Abstract
Coral reef ecosystems are continuously degraded by anthropogenic and climate change drivers, causing a widespread decline in reef biodiversity and associated goods and services. In response, active restoration methodologies and practices have been developed globally to compensate for losses due to reef degradation. Yet, most activities employ the gardening concept that uses coral nurseries, and are centered in easily-accessible reefs, with existing infrastructure, and impractical for coral reefs in remote locations. Here we evaluate the effectiveness of direct outplanting of coral micro-fragments (Pavona clavus and Pocillopora spp.) as a novel approach to restore remote reefs in the Islas Marías archipelago in the Eastern Tropical Pacific. Coral growth (height-width-tissue cover), survival percentage, extension rates (cm year−1), skeletal density (g cm−3) and calcification rates (g cm−2 year−1) were assessed over 13 months of restoration. In spite of detrimental effects of Hurricane Willa, transplants showed a greater-than-twofold increase in all growth metrics, with ~58–61% survival rate and fast self-attachment (within ~3.9 months) for studied species, with Pocilloporids exhibiting higher extension, skeletal density, and calcification rates than Pavona. While comprehensive long-term studies are required, direct transplantation methodologies of coral micro-fragments are emerging as time-effective and affordable restoration tools to mitigate anthropogenic and climate change impacts in remote and marginal reefs.
1. Introduction
Worldwide distributed coral reef ecosystems host >25% of marine life, sustain important biogeochemical and ecological functionality [,], and uphold goods and services for human wellbeing [,,], yet are continuously impacted by the cumulative effects of anthropogenic stressors and climate change drivers, causing a widespread degradation of these valuable coral reef communities [,,]. Several coral reef ecosystems revealed some resistance to the inflicted impacts, with natural recovery processes with slow recovery trajectories, yet, all providing some time for natural acclimatization to occur [,,,,,,,]. In response, the notion of active coral reef restoration as a key strategy addressing the cumulative impacts of anthropogenic and climate change has been put forward, and new coral restoration approaches have been developed globally in the last decades [,,,,,,,,,]. As it becomes questionable whether corals would be able to acclimatize quickly enough [,], the emerged active reef restoration measures have acquired further consideration as effective tools for facilitating the rehabilitation of the globally impacted coral reef ecosystems [,,,,,], and to save time, since adaptation seems to occur over slow evolutionary timescales [,]. At this point, many of the active reef restoration measures are using ecological engineering applications, gardening and farming concepts, assisted migration/colonization, assisted genetics/evolution, assisted microbiome, epigenetics, and chimerism (reviewed in [,]). Although some progress has been made in the last two decades, coral reef restoration is far from being a mature discipline, as many approaches present a local applicability or were not explored in a wide range of coral reefs [,]. Hence, the continuous assessment of restoration tools and the development of cost- and time-effective protocols are required to mitigate, or compensate for, rapid coral reef degradation [,,].
The Eastern Tropical Pacific (ETP) coral reef communities are declining, experiencing anthropogenic and climate change impacts, including several major coral bleaching and mortality events, resulting from the El Niño–Southern Oscillation (ENSO) phenomena. Live coral cover has been reduced from 33% to 7% with >90% local coral mortalities caused by these extreme thermal anomalies in the last 30 years [,,,,,,]. Yet, some ETP reefs reveal fast recovery [,,,,,,,], and life history patterns of certain branching and massive coral reef species have shown high thermal tolerance thresholds [,,,,,]. Hence, assisted coral restoration with more resistant coral genotypes can be a pantropical conservation priority, to accelerate natural recovery and, also, to restore already-degraded reefs for improved future statuses of the ETP coral reef communities.
