Effects of Toxic Metal Contamination in the Tri-State Mining District on the Ecological Community and Human Health: A Systematic Review
Abstract
1. Introduction
2. Literature Review
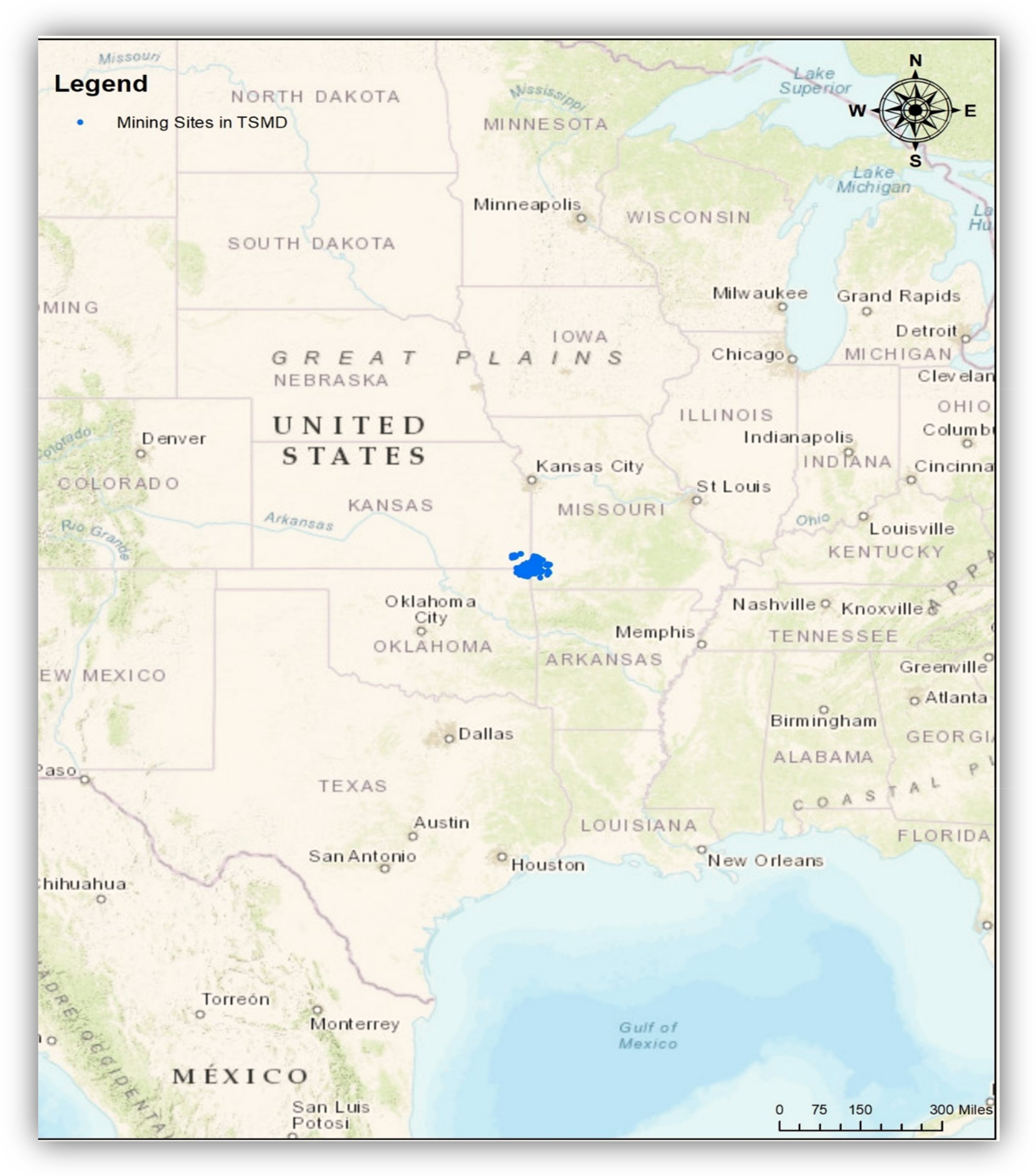
2.1. History of the TSMD
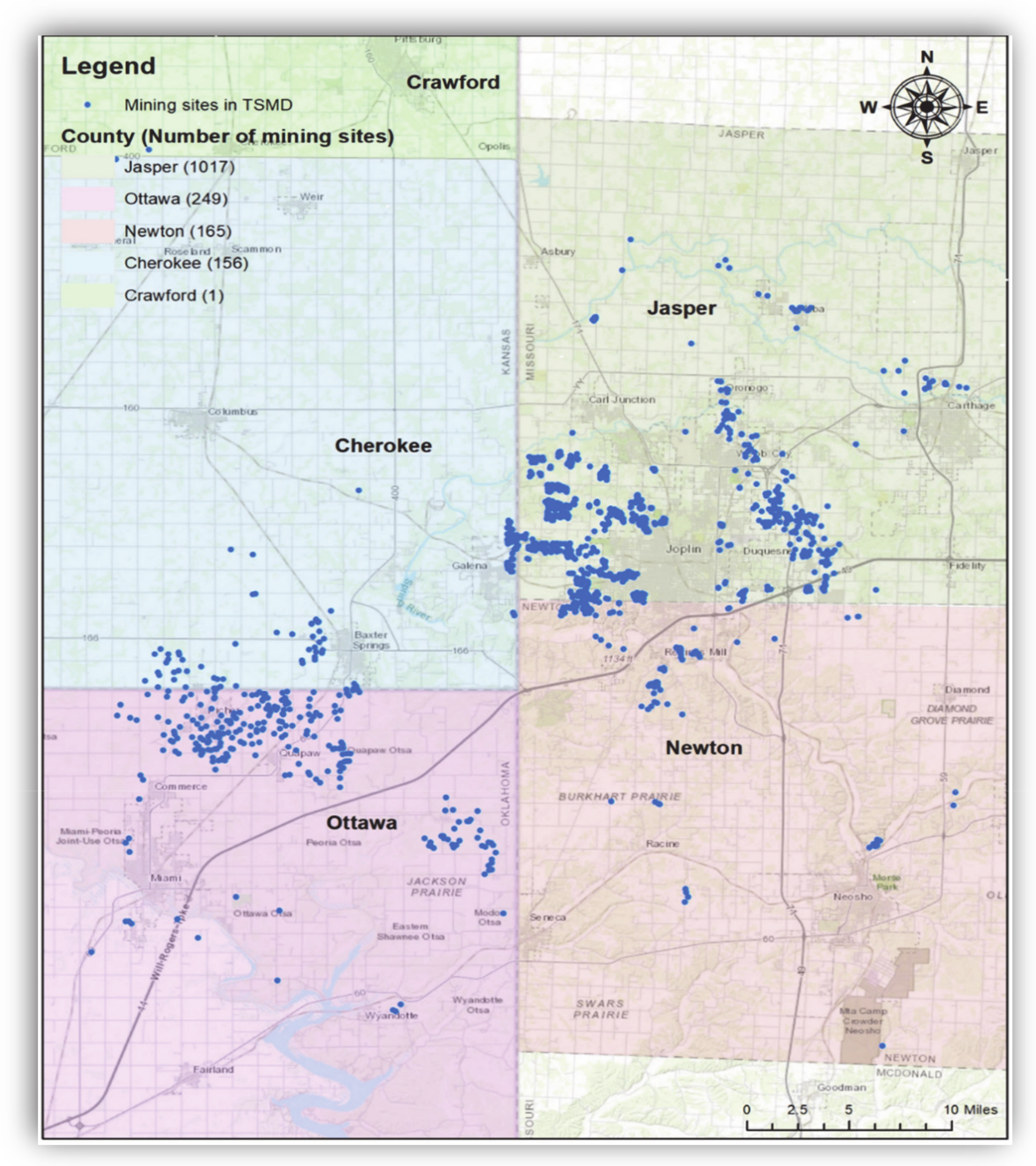
2.2. Characteristics of TSMD Superfund Sites and Their Contamination
2.2.1. Cherokee County (Kansas) Superfund Site (298 km2)
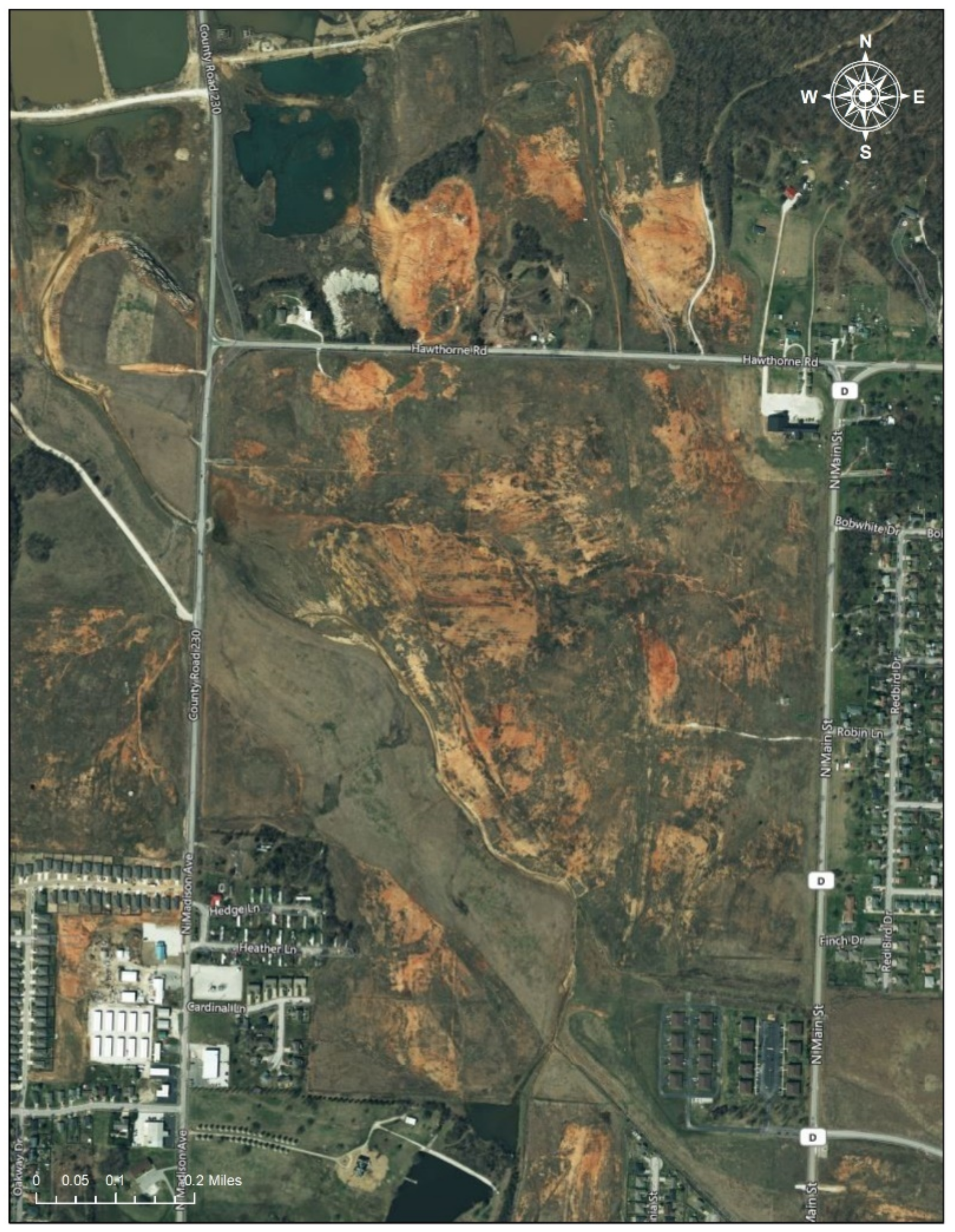
2.2.2. Jasper County (Missouri) Superfund Site (498 km2)
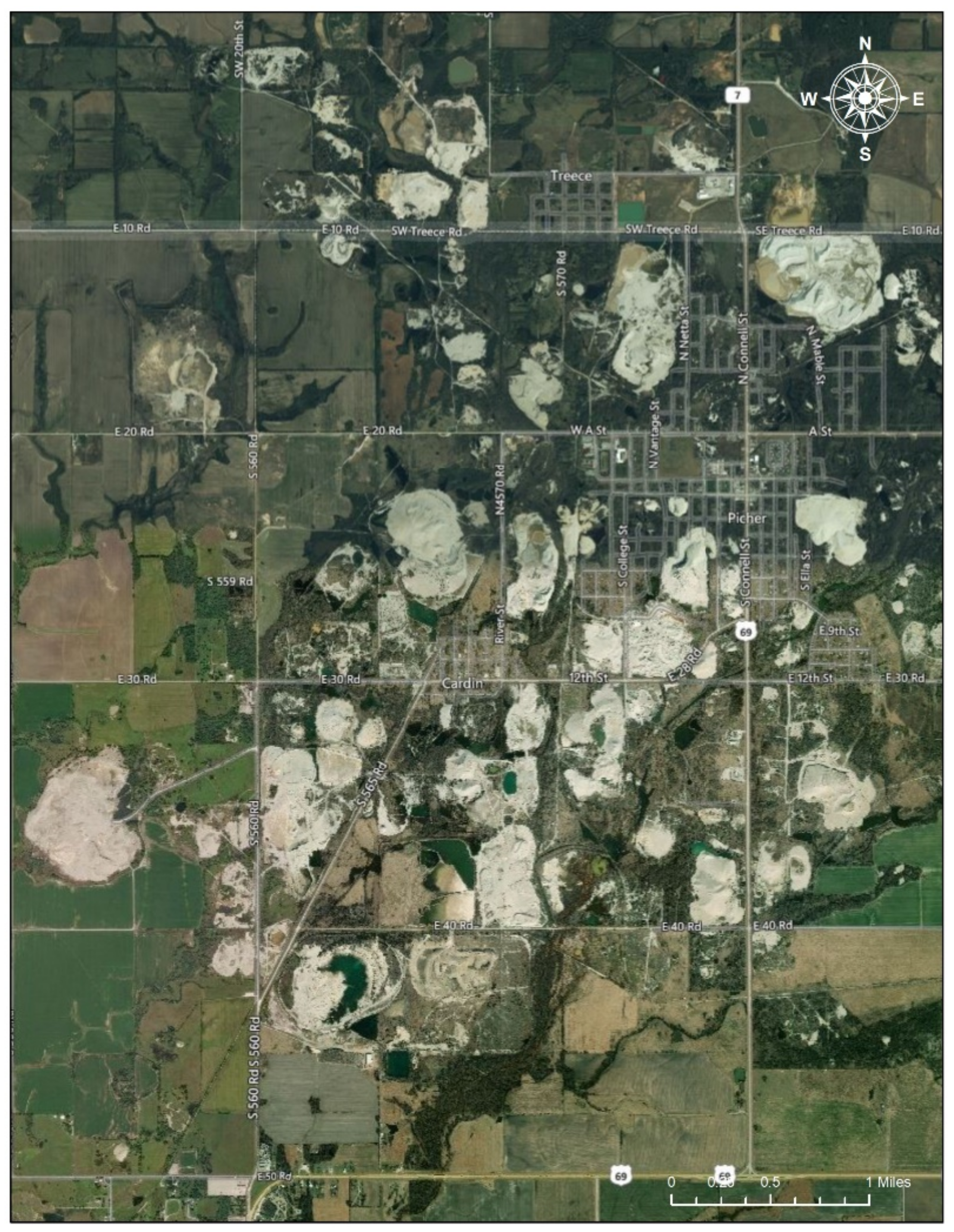
2.2.3. Tar Creek, Ottawa County (Oklahoma) Superfund Site (104 km2)
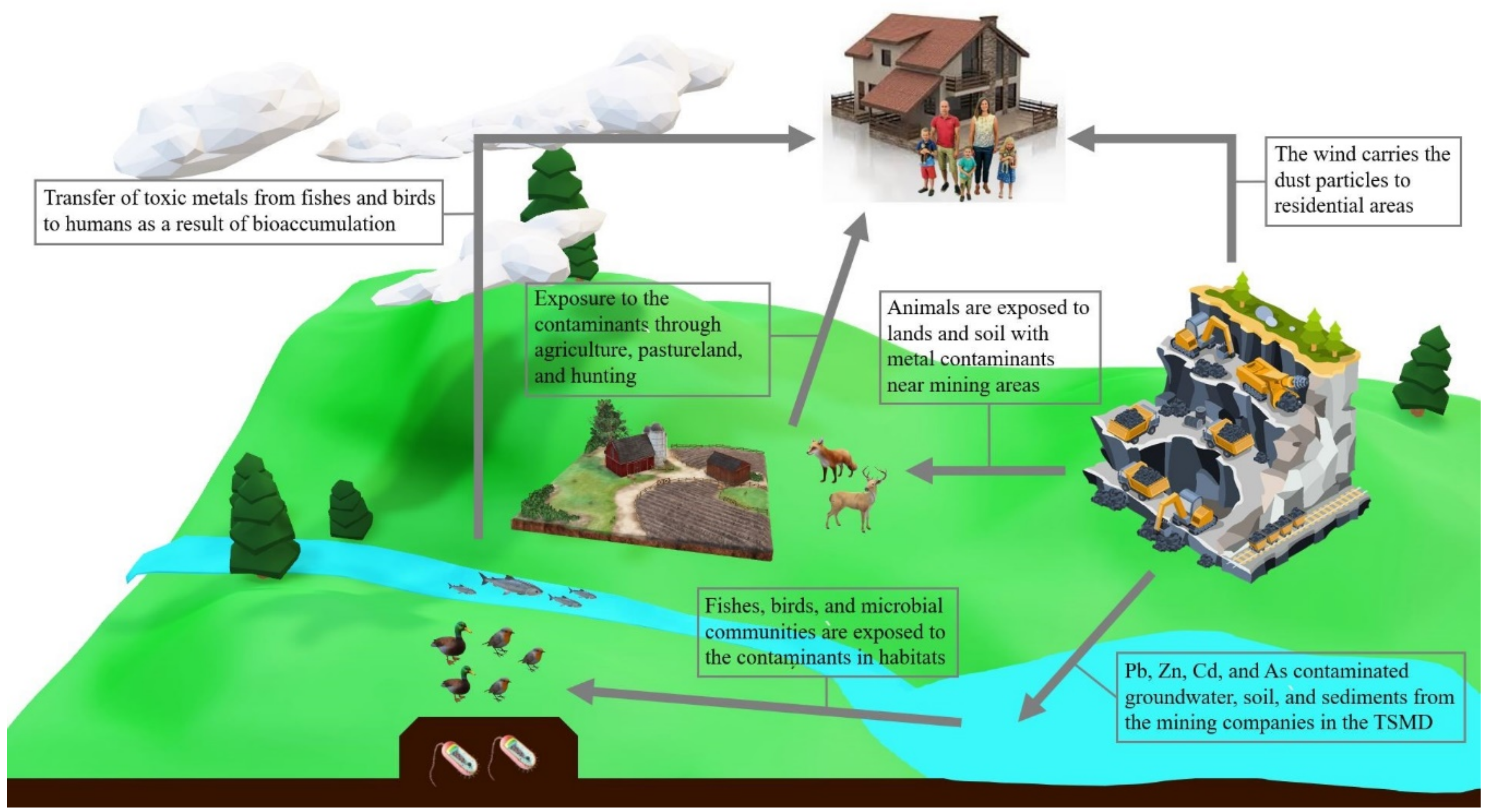
2.3. The Impact of Toxic Metal Components on Ecological Systems
2.4. The Impact of Toxic Meta Components on Human Health
3. Methods
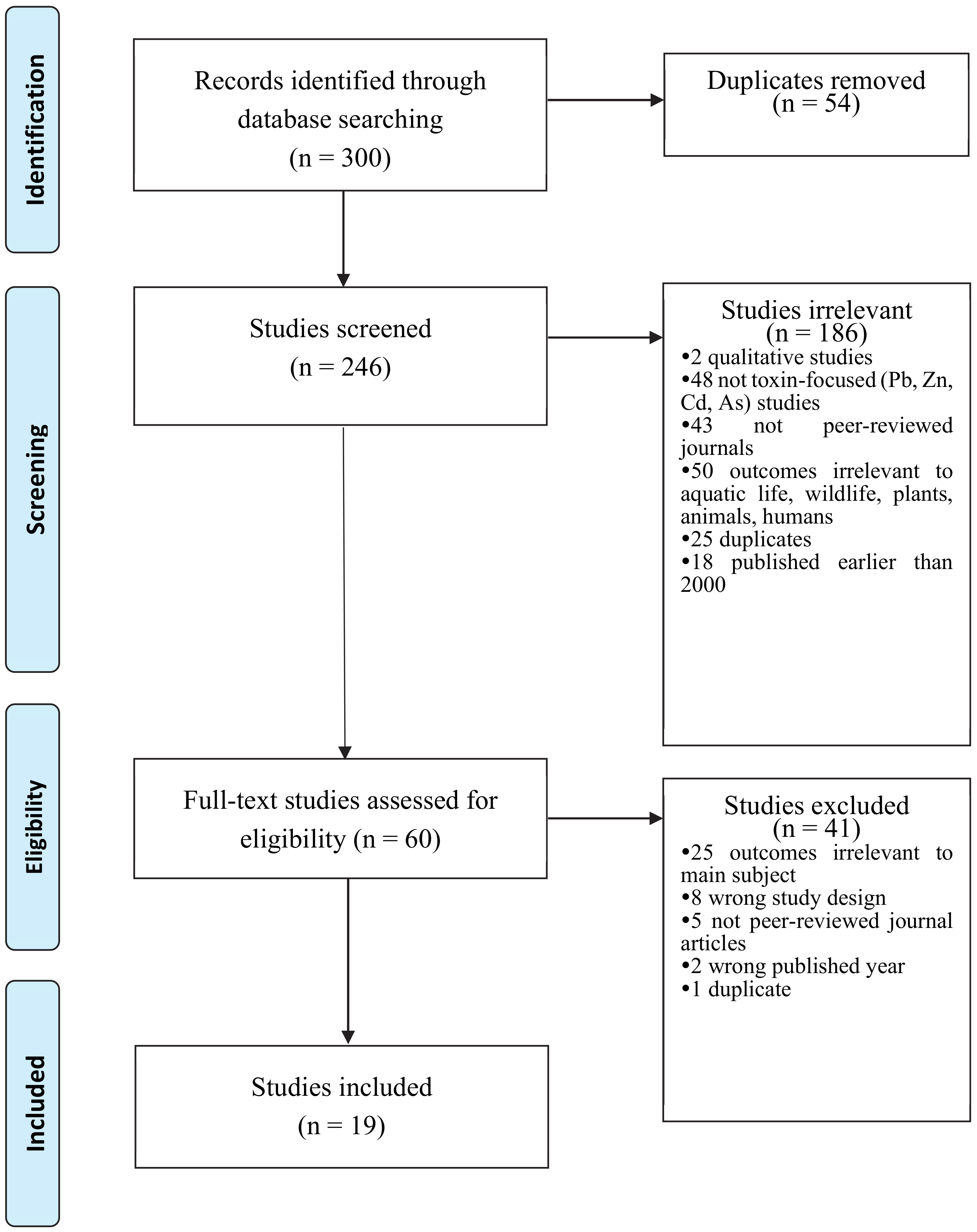
3.1. Search Strategy and Selection Procedure
3.2. Study Selection
3.3. Quality Assessment
4. Results
4.1. Risk of Bias
4.2. Characteristics of Included Studies
4.2.1. TSMD Toxic Effects on Plants
4.2.2. TSMD Toxic Effects on Aquatic Life
4.2.3. TSMD Toxic Effects on Wildlife Animals
4.2.4. TSMD Toxic Effects on Microbial Communities
4.2.5. TSMD Effects on Human Health
5. Discussion
6. Recommendations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Juracek, K.E.; Drake, K.D. Mining-Related Sediment and Soil Contamination in a Large Superfund Site: Characterization, Habitat Implications, and Remediation. Environ. Manag. 2016, 58, 721–740. [Google Scholar] [CrossRef]
- Beyer, W.N.; Dalgarn, J.; Dudding, S.; French, J.B.; Mateo, R.; Miesner, J.; Sileo, L.; Spann, J. Zinc and lead poisoning in wild birds in the tri-state mining district (Oklahoma, Kansas, and Missouri). Arch. Environ. Contam. Toxicol. 2005, 48, 108–117. [Google Scholar] [CrossRef]
- Johnson, A.W.; Gutiérrez, M.; Gouzie, D.; McAliley, L.R. State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef]
