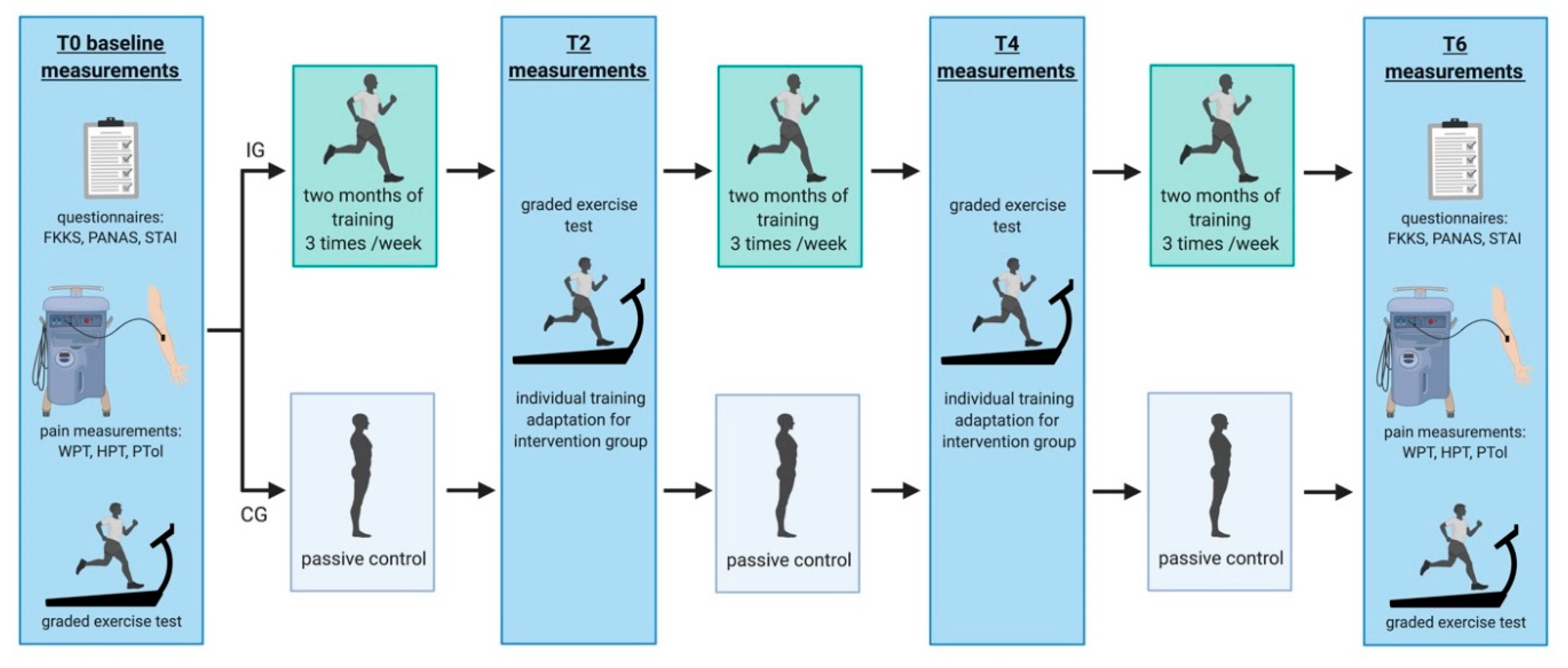
2.1. Experimental Design
2.2. Participants
2.3. Graded Exercise Test
2.4. Endurance Exercise Intervention
2.5. Frankfurt Body-Concept Scales (FKKS)
2.6. Positive and Negative Affect Schedule (PANAS)
2.7. Determination of Sensory and Heat Pain Threshold
2.8. Pain Tolerance Determination
2.9. Statistical Analysis
2.10. Within-Group Analyses
2.11. Between-Group Analyses
2.12. Correlation Analyses
3. Results
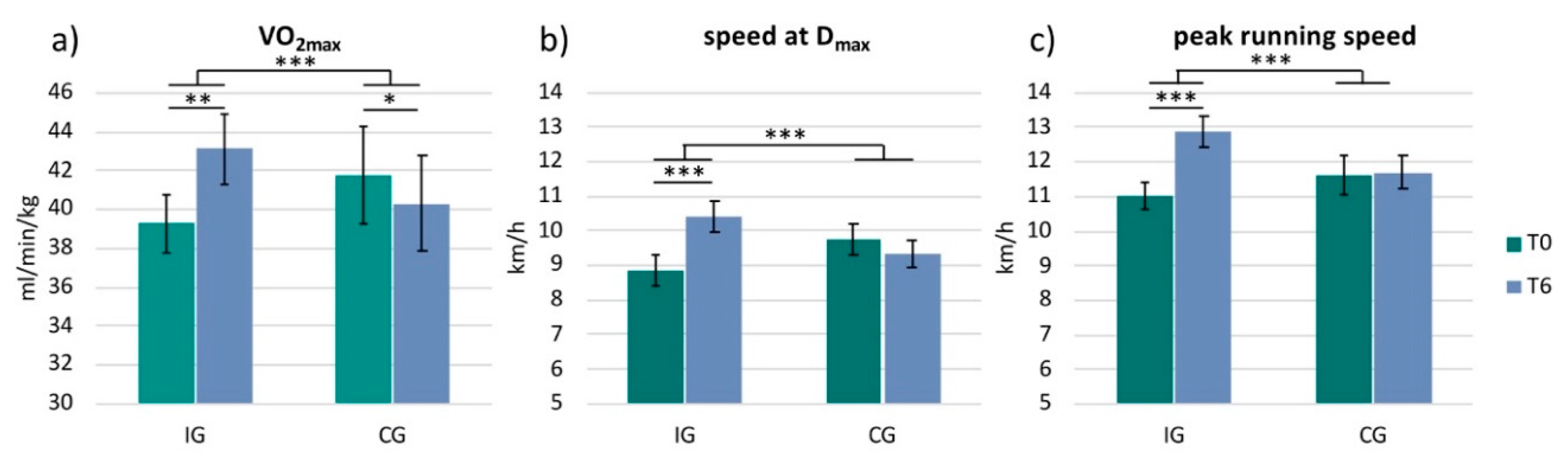
3.1. Physiological Data
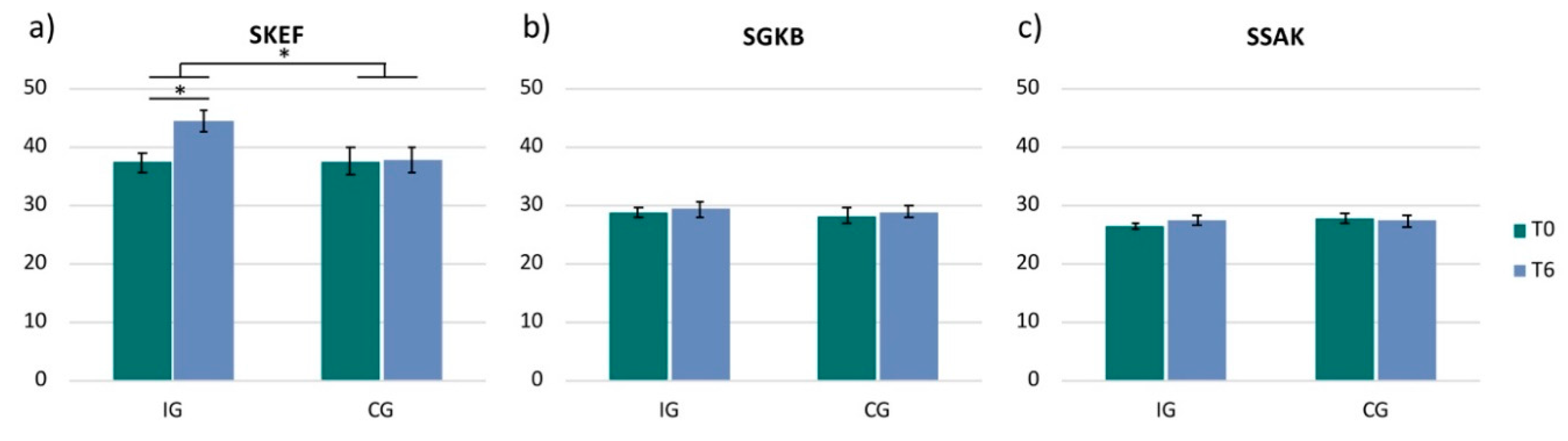
3.2. Frankfurt Body-Concept Scales
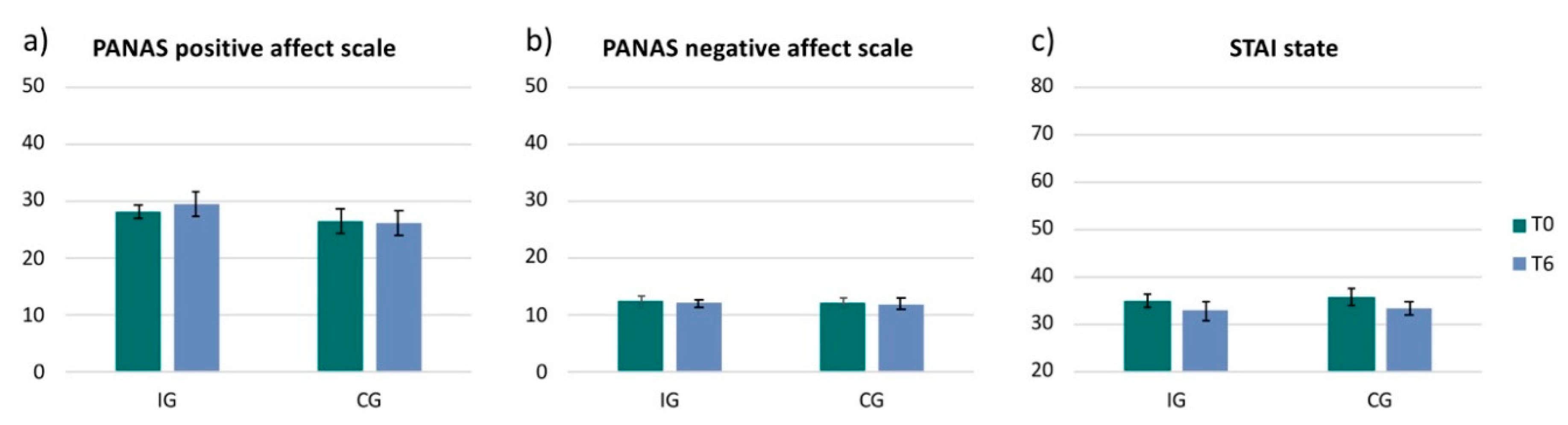
3.3. Mood and Anxiety Scales
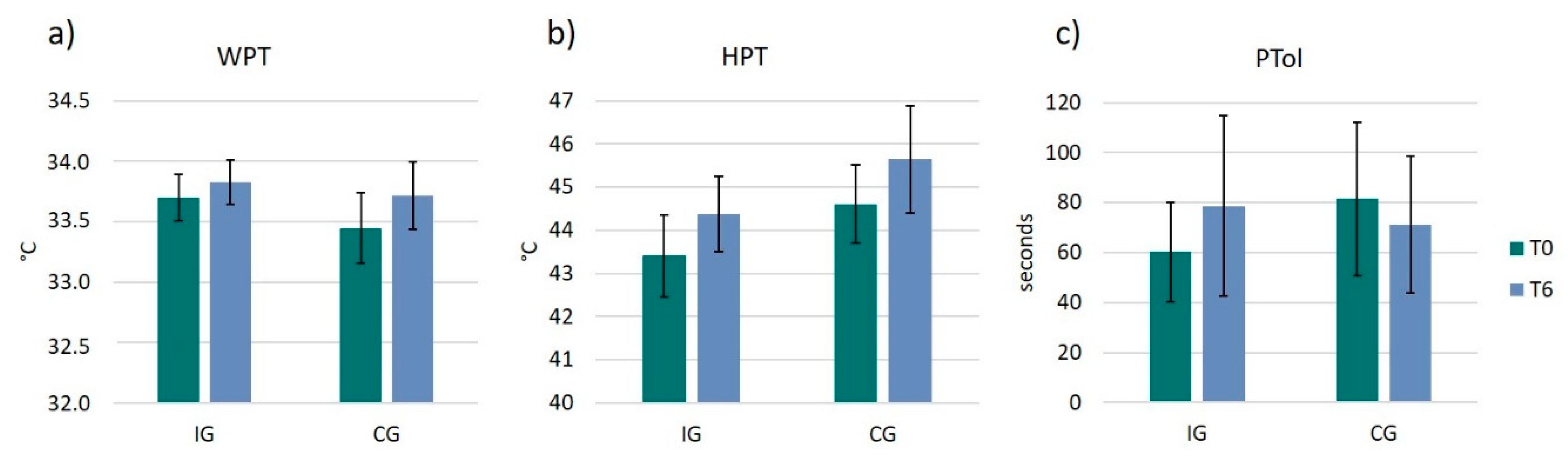
3.4. Pain Thresholds and Pain Tolerance
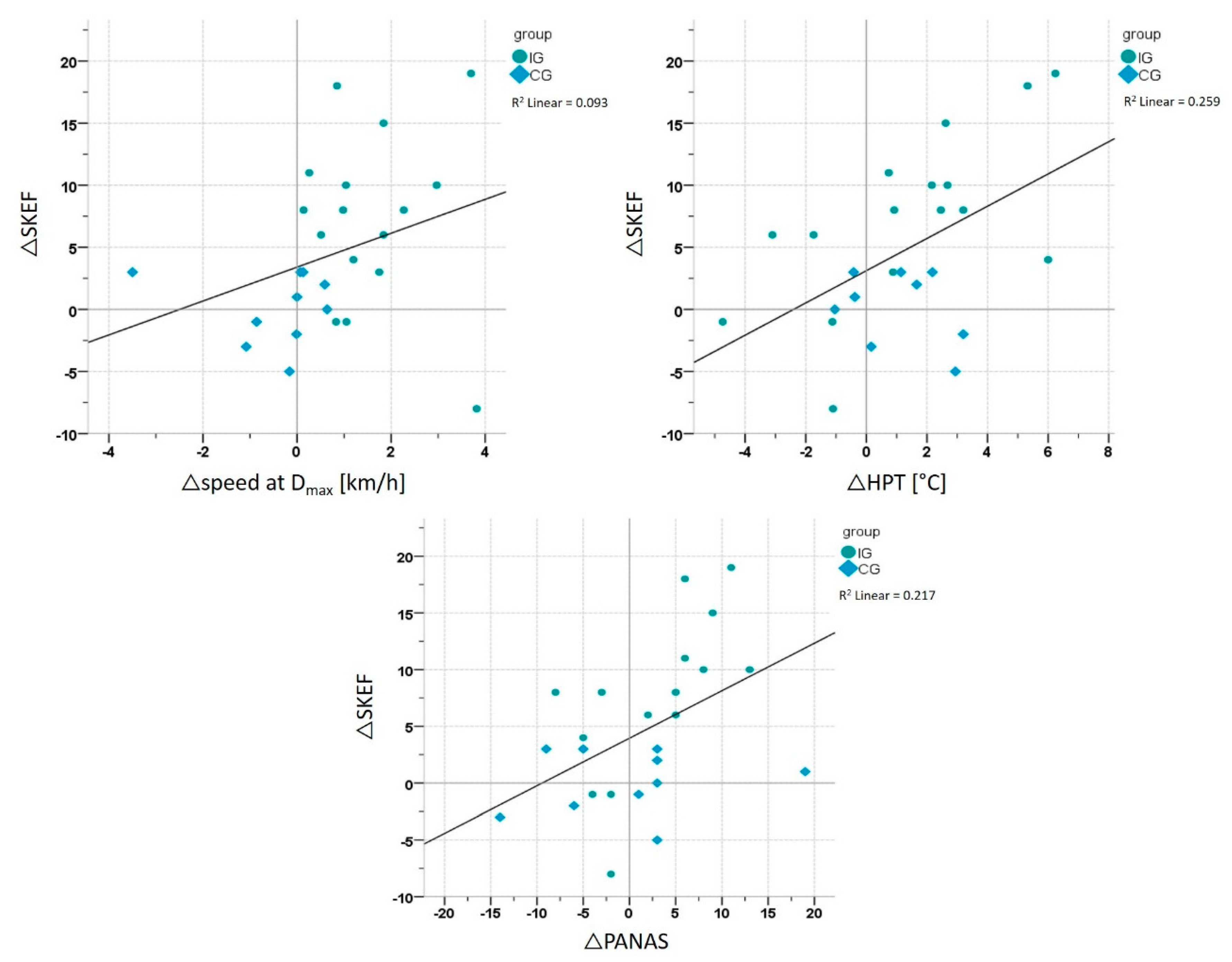
3.5. Correlation Analyses
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, Q. Beneficial effects of moderate voluntary physical exercise and its biological mechanisms on brain health. Neurosci. Bull. 2008, 24, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Onerup, A.; Arvidsson, D.; Blomqvist, A.; Daxberg, E.L.; Jivegard, L.; Jonsdottir, I.H.; Lundqvist, S.; Mellen, A.; Persson, J.; Sjogren, P.; et al. Physical activity on prescription in accordance with the Swedish model increases physical activity: A systematic review. Br. J. Sports Med. 2019, 53, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Poitras, V.J.; Gray, C.E.; Borghese, M.M.; Carson, V.; Chaput, J.P.; Janssen, I.; Katzmarzyk, P.T.; Pate, R.R.; Connor Gorber, S.; Kho, M.E.; et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 2016, 41, S197–S239. [Google Scholar] [CrossRef]