The number of active restoration projects in the ETP has increased dramatically in the last decade [,,,,,,]. Yet, more studies are required in order to integrate and enhance restoration efforts in the region and other remote and marginal reefs, where accessibility to absent or scarce infrastructures and facilities is limited, and active restoration measures are not commonly implemented [,]. In the present study, we tested the efficiency of the direct outplanting of coral micro-fragments taken from Pavona clavus (massive, slow-growing species), Pocillopora cf. eydouxi, and Pocillopora cf. effusus (branching, fast-growing species), as a practical approach to restore remote and marginal ETP coral reefs. Annual increment of coral growth parameters as height (cm), width (cm), live tissue cover (cm−2), annual extension (cm year−1), skeletal density (g cm−3), annual calcification rate (g cm−2 year−1), survival, and self-attachment rates (%) were assessed over 13 months (400 days) of restoration (2018–2019) at the Islas Marías archipelago, Mexico, in the Northeastern Tropical Pacific.
2. Materials and Methods
2.1. Study Area
The study was conducted from June 2018 to August 2019 (400 days) at Islas María Cleofas (IMC), Islas Marías Biosphere Reserve in the Central Mexican Pacific, located 132 km offshore to the nearest coast of Nayarit, Mexico (Figure 1). The Marías archipelago is a restricted zone, being a federal prison area for over 100 years and where conservation measures on the marine ecosystems have been extreme []. This insular zone acts as a hub for coral species, and as a stepping-stone for larval dispersal route across the Central to Eastern Pacific regions and between Eastern Pacific coral communities [,,]. Within this area, Islas María Cleofas harbors a high diversity of scleractinians, including branching Pocillopora species, in shallow waters (2–6 m), and massive Pavona and Porites species on deeper reefs [,]. The Marías archipelago is located in an ocean convergence zone influenced by interannual transitional ocean currents, the California current with seawater temperature (SWT) ranging from 18 to 21°C during December–April [], by the Mexican coastal current with warmer SWT (~27–31 °C) between July–November [], the seasonal upwelling during April–May [] in addition to recurrent heat waves associated with intense ENSO (both “El Niño and La Niña” phases []), and by a high frequency of tropical cyclones [].
Figure 1.
Restoration site (red star) located at Islas María Cleofas (IMC), Islas Marías archipelago in the Central Mexican Pacific (red box).
2.2. Coral Fragment Transplantation
Transplantation of coral species was performed with coral fragments taken from the main reef-building corals residing in Islas Marías, the massive species Pavona clavus and the branching species Pocillopora spp. Small portions (~3–5, less than 5% of Pavona colonies) of apical nodules and laminar edges (fragments ~25–30 cm2 each) were removed from 15 adult P. clavus colonies, using hammer and chisel. For the branching species, we collected naturally dispersed “corals of opportunity” fragments (~10–15 cm length) from 25 colonies of the morphospecies Pocillopora cf. effusus and Pocillopora cf. eydouxi. Collected fragments were removed and stained for 1 h ex situ (on board) with Alizarin Red (20 mg L−1, sigma) in aerated aquariums filled with local seawater, and translocated to the reef, where they were further fragmented to small coral tissue segments of sizes >3 cm−2 (coral micro-fragments), using a small, sharp chisel, following the recommendation by Forsman et al. []. We obtained 78 micro-fragments from P. clavus with a mean size of 4.5 cm2, and 3.2 cm2 for Pocillopora micro-fragments (n = 76) that were randomly arrayed along two plots at 5 m depth of ~6 m−2 each, with distances of ~5–10 cm between fragments. The fragments were glued to the natural substrate (limestone rock), previously cleaned manually by brushes (Figure 2A and Figure 3A), using a water-resistant silicon adhesive (MS Express, Fischer).
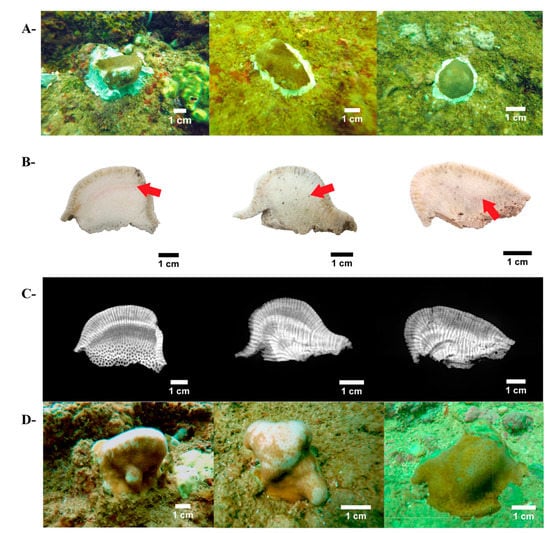
Figure 2.