- Coolon, J.D.; Jones, K.L.; Narayanan, S.; Wisely, S.M. Microbial ecological response of the intestinal flora of Peromyscus maniculatus and P. leucopus to heavy metal contamination. Mol. Ecol. 2010, 19 (Suppl. S1), 67–80. [Google Scholar] [CrossRef]
- Allert, A.L.; DiStefano, R.J.; Schmitt, C.J.; Fairchild, J.F.; Brumbaugh, W.G. Effects of mining-derived metals on riffle-dwelling crayfish in southwestern Missouri and southeastern Kansas, USA. Arch. Environ. Contam. Toxicol. 2012, 63, 563–573. [Google Scholar] [CrossRef][Green Version]
- Lynch, R.A.; Malcoe, L.H.; Skaggs, V.J.; Kegler, M.C. The Relationship between Residential Lead Exposures and Elevated Blood Lead Levels in a Rural Mining Community. J. Environ. Health 2000, 63, 9–15. [Google Scholar]
- Gulson, B.L. Tooth Analyses of Sources and Intensity of Lead Exposure in Children. Environ. Health Perspect. 1996, 104, 306–312. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Weitzman, M.; Winter, N.L.; Eberly, S.; Yakir, B.; Tanner, M.; Emond, M.; Matte, T.D. Lead-contaminated house dust and urban children’s blood lead levels. Am. J. Public Health 1996, 86, 1416–1421. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Roghmann, K.J. Pathways of lead exposure in urban children. Environ. Res. 1997, 74, 67. [Google Scholar] [CrossRef]
- The Tar Creek Trustee Council. Natural Resource Progrmmatic Restoration Plan and Environmental Assessment; U.S. Fish & Wildlife Service, Department of the Interior: Washington, DC, USA, 2017. Available online: https://www.fws.gov/southwest/es/oklahoma/Documents/Contaminants/Final%20draft_TarCreek%20ProgrammaticRP_EA.pdf (accessed on 30 June 2020).
- Gagnon, G. Distribution system water quality. J. Am. Water Work. Assoc. 2012, 104, 6668. [Google Scholar]
- Delvaux, I.; Van Cauwenberghe, J.; Den Hond, E.; Schoeters, G.; Govarts, E.; Nelen, V.; Baeyens, W.; Van Larebeke, N.; Sioen, I. Prenatal exposure to environmental contaminants and body composition at age 7–9 years. Environ. Res. 2014, 132, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Schaider, L.A.; Ettinger, A.S.; Wright, R.O.; Shine, J.P.; Spengler, J.D. Metal sources and exposures in the homes of young children living near a mining-impacted Superfund site. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J.R.; Brady, L.L.; Wilson, F.W. Study of Stability Problems and Hazard Evaluation of the Kansas Portion of the Tri-state Mining Area (Contract 10100131 Report); U.S. Bureau of Mines Open-file Report 75–83, 193. P. (Also Kansas Geological Survey Open-file Report 83-2); United States Bureau of Mines: Washington, DC, USA, 1983.
- Gibson, A.M. Wilderness Bonanza: The Tri-State District of Missouri, Kansas, and Oklahoma; University of Oklahoma: Norman, OK, USA, 1972. [Google Scholar]
- Perry, P.M.; Pavlik, J.W.; Sheets, R.W.; Biagioni, R.N. Lead, cadmium, and zinc concentrations in plaster and mortar from structures in Jasper and Newton Counties, Missouri (Tri-State Mining District). Sci. Total Environ. 2005, 336, 275–281. [Google Scholar] [CrossRef]
- Pope, L.M. Assessment of Contaminated Streambed Sediment in the Kansas Part of the Historic Tri-State Lead and Zinc Mining District, Cherokee County, 2004; Scientific Investigation Report 2005–5201; U.S. Fish & Wildlife Service and the Kansas Department of Health and Environment: Manhattan, KS, USA, 2005. Available online: https://pubs.usgs.gov/sir/2005/5251/report.pdf (accessed on 20 May 2020).
- Peebles, J. Special analysis of mining related sediment contamination of Turkey Creek Watershed in the Tri-state mining district, Joplin (MS Thesis), Missouri State University, Springfield MO. In Proceedings of the 2013 GSA Annual Meeting in Denver, Springfield, MO, USA, 27–30 December 2013. [Google Scholar]
- United State Code. Chapter 1: Subject Matter and Scope of Copyright; USA Government: Washington, DC, USA, 2020. [Google Scholar]
- Hu, H.; Shine, J.; Wright, R.O. The challenge posed to children’s health by mixtures of toxic waste: The Tar Creek superfund site as a case-study. Pediatr. Clin. N. Am. 2007, 54, 155–175. [Google Scholar] [CrossRef]