- Catalan-Matamoros, D.; Gomez-Conesa, A.; Stubbs, B.; Vancampfort, D. Exercise improves depressive symptoms in older adults: An umbrella review of systematic reviews and meta-analyses. Psychiatry Res. 2016, 244, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.V.; Pezarat-Correia, P.; Vaz, J.R.; Silva, L.; Almeida, I.D.; Heffernan, K.S. Impact of Exercise Training on Physiological Measures of Physical Fitness in the Elderly. Curr. Aging Sci. 2016, 9, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Galuska, D.A.; Serdula, M.K.; Jones, D.A. Attempting to lose weight: Specific practices among U.S. adults. Am. J. Prev. Med. 2004, 26, 402–406. [Google Scholar] [CrossRef]
- Fallon, E.A.; Harris, B.S.; Johnson, P. Prevalence of body dissatisfaction among a United States adult sample. Eat. Behav. 2014, 15, 151–158. [Google Scholar] [CrossRef]
- Grogan, S. Body image and health: Contemporary perspectives. J. Health Psychol. 2006, 11, 523–530. [Google Scholar] [CrossRef]
- Bailey, K.A.; Gammage, K.L.; van Ingen, C. How do you define body image? Exploring conceptual gaps in understandings of body image at an exercise facility. Body Image 2017, 23, 69–79. [Google Scholar] [CrossRef]
- Campbell, A.; Hausenblas, H.A. Effects of exercise interventions on body image: A meta-analysis. J. Health Psychol. 2009, 14, 780–793. [Google Scholar] [CrossRef]
- Markey, C.H.; Dunaev, J.L.; August, K.J. Body image experiences in the context of chronic pain: An examination of associations among perceptions of pain, body dissatisfaction, and positive body image. Body Image 2020, 32, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.S.; Winett, R.A.; Wojcik, J.R.; Williams, D.M. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: Social support, self-efficacy, outcome expectations and self-regulation in the guide-to-health trial. J. Health Psychol. 2010, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Sonstroem, R.J.; Harlow, L.L.; Josephs, L. Exercise and self-esteem: Validity of model expansion and exercise associations. J. Sport Exerc. Psychol. 1994, 16, 29–42. [Google Scholar] [CrossRef]