The use of micro-fragments of Pavona clavus from Islas Marías Cleofas for coral reef restoration. (A): micro-fragments at onset, just glued to the substrates; (B): coral skeleton slabs exhibiting Alizarin red marks (red arrows); (C): X-ray images of coral skeletons displaying annual bands pattern (light/dark shade = low/high density): (D): final coral size of the same colonies as in part A, 13 months later.
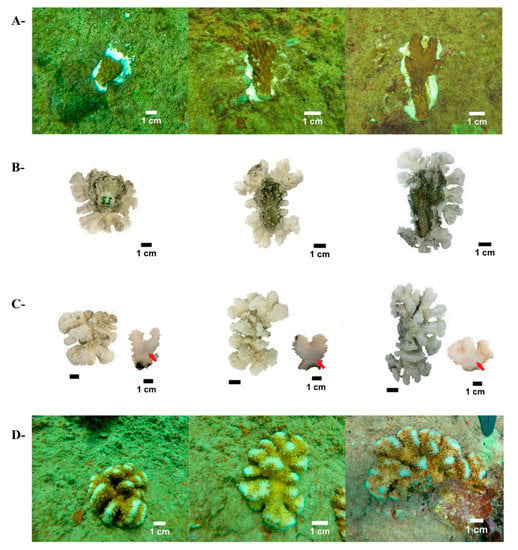
Figure 3.
The use of micro-fragments of Pocillopora spp. from Islas Marías Cleofas for coral reef restoration. (A): micro-fragments at onset, just glued to the substrates; (B): coral skeletons comparing initial and final branches (bottom-side view); (C): coral skeleton upside view and coral branch slabs exhibiting Alizarin red marks (red arrows); and (D): final coral size of the same colonies as in part A, 13 months later.
2.3. Coral Growth Metrics
Each of the 154 micro-fragments were tagged, and information was gathered on growth, survival, and attachment efficiency at three time points (at day 0, 32, 252, and 400). For coral growth, maximum height increase (h) was determined by the longest apical distance (cm) from base to the top of the colony, and maximum width (w) referred as the perpendicular length distance (cm) to the height axis, measured in situ with calipers (precision: 0.05 mm). Increment of live tissue surfaces (cm−2) was calculated using mean “h” and “w” values for all corals. The number of new adjacent branches in Pocillopora was also noted. Coral survival (%) was determined as the percentage of fragments with continuous live tissue growth along the experiment. Seawater temperature (SWT) was registered in situ daily, using underwater temperature loggers (HOBO) installed at the study location.
2.4. Calcification Rates and Annual Banding Measurements
After 13 months under natural conditions, 28 developing coral colonies (Pavona clavus (n = 15) and Pocillopora spp. (n = 13)) were collected (August 2019) and transported to the laboratory, their tissues were removed with fresh water jets, and skeletons were dried using pressurized air. Dried skeletons were then placed in a conventional oven at 75 °C for 3 h, in order to eliminate organic residues.
To determine annual banding and skeletal parameters for massive corals, each colony was cut into slices of 8–10 mm width, using a tipped-diamond saw blade (Qep) with fresh water as lubricant. Coral slices were X-rayed using a Philips X-ray machine (Mobile Diagnost Opta), set at 50 kv for 20 mAs at 1.8 m distance from the X-ray source (Figure 2C). In each X-ray, a wedge of the bivalve Tridacna maxima was used as a bulk density standard (2.77 g cm−3). X-ray images (75 dpi) were corrected using Duprey et al.’s [] protocol, in order to eliminate irradiation bias (heel effect and square law) that may affect calculating density data. Afterward, the corrected images were analyzed, and skeletal density values were obtained (g CaCO3 cm−3) using the software ImageJ ver. 1.52s (National Institute of Mental Health, Bethesda, Maryland, MD, USA) (https://imagej.nih.gov/ij/), following Carricart-Ganivet and Barnes []. For annual extension rates (cm year−1), vertical distances were measured from the Alizarin red mark to the uppermost edge of each colonial skeleton with a digital caliper (Truper 0.001mm precision; Figure 2B). Annual coral calcification rates (g CaCO3 cm−2 year−1) were calculated by the product of mean annual extension rate and skeletal density [].