- Robertson, D. Hard as the Rock Itself; University Press of Colorado: Louisville, CO, USA, 2006. [Google Scholar]
- Fowler, B.A. Molecular Biological Markers for Toxicology and Risk Assessment; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Pierzynski, G.M.; Vaillant, G.C. Remediation to reduce ecological risk from trace element contamination: A decion case study. J. Nat. Resour. Life Sci. Educ. 2006, 35, 85–94. [Google Scholar] [CrossRef]
- Wang, N.; Chen, C.; Nie, X.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; et al. Blood lead level and its association with body mass index and obesity in China-Results from SPECT-China study. Sci. Rep. 2015, 5, 18299. [Google Scholar] [CrossRef]
- Faulk, C.; Barks, A.; Liu, K.; Goodrich, J.M.; Dolinoy, D.C. Early-life lead exposure results in dose- and sex-specific effects on weight and epigenetic gene gene regulation in weanling mice. Epigenomics 2014, 5, 487–500. [Google Scholar] [CrossRef]
- Leasure, J.L.G.A.; Chaney, S.; Johnson, J.E., Jr.; Pothakos, K.; Lau, Y.S.; Fox, D. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008, 116, 355–361. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Jamali, M.K.; Arain, M.B.; Jalbani, N.; Baig, J.A.; Sarfraz, R.A. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res. Clin. Pract. 2008, 80, 280–288. [Google Scholar] [CrossRef]
- Chen, Y.W.; Yang, C.Y.; Huang, C.F.; Hung, D.Z.; Leung, Y.M.; Liu, S.H. Heavy metals, islet function and diabetes development. Islets 2014, 1, 169–176. [Google Scholar] [CrossRef]
- Moon, S.S. Association of lead, mercury and cadmium with diabetes in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabetes Med. 2013, 30, e143–e148. [Google Scholar] [CrossRef]
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2004; Available online: https://era.library.ualberta.ca/items/48b9b989-c221-4df6-9e35-af782082280e/view/a1cffdde-243e-41c3-be98-885f6d4dcb29/standard_quality_assessment_criteria_for_evaluating_primary_research_papers_from_a_variety_of_fields.pdf (accessed on 11 June 2020).