- Sonstroem, R.J.; Morgan, W.P. Exercise and self-esteem: Rationale and model. Med. Sci. Sports Exerc. 1989, 21, 329–337. [Google Scholar] [CrossRef]
- Lotze, M.; Moseley, G.L. Role of distorted body image in pain. Curr. Rheumatol. Rep. 2007, 9, 488–496. [Google Scholar] [CrossRef]
- Moseley, G.L. I can’t find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain 2008, 140, 239–243. [Google Scholar] [CrossRef]
- Martin, A.; Svaldi, J. Körperbild und Körperbildstörungen. Psychotherapeut 2015, 60, 475–476. [Google Scholar] [CrossRef]
- Lepage, M.L.; Crowther, J.H. The effects of exercise on body satisfaction and affect. Body Image 2010, 7, 124–130. [Google Scholar] [CrossRef]
- Martin Ginis, K.A.; Eng, J.J.; Arbour, K.P.; Hartman, J.W.; Phillips, S.M. Mind over muscle? Sex differences in the relationship between body image change and subjective and objective physical changes following a 12-week strength-training program. Body Image 2005, 2, 363–372. [Google Scholar] [CrossRef]
- Tylka, T.L.; Wood-Barcalow, N.L. What is and what is not positive body image? Conceptual foundations and construct definition. Body Image 2015, 14, 118–129. [Google Scholar] [CrossRef]
- Hausenblas, H.A.; Fallon, E.A. Exercise and body image: A meta-analysis. Psychol. Health 2006, 21, 33–47. [Google Scholar] [CrossRef]
- Duijts, S.F.; Faber, M.M.; Oldenburg, H.S.; van Beurden, M.; Aaronson, N.K. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—A meta-analysis. Psychooncology 2011, 20, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Martin Ginis, K.A.; Strong, H.A.; Arent, S.M.; Bray, S.R.; Bassett-Gunter, R.L. The effects of aerobic-versus strength-training on body image among young women with pre-existing body image concerns. Body Image 2014, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Cowles, M. Body image and exercise: A study of relationships and comparisons between physically active men and women. Sex. Roles 1991, 25, 33–44. [Google Scholar] [CrossRef]