Annual growth parameters for Pocillopora spp. were obtained from a subset of colony branches, which were cut below the Alizarin red marks and sliced using a handheld rotary cutting tool (Dremel 4000). Coral slices were photographed with a Canon Powershot XS500 camera (Canon Inc., New York, NY, USA), and the images were analyzed with ImageJ. Extension rates were determined by measuring the distances from the stain mark lines to the uppermost axes, in 3–6 branches for each colony (Figure 3C). Skeletal densities were measured in the same subset of branches using the buoyant weight method [], and estimated as the mean dry weight (mass) divided by the differences between wet weight (water displaced) and dry weight. Coral calcification rates were calculated as for massive colonies, using the mean extension and density data set for each colony.
2.5. Data Analysis
Mean average values of coral parameters (± standard deviation) were calculated, and all data were tested for normality (Shapiro–Wilk; p < 0.005) and homoscedasticity (Levene; p < 0.05). As data were not distributed normally nor homogenously, the non-parametric Kaplan–Meier function [] was used to compare survival between branching and massive corals. Non-parametric ANOVA based on ranks (Kruskal–Wallis) was used to assess difference of coral growth metrics (density, extension, and calcification) among species. Simple linear regression tests were used to determine relationships between coral parameters, and the relation of coral growth with SWT. The constant variance and normality of residuals were evaluated for each regression. Statistical analyses were conducted using Sigma Plot Ver. 11 software (Systat Software, Inc., Chicago, IL, USA), with a confidence interval of 95% (α = 0.005).
3. Results
A total of 154 coral micro-fragments were produced and outplanted within 3 days (30 h teamwork of two people), with five micro-fragments outplanted per hour/person. The total cost of the whole restoration process (installation, materials, and monitoring cost) was US$605 (~US$4.00 per outplanted coral, excluding indirect expenses (travel, accommodation, food supplies, and scuba gear costs). Pooled survivorship after 13 months was 60% (Figure 4), with no significant difference between Pocillopora spp. (58%) and Pavona (61%) corals (log rank, X2 = 0.333; p = 0.564). An increase in mortality (from 98% survivorship to 70% for Pavona and Pocillopora species) was observed following Hurricane Willa [], which passed over the Islas Marías archipelago in October 2018, including the restoration site (Figure 4). Even though the accumulated mortality was modest, most (30%) dead fragments were dislodged, most probably by the storm, and were lost. Only 10% of micro-fragments died during the 13-month study period, following which they were fouled by macroalgae.
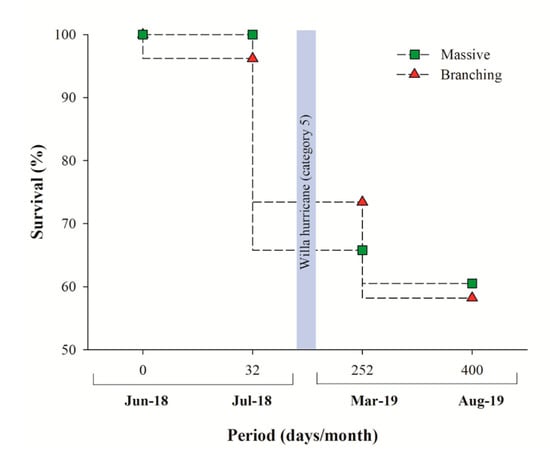
Figure 4.
Coral survival (%) for Pavona clavus (green squares) and Pocillopora species (red triangles) during the 13-month in situ period. The vertical grey bar depicts Hurricane Willa date (118 restoration days).