- Fernández-Espínola, C.; Abad Robles, M.T.; Giménez Fuentes-Guerra, F.J. Small-Sided Games as a Methodological Resource for Team Sports Teaching: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 1884. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Buck, C.; Freiberger, E.; Murphy, M.; Brug, J.; Cardon, G.; O’Donoghue, G.; Pigeot, I.; Oppert, J.-M.; DEDIPAC Consortium. Systematic literature review of determinants of sedentary behaviour in older adults: A DEDIPAC study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 127. [Google Scholar] [CrossRef]
- Serra-Olivares, J.; González-Víllora, S.; García-López, L.M.; Araújo, D. Game-Based Approaches’ Pedagogical Principles: Exploring Task Constraints in Youth Soccer. J. Hum. Kinet. 2015, 46, 251–261. [Google Scholar] [CrossRef]
- Beyer, W.N.; Franson, J.C.; French, J.B.; May, T.; Rattner, B.A.; Shearn-Bochsler, V.I.; Warner, S.E.; Weber, J.; Mosby, D. Toxic Exposure of Songbirds to Lead in the Southeast Missouri Lead Mining District. Arch. Environ. Contam. Toxicol. 2013, 65, 598–610. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Zota, A.R.; Amarasiriwardena, C.J.; Hopkins, M.R.; Schwartz, J.; Hu, H.; Wright, R.O. Maternal Arsenic Exposure and Impaired Glucose Tolerance during Pregnancy. Environ. Health Perspect. 2009, 117, 1059–1064. [Google Scholar] [CrossRef]
- Malcoe, L.H.; Lynch, R.A.; Kegler, M.C.; Skaggs, V.J. Lead Sources, Behaviors, and Socioeconomic Factors in Relation to Blood Lead of Native American and White Children: A Community-Based Assessment of a Former Mining Area. Environ. Health Perspect. 2002, 110, 221–231. [Google Scholar] [CrossRef]
- Neuberger, J.S.; Hu, S.C.; Drake, K.D.; Jim, R. Potential health impacts of heavy-metal exposure at the Tar Creek Superfund site, Ottawa County, Oklahoma. Environ. Geochem. Health 2009, 31, 47. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Carpenter, J.W.; Nietfeld, J.C.; Miesner, J.F. Adverse health effects in Canada geese (Branta canadensis) associated with waste from zinc and lead mines in the Tri-State Mining District (Kansas, Oklahoma, and Missouri, USA). J. Wildl. Dis. 2011, 47, 650–660. [Google Scholar] [CrossRef]
- Brumbaugh, W.G.; Schmitt, C.J.; May, T.W. Concentrations of cadmium, lead, and zinc in fish from mining-influenced waters of northeastern Oklahoma: Sampling of blood, carcass, and liver for aquatic biomonitoring. Arch. Environ. Contam. Toxicol. 2005, 49, 76–88. [Google Scholar] [CrossRef]
- Garvin, E.M.; Bridge, C.F.; Garvin, M.S. Edible wild plants growing in contaminated floodplains: Implications for the issuance of tribal consumption advisories within the Grand Lake watershed of northeastern Oklahoma, USA. Environ. Geochem. Health 2018, 40, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.J.; Whyte, J.J.; Brumbaugh, W.G.; Tillitt, D.E. Biochemical effects of lead, zinc, and cadmium from mining on fish in the Tri-States District of northeastern Oklahoma, USA. Environ. Toxicol. Chem. 2005, 24, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Struckhoff, M.A.; Stroh, E.D.; Grabner, K.W. Effects of mining-associated lead and zinc soil contamination on native floristic quality. J. Environ. Manag. 2013, 119, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.L.; Janz, D.M. Tissue-specific HSP70 levels and reproductive physiological responses in fishes inhabiting a metal-contaminated creek. Arch. Environ. Contam. Toxicol. 2003, 45, 110–120. [Google Scholar] [CrossRef]
- Beattie, R.E.; Henke, W.; Campa, M.F.; Hazen, T.C.; McAliley, L.R.; Campbell, J.H.; Oak Ridge National Lab; Lawrence Berkeley National Lab. Variation in microbial community structure correlates with heavy-metal contamination in soils decades after mining ceased. Soil Biol. Biochem. 2018, 126, 57–63. [Google Scholar] [CrossRef]
- Phelps, K.L.; McBee, K. Ecological Characteristics of Small Mammal Communities at a Superfund Site. Am. Midl. Nat. 2009, 161, 57–68. [Google Scholar] [CrossRef]
- Besser, J.M.; Ingersoll, C.G.; Brumbaugh, W.G.; Kemble, N.E.; May, T.W.; Wang, N.; MacDonald, D.D.; Roberts, A.D. Toxicity of sediments from lead-zinc mining areas to juvenile freshwater mussels (Lampsilis siliquoidea) compared to standard test organisms. Environ. Toxicol. Chem. 2015, 34, 626–639. [Google Scholar] [CrossRef]
- Hays, K.A.; McBee, K. Population Demographics of Red-Eared Slider Turtles (Trachemys scripta) from Tar Creek Superfund Site. South Am. J. Herpetol. 2010, 44, 441–446. [Google Scholar] [CrossRef]
- Schmitt, C.J.; Brumbaugh, W.G.; Linder, G.L.; Hinck, J.E. A screening-level assessment of lead, cadmium, and zinc in fish and crayfish from Northeastern Oklahoma, USA. Environ. Geochem. Health 2006, 28, 445–471. [Google Scholar] [CrossRef]
- Gasser, U.G.; Walker, W.J.; Dahlgren, R.A.; Borch, R.S.; Burau, R.G. Lead Release from Smelter and Mine Waste Impacted Materials under Simulated Gastric Conditions and Relation to Speciation. Environ. Sci. Technol. 1996, 30, 761–769. [Google Scholar] [CrossRef]
- Cook, M.; Chappell, W.R.; Hoffman, R.E.; Mangione, E.J. Assessment of blood lead levels in children living in a historic mining and smelting community. Am. J. Epidemiol. 1993, 137, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, T.; Sokołowska, D.; Kulka, E. Health risk assessment in children exposed to lead compounds in the vicinity of mine-smelter plant “Orzeł Biały”. Pol. J. Occup. Med. Environ. Health 1993, 6, 71. [Google Scholar] [PubMed]
- Gulson, B.L.; Davis, J.J.; Mizon, K.J.; Korsch, M.J.; Law, A.J.; Howarth, D. Lead Bioavailability in the Environment of Children: Blood Lead Levels in Children Can Be Elevated in a Mining Community. Arch. Environ. Health Int. J. 1994, 49, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Murgueytio, A.M.; Evans, R.G.; Roberts, D. Relationship between soil and dust lead in a lead mining area and blood lead levels. J. Expo. Anal. Environ. Epidemiol. 1998, 8, 173. [Google Scholar]
- Kozlov, M.W.T. Population densities and diversity of Calliphoridae (Diptera) around a nickel-copper smelter at Monchegorsk, Northwestern Russia. Èntomol. Fenn. 2002, 13, 98–104. [Google Scholar] [CrossRef]
- Verneaux, J.; Schmitt, A.; Verneaux, V.; Prouteau, C. Benthic insects and fish of the Doubs River system: Typological traits and the development of a species continuum in a theoretically extrapolated watercourse. Hydrobiologia 2003, 490, 63–74. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Recycling, Reuse and Rehabilitation of Mine Wastes. Entomol. Fenn. 2011, 13, 98–104. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals--concepts and applications. Chemosphere 2013, 91, 869. [Google Scholar] [CrossRef]
- Bansah, K.J.A. Factors Contributing to Karst Development in Southwestern Missouri, USA. In Proceedings of the Symposium on the Application of Geophysics to Engineering and Environmental Problems 2017, Denver, CO, USA, 19–23 March 2017; p. 399. [Google Scholar]
- Steele-Valentín, K.M.; Padilla, I.Y. Assessment of potential exposure pathways in karst groundwater systems in Vega Alta, Puerto Rico using geographic information systems. In Proceedings of the Seventh LACCEI Latin American and Caribbean Conference for Engineering and Technology (LACCEI’2009)—Energy and Technology for the Americas: Education, Innovation, Technology and Practice, San Cristóbal, Venezuela, 2–5 June 2009; Available online: http://www.laccei.org/LACCEI2009-Venezuela/p223.pdf (accessed on 30 August 2020).