- Swami, V.; Weis, L.; Barron, D.; Furnham, A. Positive body image is positively associated with hedonic (emotional) and eudaimonic (psychological and social) well-being in British adults. J. Soc. Psychol. 2018, 158, 541–552. [Google Scholar] [CrossRef]
- Gillen, M.M. Associations between positive body image and indicators of men’s and women’s mental and physical health. Body Image 2015, 13, 67–74. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Hoffman, D.R. Exercisers achieve greater acute exercise-induced mood enhancement than nonexercisers. Arch. Phys. Med. Rehabil. 2008, 89, 358–363. [Google Scholar] [CrossRef]
- Hallgren, M.A.; Moss, N.D.; Gastin, P. Regular exercise participation mediates the affective response to acute bouts of vigorous exercise. J. Sports Sci. Med. 2010, 9, 629–637. [Google Scholar]
- Focht, B.C.; Garver, M.J.; Cotter, J.A.; Devor, S.T.; Lucas, A.R.; Fairman, C.M. Affective Responses to Acute Resistance Exercise Performed at Self-Selected and Imposed Loads in Trained Women. J. Strength Cond. Res. 2015, 29, 3067–3074. [Google Scholar] [CrossRef]
- Hopkins, M.E.; Davis, F.C.; Vantieghem, M.R.; Whalen, P.J.; Bucci, D.J. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience 2012, 215, 59–68. [Google Scholar] [CrossRef]
- Smith, J.C. Effects of emotional exposure on state anxiety after acute exercise. Med. Sci. Sports Exerc. 2013, 45, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Heggelund, J.; Kleppe, K.D.; Morken, G.; Vedul-Kjelsas, E. High aerobic intensity training and psychological States in patients with depression or schizophrenia. Front. Psychiatry 2014, 5, 148. [Google Scholar] [CrossRef] [PubMed]