The Pocillopora growth measurements revealed a 183% linear extension and a 253% width increase, compared to initial sizes, and with average extension highs of 4.16 ± 1.02 cm (eight new branches developed from a single branch, one-year growth values; Figure 5, Table 1), 4.25 ± 1.39 cm for widths, and surface area of 5.27 ± 1.96 cm−2 (increased by 464%). Pavona clavus hemispherical structures increased by 158% and 174% in height and width, respectively. It presented an average growth rate of 1.66 ± 1.22 cm for height, and 1.65 ± 1.40 cm for width, a mean accumulated surface area of 5.27 ± 1.96 cm−2, and live coral tissue increase of 237%, with respect to initial measurements (Figure 5, Table 1). We further documented that live coral tissues completely overgrew the silicon-adhesive lugs by day 32, primarily in Pocillopora fragments and almost all (96%) of the micro-fragments (Pocillopora and Pavona) were self-attached to natural substrata within 3.9 months (range 1–8 months; Figure 5, Table 1).
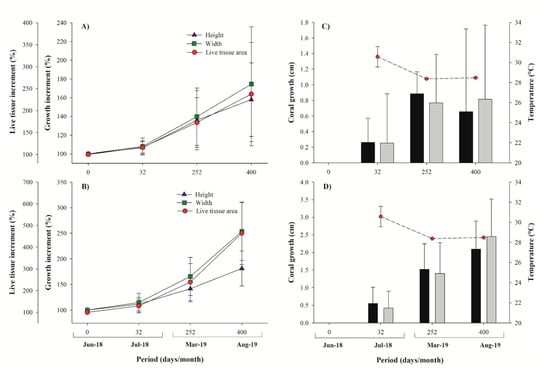
Figure 5.
Micro-fragments growth measurements. Percentage of growth increment (% ± SD) of Pavona clavus (A) and Pocillopora spp. (B). Mean coral growth (cm ± SD) and seawater temperature at each interval time (days) for Pavona clavus (C) and Pocillopora spp. (D).

Table 1.
Growth parameters (±SD). Height (H), width (W), live tissue (LT), and attachment rates (AR) for Pocillopora spp. and Pavona clavus, along three time points.
Yearly mean extension rates for Pocillopora spp. were 2.74 ± 0.33 cm year−1, with skeletal density of 2.19 ± 0.14 g cm−3, and calcification rate of 6.02 ± 0.93 g cm−2 year−1, compared to mean extension rates of 0.92 ± 0.16 cm year−1, skeletal density of 1.39 ± 0.25 g cm−3, and calcification rate of 1.25 ± 0.21 g cm−2 year−1 for P. clavus (Table 1). As expected, P. clavus revealed significantly lower growth rates, as compared to Pocillopora species in all three parameters: extension (H = 80.568: p < 0.001), skeletal density (H = 82.211: p < 0.001), and calcification rate (H = 81.397: p < 0.001) (Figure 6). For the Pocillopora species, we found positive correlations between extension and calcification rate (R2 = 0.82: p < 0.001) and density vs. calcification (R2 = 0.42: p < 0.001), but not for extension vs. density (R2 = 0.07: p = 0.005). In P. clavus, the calcification rates were positively correlated with extension rates (R2 = 0.24: p = 0.002) and skeletal densities (R2 = 0.20: p = 0.006), yet extension rates and skeletal densities were negatively correlated (R2 = 0.29: p < 0.001). Mean annual seawater temperature was 28.71 ± 2.38 °C, with a minimum of 25.03 °C during winter season and a maximum of 31.67 °C during summer. The SWT data was positively correlated with coral growth values of Pocillopora spp. (R2 = 0.49: p < 0.001) and with live tissue values of P. clavus (R2 = 0.12, p = 0.013).
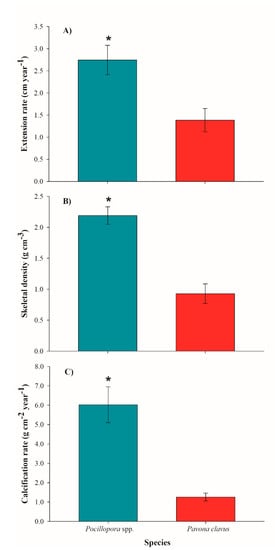
Figure 6.