- Deeb, R.; Hawley, E.; Kell, L.; O’Laskey, R. Assessing Alternative Endpoints for Groundwater Remediation at Contaminated Sites; ESTCP Project Report ER-200832 Environmental Security Technology Certification Program (ESTCP); ARCADIS/Malcolm Pirnie, Inc.: Arlington, VA, USA, 2011; p. 233. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a544571.pdf (accessed on 1 September 2020).
| Study | Purpose | Research Design/Sampling Method | Key Predictor Variable (IV), Measurement | Key Outcome Variables (DV), Measurement | Sample (Subject/Location) | Statistical Methods | Findings | |
|---|---|---|---|---|---|---|---|---|
| 1 | Allert et al. (2012) | Characterize physical habitat and water quality; evaluate the potential effects of metals in crayfish and carnivorous wildlife. | Quantitative; comparisons in different sites (reference, mining, downstream). | Pb, Zn, and Cd in surface water, sediment, detritus, and crayfish. | The concentration of Pb, Zn, and Cd. | n = 24/site. Subsamples of water, sediments, detritus, and crayfish from 3 metal-contaminated sites in Spring River of southwestern Missouri (MO) and southeastern Kansas (KS). | Nested analysis of variance (ANOVA) for finding group differences of areas with site considered a fixed effect; Linear regression using PROC REG was used for crayfish densities. | 1. Mean densities of crayfish at mining sites were lower than reference sites. 2. Mean concentrations of metal materials were significantly correlated and greater at mining and downstream sites than at reference sites. 3. Sediment probable-effects quotients and surface water toxic units were significantly correlated, indicating the risk of toxicity to aquatic biota at several sites. 4. Metals concentrations in crayfish at several sites exceeded concentrations considered toxic to carnivorous wildlife. |
| 2 | Beattie et al. (2018) | Understand changes in microbial community structures due to regional metals contamination (Pb, Zn, Cd, AI, and Mg). | Quantitative; topsoil samples were collected using an ethanol-cleaned metal hand spade at intervals of 0.32 km in each of the cardinal directions (n = 100) extending from and directly from mine tailings in chat piles. | Soil pH, moisture, and heavy-metal concentrations of Topsoil samples. | 16S ribosomal RNA gene sequences and quantitative PCR calculations of Bacteria and Archaea. | Topsoil samples (n = 111; 10 cm depth) were collected within an 8.05 km radius of Picher in Ottawa County, Oklahoma (OK). | Analyzing concentrations of 20 metals with EPA method using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). | 1. Bacteria were negatively and significantly correlated with Pb, Cd, Zn, and Mg. 2. Archaea was only significantly and positively correlated with pH. 3. There were significantly different microbial communities in chat and west transect samples. 4. There were significantly impacted toxic components and distribution of individual Operational Taxonomic Unit (OUT). |
| 3 | Besser et al. (2015) | Examine chronic effects of sediment toxicity on freshwater mussels’ survival, growth, and biomass (also known as, amphipod toxicity test). | Longitudinal and quantitative; in 2006, sediment was collected using a Petit Birge-Ekman grab sampler. 2 precleaned 20-L polyethylene sample buckets were filled one third with site water, and a benthos wash bucket with stainless steel screen bottom was placed inside a sample bucket; Juvenile amphipods, midge larvae, larval mussels | Toxic materials in sediments. | Survival, growth (mean weight), biomass (total weight per replicate) for each species. | Tri-State (n = 58–65), Southeast MO (n = 16). Every 20 L of bulk sediments and porewater (wet sieved to <2 mm particle diameter) were collected from Tri-State sites, southeast MO, and reference sites; juvenile amphipods, midge larvae, and larval mussels | Toxicity test using ANOVA, EC 10 (for metal mixture), and principal components analysis to evaluate relationships among metal concentrations, sediment characteristics, and responses in toxicity tests. | 1. The frequency of highly toxic responses in Tri-State sediments was greater for amphipod survival (25% of samples), midge biomass (20%), and mussel survival (14%). 2.The frequency of highly toxic responses in southeast Missouri sediments, the frequency of highly toxic was greater for mussel biomass (25%), and amphipod biomass (13%). 3. Thresholds for metal toxicity to mussels were lower for southeast MO sediments than for Tri-State sediments. 4. Southeast MO sites with toxic sediments had 2 or fewer live mussel taxa, compared with 7 to 26 taxa at reference sites. |
| 4 | Beyer et al. (2005) | Determine if the habitat of the TSMD has been contaminated by the espousal of toxic concentrations of metals. | Quantitative; wild birds were selected in chat piles. | Toxic materials in tissues and blood of sample birds. | Biological functions and external signs of poisoning. | An experimental group (13 species) were collected from the TSMD area from December 2000 and August 2001, and a reference group (the same species) was collected from uncontaminated sites (Neosho Wildlife Area, St. Paul, KS, and cliffs in Chestertown, Maryland (MD). | Toxic materials from samples of tissues were quantified by ICPMS/ICP-ES; blood samples were analyzed for ALAD activity. | 1. American robins, northern cardinals, and waterfowl had higher Pb tissue concentrations (p < 0.05) than Pb tissue concentrations from reference birds. 2. Mean activities of the Pb-sensitive enzyme delta-aminolaevulinic acid dehydratase (ALAD) were decreased by >50% in red blood cells in these birds (p < 0.05). 3. Some birds had tissue concentrations of Pb that have been associated with impaired biological functions and external signs of poisoning. 4. Zn in the liver and kidney of waterfowl were significantly higher (p < 0.05) than reference birds. |
| 5 | Beyer et al. (2013) | Estimate the potential exposure of songbirds to Pb in southeastern MO. | Quantitative; earthworms, soil, 34 adult, and juvenile songbirds collected from southeastern MO were collected, reference songbirds remote from Pb mining; one composite sample of eight soil cores was collected at each site. | Earthworms associated with Pb concentrations of soil. | Mean tissue Pb concentrations in songbirds. | All songbirds (reference = 39, mining site = 34). Birds were captured at least 4 weeks after spring migration. | Blood (1% of body weight) of birds was taken with a mic needle. Red-blood cell ALAD activity was measured. The soil samples from the sites were quantified for detecting toxic materials; the tissue metal concentrations and ALAD activities in songbirds from mining sites were compared with reference birds using ANOVA. | 1. Mean tissue Pb concentrations in songbirds from the contaminated areas were greater (p < 0.05) than those in songbirds from the reference site. 2. Out of the total birds (n = 34), 22 had hepatic Pb concentrations consistent with adverse physiological effects, 3 with systemic toxic effects, and 4 with life-threatening toxic effects. 3. Acid-fast renal intranuclear inclusion bodies, indicative of Pb poising, were detected in kidneys from 2. 4. Mean activity of the ALAD in the red blood cell, a well-established bioindicator of Pb in birds, was decreased by 58–82% in songbirds from the mining sites. |