- Koltyn, K.F. Analgesia following exercise: A review. Sports Med. 2000, 29, 85–98. [Google Scholar] [CrossRef]
- Koltyn, K.F.; Brellenthin, A.G.; Cook, D.B.; Sehgal, N.; Hillard, C. Mechanisms of exercise-induced hypoalgesia. J. Pain 2014, 15, 1294–1304. [Google Scholar] [CrossRef]
- Naugle, K.M.; Fillingim, R.B.; Riley, J.L., 3rd. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Geva, N.; Defrin, R. Enhanced pain modulation among triathletes: A possible explanation for their exceptional capabilities. Pain 2013, 154, 2317–2323. [Google Scholar] [CrossRef]
- Tesarz, J.; Schuster, A.K.; Hartmann, M.; Gerhardt, A.; Eich, W. Pain perception in athletes compared to normally active controls: A systematic review with meta-analysis. Pain 2012, 153, 1253–1262. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Hoffman, D.R. Does aerobic exercise improve pain perception and mood? A review of the evidence related to healthy and chronic pain subjects. Curr. Pain Headache Rep. 2007, 11, 93–97. [Google Scholar] [CrossRef]
- Deusinger, I.M. Frankfurter Körperkonzeptskalen; Hogrefe: Göttingen, Germany, 1998. [Google Scholar]
- Levenig, C.G.; Kellmann, M.; Kleinert, J.; Belz, J.; Hesselmann, T.; Hasenbring, M.I. Body image is more negative in patients with chronic low back pain than in patients with subacute low back pain and healthy controls. Scand. J. Pain 2019, 19, 147–156. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Schmidt, K.H.; Metzler, P. Wortschatztest: WST; Beltz Test GmbH: Weinheim, Germany, 1992. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait. Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar]
- Hautzinger, M.; Bailer, M.; Worall, H.; Keller, F. Beck-Depressions-Inventar (BDI); Verlag Hans Huber: Bern, Switzerland, 1994. [Google Scholar]
- Hollmann, W.; Knigge, H.; Knicker, A.; Strüder, H.K. Methods for Measurement of Physical Fitness and Training Recommendations in Studies on Humans. In Functional Neuroimaging in Exercise and Sport Sciences; Boecker, H., Hillman, C.H., Scheef, L., Strüder, H.K., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [PubMed]
- Bishop, D.; Jenkins, D.G.; Mackinnon, L.T. The relationship between plasma lactate parameters, Wpeak and 1-h cycling performance in women. Med. Sci. Sports Exerc. 1998, 30, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.W.; Tausch, A. Untersuchungen mit einer deutschen Version der „Positive and Negative Affect Schedule“ (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar]
- Bar, K.J.; Greiner, W.; Letsch, A.; Kobele, R.; Sauer, H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. J. Psychiatr Res. 2003, 37, 345–353. [Google Scholar] [CrossRef]
- Bassett, D.R., Jr.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Osumi, M.; Imai, R.; Ueta, K.; Nobusako, S.; Morioka, S. Negative body image associated with changes in the visual body appearance increases pain perception. PLoS ONE 2014, 9, e107376. [Google Scholar] [CrossRef]
- Lahart, I.; Darcy, P.; Gidlow, C.; Calogiuri, G. The Effects of Green Exercise on Physical and Mental Wellbeing: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 1352. [Google Scholar] [CrossRef]
- Rosenbaum, S.; Sherrington, C. Is exercise effective in promoting mental well-being in older age? A systematic review. Br. J. Sports Med. 2011, 45, 1079–1080. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Cooney, G.M.; Dwan, K.; Greig, C.A.; Lawlor, D.A.; Rimer, J.; Waugh, F.R.; McMurdo, M.; Mead, G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Kvam, S.; Kleppe, C.L.; Nordhus, I.H.; Hovland, A. Exercise as a treatment for depression: A meta-analysis. J. Affect. Disord. 2016, 202, 67–86. [Google Scholar] [CrossRef] [PubMed]