| 6 | Brumbaugh et al. (2005) | Assess the surface-and groundwater contamination in the Spring River Neosho River (SR-NR) system of northeastern OK. | Quantitative: 74 fish from 6 locations in the SR–NR system were collected (e.g., catfish, bass, and white crappie). | Pb, Cd, and Zn in tissues, blood, and liver of sample fish. | High levels of toxic contaminations in fish. | Sample size is 74. Sample fish were collected from TSMD-affected portions of the SR and NR in northeastern OK; 4 specimens of each of three primary species were targeted at each OK site (i.e., common carp, largemouth bass, and channel catfish). | The ICP-MS was programmed to determine Zn, Cd, and Pb; for each variable, species-station arithmetic means and standard errors were computed; ANOVA was conducted for each variable; a one-way ANOVA was considered for testing for differences among collection sites; Fisher’s protected LSD was used for differences among individual sites. | 1. Cd and Pb in carp and catfish from OK and Pb in carp and catfish from MO were elevated. 2. Zn in bass and crappie were low. 3. Variability was high for Cd in all three tissues of crap; differences between sites were significant only for blood, even though the mean liver was 100-fold greater than those in blood. 4. Blood concentrations of Cd and Pb were positively correlated with the concentration of the same element in crap and catfish, and the corresponding multiple regression models were highly significant. |
| 7 | Coolon et al. (2010) | Examine the effect of residual contamination on rodent and the microbial communities at remediated sites in the TSMD. | Quantitative; using 25 trap stations per site, trapped a representation of the small mammal community; tapped 10 Peromyscus. maniculatus and 6 Permyscus. leucopus, euthanized and necropsied in the field. | Heavy metal exposure. | Bacterial community diversity (soil bacteria). | 10 rodents’ pair (2 species) from the TSMD and non-TSMD. | Massively parallel sequencing (MPS) technologies to sequence bacterial 16S DNA amplified from the soil and mouse intestines. | 1. Rodents on the remediated site had reduced body mass, smaller body size and lower body fat than animals on reference sites. 2. Bacterial communities in both the soil and Peromyscus spp. Gastrointestinal tracts had no difference in diversity between reference and remediated sites, but assemblages differed in response to contamination. |
| 8 | Ettinger et al. (2009) | The association between the level of arsenic and impaired glucose tolerance. | Correlational and quantitative; screening pregnant women during prenatal visits at the hospital in Ottawa county. | Toxic elements in blood and hair. | Blood glucose. | 532 pregnant women/The Tar Creek Superfund Site. | Univariate, bivariate, and logistic regression analyses. | 1. The concentration of arsenic was found between 0.2 and 24.1 ug/L (ppb) and 1.1 to 724.4 ng/g (ppb) in blood and hair, respectively. 2. One-hour glucose levels ranged from 40 to 284 mg/dL; impaired glucose tolerance was observed in 11.9% of women as using standard screening criterion (>140 mg/dL). 3. Women in the highest quartile of blood arsenic exposure had 2.8 higher odds of impaired GTT than women in the lowest quartile of exposure. |
| 9 | Garvin et al. (2018) | Determine metal concentrations in consumed plant species in the TSMD. | Quantitative; collect 36 species of edible plants and soil from floodplain areas, plants from reference sites. | Cd, Pb, and Zn in plants. | Cd, Pb, and Zn in soil. | The sample size is 210; 36 species edible plants from various floodplain areas for tribal communities in the TSMD soil samples. | ICP-MS; Spearman rank correlation. | 1. A significantly positive correlation between metal concentrations in plant tissues and soil. 2. A significant difference in metal concentration distribution between reference and impacted plant samples. |
| 10 | Hays & McBee (2010) | Investigate the effect of Pb and Zn on the ecology of Red-Eared Slider Turtles. | Quantitative; out of 327 turtles, 293 individuals were used to determine sex ratios, SDI, and average sizes. | Toxic elements in the TSMD. | Body size, sex ratios, sexual dimorphism indices, and recapture and survival rates. | 293 Trachemys scripta (165 males, 128 females) from Tar Creek Superfund Site (TCSFS) and reference cites (Sequoyah National Wildlife Refuge, Lake Carl Blackwell). | Chi-square test, goodness-of-fit tests in RELEASE, saturated global model, adjusted Akaike’s Information Criterion. | 1. Sex ratios were female-biased at TCSFS and Lake Carl Blackwell and male-biased at Sequoyah National Wildlife Refuge. 2. Degree of sexual size dimorphism differed among the 3 sites. 3. Male turtle was significantly larger at Lake Carl Blackwell than at reference sites. 4. Females from TCSFS were significantly larger than females from Lake Carl Blackwell. 5. Survival and recaptures rates did not differ significantly in two different areas. |
| 11 | Lynch et al. (2000) | Examine the independent contributions of various lead sources to elevate blood lead levels in area children. | Quantitative; (a representative random sample of Native American and white households residing within the study area). | Level of lead in sources (from the soil, dust, and paint). The paint was measured using protocols of the U.S. Department of Housing and Urban Development and the U.S. Environmental Protection Agency. | Elevated blood lead level measured by micrograms per deciliter (10 μg/dL). | n = 244, Native American and white residents within 31 contiguous census blocks in northeastern Ottawa County, Oklahoma. | Logistic regression using SAS and EpiInfo to estimate associations between environmental exposures and elevated blood lead levels. | 1. Floor dust, yard soil, interior paints, and location of residence were independently associated with elevated blood lead levels. |
| 12 | Malcoe et al. (2002) | Examine the effects of lead sources on blood lead concentrations (BPbs) in rural children. | Quantitative; (a population-based, representative sample of Native American and white children in the study area). | Paint indices measured as Index Value = (Pb Concentration) × (Size of Sample Area) × (Deterioration Value). Socioeconomic and behavioral measures were used from interview data. | Blood lead concentrations measured by micrograms per deciliter (10 μg/dL). | n = 224, Native American and white children in Ottawa County, Oklahoma. | Non-parametric Wilcoxon rank-sum test and multiple linear regression using SAS for BPb variability. | 1. Soil and dust lead derived largely from mining waste pose a health hazard to Native American and white children, and that current residential dust lead standards are insufficient to protect children adequately. 2. Poor children were especially vulnerable to lead exposures suggests that residential standards should consider interactions among socioeconomic conditions and lead sources if environmental justice is to be achieved. |
| 13 | Neuberger et al. (2009) | Examine the potential impact of exposure to heavy metals and health problems. | Quantitative; (secondary data obtained from the Oklahoma State Department of Health). | Geographic comparisons (exposed areas of Ottawa County vs. unexposed areas). | Mortality outcomes (lung cancer, Tuberculosis, Bronchitis, emphysema, asthma, kidney disease, hypertension, stroke, and heart disease), health outcomes in the first year of life (low birth weight, infant mortality, and infant mortality excluding infectious diseases). | n = not clear, residents at or near the Superfund site in Ottawa County, Oklahoma. | Standardized Mortality Ratio (SMR) (observed versus expected mortality calculation by ratio) and a Poisson model. | 1. Excess mortality was found for stroke and heart disease when comparing the exposed County to the state but not when comparing the exposed cities to the nonexposed rest of the County. |
| 14 | Phelps & McBee (2009) | Determine ecological characteristics of small mammal communities inhabiting a heavy metal contaminated site, Tar Creek Superfund Site, compared to reference sites located in northeastern Oklahoma over a 2-year timeframe. | Quantitative; (small mammal communities were sampled at two locations within Tar Creek Superfund Site (TCSFS) and two uncontaminated reference sites). | Geographic comparisons (two locations within TCSFS vs. two uncontaminated sites). | Species diversity measured using Simpson’s diversity index, rank abundance analysis measured using Southwood and Henderson (2000)’s method, and differences in community composition among sites evaluated using detrended correspondence analysis (Ter Braak and Smilauer, 2002). | n = not clear, small mammal communities within TCSFS and outside the immediate area surrounding TCSFS. | Simpson’s diversity index, rank abundance analysis, and detrended correspondence analysis with GANOCO. | 1. Tar Creek Superfund. The site had reduced species diversity, including richness and evenness, compared to the reference sites. 2. Species composition was different between contaminated sites and reference sites, as evidenced by detrended correspondence analysis, with contaminated sites being more similar to each other than to either reference site. 3. No direct link between site contamination and disparities among most ecological characteristics could be established. |
| 15 | Schmitt et al. (2005) | Examine biochemical effects of lead, zinc, and cadmium from mining on fish in the TSMD. | Quantitative; (fish representing six species were collected from six sites on the Spring and Neosho Rivers, and additional samples from the Big River). | Concentrations of Zn, Cd, Pb, and iron in the blood of six species of fish. | d-aminolevulinic acid dehydratase (ALAD) activity, and Hb-adjusted ALAD activity (ALAD/Hb) in the blood of fishes. | n = 74, fish representing six species were collected from six sites on the Spring and Neosho Rivers, and additional samples from the Big River. | One-way ANOVA using the site as a fixed effect and stepwise multiple linear regression using SAS. | 1. ALAD activity was inhibited by more than 50% in catfish from several TSMD sites, which is evidence that Pb is both bioavailable, and active biochemically. |
| 16 | Struckhoff et al. (2013) | Examine the effects of mining-associated lead and zinc soil contamination on native floristic quality. | Quantitative; (plant communities were sampled in three strata. | Soil concentrations of lead and zinc measured as mg/kg. | Mean C and Floristic Quality Index (FQI) measured using Floristic Quality Assessment methods (Swink and Wilhelm, 1994). | n = 76 (Mean C = 38, FQI = 38), plant communities in the southeast Missouri Mining District. | Least-square regression trees to identify variables that best explain variation in Mean C and FQI and univariate regression. | 1. Significant negative relationships between both Mean C and FQI. |
| 17 | Merwe et al. (2011) | Assess the presence of preclinical lesions, metals accumulation in tissues, and physiologic markers of adverse health effects associated with metals exposure on Canada geese. | Quantitative; (birds were collected by the U.S. Fish and Wildlife Service personnel). | Geographic locations (four mine waste-exposed sites vs. a reference site). | Lead and zinc concentrations in bird tissues. | n = 28, Canada geese from the TSMD and the reference site at a farm pond 1.6 km northeast of Neosho State Fishing Lake, Kansas. | One-way ANOVA for data with normality and Kruskal–Wallis One-way ANOVA for data with failed normality using SigmaPlot. | 1. Elevated tissue lead concentrations and inhibited blood ALAD enzyme activities were consistently found in birds at all contaminated sites. |
| 18 | Yoo & Janz (2003) | Determine HSP70 protein expression in head kidney, liver, gill, and ovarian tissues; and examine reproductive physiological responses in female fishes exposed chronically to sublethal metal concentrations. | Quantitative; (two fish species were collected in pre-spawning period and recrudescence period). | Season (spring and winter) and site (Tar Creek and Lytle Creek) | The 70-kDa stress protein family (HSP70) level. | n = not clear, Bluegill Sunfish and Black Bullhead from Tar Creek (study site) and Lytle Creek (reference site). | Two-way ANOVA and student t-tests to detect differences between reference and metal-exposed fish. | 1. HSP70 expression was consistently elevated in the head kidney of both fish species collected at Tar Creek in comparison to fish collected from the reference creek. 2. In contrast, no consistent differences were observed in HSP70 expression in liver, gill, or ovarian tissues between sites |
| 19 | Schmitt et al. (2006) | Evaluate potential human and ecological risks associated with metals in fish and crayfish from mining in the TSMD. | Quantitative; (fish of six frequently consumed species collected from the Oklahoma waters of the Spring River and Neosho River). | Metals contaminations in aquatic organisms in Spring River vs. Neosho River. | Diets of Native Americans and wildlife, potential hazards of Pb, Zn, and Cd in these organisms to fish, wildfire, and humans. | n = varied by species (5–60 animals), fish and crayfish samples form Spring River and Neosho River. | Separate one-way ANOVA. | 1. Metals concentration were typically higher in samples from sites most heavily affected by mining and lowest in reference samples. Within the TSMD, most metals concentration were higher at sites on the SR than on the NR and were typically highest in common carp and crayfish than in other taxa. |
| Studies | Objective | Design | Method | Subjects | Random | Blinding Investigations | Blinding Subjects | Measures Outcomes | Sample Size | Analytic Methods | Variance | Controlled for Confounding | Results | Conclusions | Quality Score (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 2 | 1 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 90.9 |
| 2 | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 3 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 4 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| 5 | 2 | 2 | 1 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 6 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| 7 | 2 | 2 | 1 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 90.9 |
| 8 | 2 | 2 | 1 | 2 | 0 | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 87.5 |
| 9 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 90.9 |
| 10 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 95.4 |
| 11 | 2 | 2 | 1 | 2 | 2 | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 95.8 |
| 12 | 2 | 2 | 1 | 2 | 2 | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 95.8 |
| 13 | 2 | 2 | 1 | 2 | 0 | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 83.3 |
| 14 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
| 15 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 16 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 17 | 2 | 2 | 1 | 1 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 86.3 |
| 18 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 95.4 |
| 19 | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Noh, K.; Min, J.J.; Rupar, C. Effects of Toxic Metal Contamination in the Tri-State Mining District on the Ecological Community and Human Health: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6783. https://doi.org/10.3390/ijerph17186783
Park H, Noh K, Min JJ, Rupar C. Effects of Toxic Metal Contamination in the Tri-State Mining District on the Ecological Community and Human Health: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(18):6783. https://doi.org/10.3390/ijerph17186783
Chicago/Turabian StylePark, Hyejoon, Keeyoon Noh, Jihyun Jane Min, and Christopher Rupar. 2020. "Effects of Toxic Metal Contamination in the Tri-State Mining District on the Ecological Community and Human Health: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 18: 6783. https://doi.org/10.3390/ijerph17186783
APA StylePark, H., Noh, K., Min, J. J., & Rupar, C. (2020). Effects of Toxic Metal Contamination in the Tri-State Mining District on the Ecological Community and Human Health: A Systematic Review. International Journal of Environmental Research and Public Health, 17(18), 6783. https://doi.org/10.3390/ijerph17186783