- McAuley, E.; Blissmer, B. Self-efficacy determinants and consequences of physical activity. Exerc. Sport Sci. Rev. 2000, 28, 85–88. [Google Scholar]
- McAuley, E.; Szabo, A.; Gothe, N.; Olson, E.A. Self-efficacy: Implications for Physical Activity, Function, and Functional Limitations in Older Adults. Am. J. Lifestyle Med. 2011, 5, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, C.M.; Fortier, M.; Sweet, S.; O’Sullivan, T.; Hogg, W.; Reid, R.D.; Sigal, R.J. Explaining physical activity levels from a self-efficacy perspective: The physical activity counseling trial. Ann. Behav. Med. 2007, 34, 323–328. [Google Scholar] [CrossRef]
- Rejeski, W.J.; Miller, M.E.; Foy, C.; Messier, S.; Rapp, S. Self-efficacy and the progression of functional limitations and self-reported disability in older adults with knee pain. J. Gerontol. B Psychol. Sci. Soc. Sci. 2001, 56, S261–S265. [Google Scholar] [CrossRef]
- McAuley, E.; Talbot, H.M.; Martinez, S. Manipulating self-efficacy in the exercise environment in women: Influences on affective responses. Health Psychol. 1999, 18, 288–294. [Google Scholar] [CrossRef]
- Biddle, S.; Mutrie, N.; Gorely, T. Psychology of Physical Activity: Determinants, Well-Being and Interventions; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]

| Intervention Group (n = 16) | Control Group (n = 10) | |
|---|---|---|
| Age (years) | 24.5 ± 4.3 | 23.7 ± 4.2 |
| Height (cm) | 173.4 ± 12.0 | 176.9 ± 7.9 |
| Mass (kg) | 70.2 ± 15.8 | 71.2 ± 14.1 |
| EHI | 75.2 ± 17.3 | 79.5 ± 13.3 |
| BMI | 23.3 ± 3.8 | 22.7 ± 3.6 |
| VO2max (mL/min/kg) | 39.3 ± 5.3 a | 41.7 ± 7.5 |
| Speed at Dmax (km/h) | 8.9 ± 1.9 | 9.8 ± 1.4 |
| HRmax (bpm) | 198.5 ± 7.9 | 200.8 ± 8.5 |
| Peak running speed (km/h) | 11.0 ± 1.6 | 11.5 ± 1.8 |
| Education (years) | 16.4 ± 3.2 | 15.8 ± 3.1 a |
| GVT | 107.3 ± 10.3 | 107.3 ± 8.8 |
| BDI | 2.5 ± 3.1 | 1.4 ± 1.5 |
| STAI trait | 34.8 ± 10.1 | 31.4 ± 6.1 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurer, A.; Deckert, S.; Levenig, C.; Schörkmaier, T.; Stangier, C.; Attenberger, U.; Hasenbring, M.; Boecker, H. Body Image Relates to Exercise-Induced Antinociception and Mood Changes in Young Adults: A Randomized Longitudinal Exercise Intervention. Int. J. Environ. Res. Public Health 2020, 17, 6801. https://doi.org/10.3390/ijerph17186801
Maurer A, Deckert S, Levenig C, Schörkmaier T, Stangier C, Attenberger U, Hasenbring M, Boecker H. Body Image Relates to Exercise-Induced Antinociception and Mood Changes in Young Adults: A Randomized Longitudinal Exercise Intervention. International Journal of Environmental Research and Public Health. 2020; 17(18):6801. https://doi.org/10.3390/ijerph17186801
Chicago/Turabian StyleMaurer, Angelika, Sebastian Deckert, Claudia Levenig, Theresa Schörkmaier, Carolin Stangier, Ulrike Attenberger, Monika Hasenbring, and Henning Boecker. 2020. "Body Image Relates to Exercise-Induced Antinociception and Mood Changes in Young Adults: A Randomized Longitudinal Exercise Intervention" International Journal of Environmental Research and Public Health 17, no. 18: 6801. https://doi.org/10.3390/ijerph17186801
APA StyleMaurer, A., Deckert, S., Levenig, C., Schörkmaier, T., Stangier, C., Attenberger, U., Hasenbring, M., & Boecker, H. (2020). Body Image Relates to Exercise-Induced Antinociception and Mood Changes in Young Adults: A Randomized Longitudinal Exercise Intervention. International Journal of Environmental Research and Public Health, 17(18), 6801. https://doi.org/10.3390/ijerph17186801