Annual growth parameters (±SD) for Pocillopora spp. and Pavona clavus. (A): annual extension rates; (B): skeletal density; and (C): calcification rates. Asterisks (*) denote significance differences between species (p < 0.001).
4. Discussion
Global climate change and local-scale anthropogenic stressors have caused the unprecedented decline in coral reefs in the last three decades [,]. As natural recovery is slow, the emerging cost- and time-effective ecological restoration tools are of prime importance to mitigate coral reef degradation, and to boost reef biodiversity and ecosystem functionality recovery [,,,]. The literature further reveals two general attitudes that are employed: the simpler and cheapest way of direct transplantation of corals fragments to degraded reef [,,] and the “coral gardening” approach that requires additional work and extended periods for coral transplantation through an intermediate aquaculture nursery phase, where coral fragments are cultured until reaching suitable transplantation sizes [,,,]. Many of the widely-used and efficient restoration techniques, such as the coral gardening and farming tenet [,,,,,,], are time-consuming, and need constant maintenance of farmed corals and accessibility to infrastructure and facilities. This is impractical in cases, where, for example, restoration activities are performed in remote reefs or under unfavorable conditions, further highlighting the need to expand the restoration toolbox and to adapt alternative approaches, such as direct transplantation.
The direct transplantation approach, while bypassing the need for established infrastructure and the creation of stock material for transplantation, has been criticized for results inconsistency, in terms of efficiency and effectiveness [,]. The production of micro-fragments (either for massive or branching corals) is commonly manifested in costly land-based coral farms (including cost for tanks, electricity, technicians, water-pumps, filters, and infrastructure maintenance) along extended periods that, with transportation and outplanting tools, may sum to US$13.00–61.00 per coral outplanted ([], but also see []). Yet, there is no evidence that land-based coral farms enhance survivorship and growth rates after outplanting [,,]. In contrast, the in situ production and direct transplantation approach, as presented in this study, is cheaper and more practical in terms of time (in the present work, US$4.00 per coral and 5 transplants per h per person), bypassing the use of nursery phase or the addition of artificial materials and substratum stabilization [,,]. This is a real time-saving, effective and low-cost approach, with scalable options, preferable for remote reef.
The overall first 13 months survivorship (60%) is similar to results obtained in previous, more time-consuming restoration approaches [,,,,,,,,,], even though the actual survival is much higher (about 90%), as most coral mortality was associated with fragment dislodgements (30%) due to the Willa hurricane forces, reflecting the impacts of natural catastrophes on other restoration projects [,]. The 10% of natural coral mortality is most probably due to competition with macroalgae (Padina sp.) overgrowth at the end of the restoration period (Figure 4). Thus, this study’s results further reveal a higher survival (20–40%), as compared to one year of outplanting in studies that use coral micro-fragmentation [,,].
After one-year in situ, Pocillopora transplants showed a twofold increase in coral sizes (height and length), and Pavona clavus colonies almost doubled in width and length, and augmented 2.5-times in live tissue cover (Figure 2 and Figure 3). Notwithstanding, while the branching species grew three times faster than the massive species, the growth rates of all coral species used here revealed similar growth rates to previous successful restoration programs in the ETP region [,,,,]. This fact is consistent with the tenet that micro-fragments and coral nubbins exhibit faster growth rates, compared to large fragments or whole colonies, a trait developed to avoid competition impacts or to reduce partial predation sways [,,,,]. This trait further facilitates coral nubbins self-attachment on all types of substrata [,].
In the same way, annual extension (2.74 cm year−1) and calcification rates (6.03 g cm−2 year−1) obtained for Pocillopora spp. are similar to other documented outcomes in the ETP region [,,,,,]. The same applies to Pavona, one of the fastest growing massive coral species in the ETP region [,,,,,,]. The consistency in the comparisons is further applied to coral density for branching (1.90–2.65 g cm−3) and massive (0.97–1.95 g cm−3) species [,,,]. Our results thus reveal the possible equal calcification patterns for both extension rates and skeletal density in coral nubbins, a different calcification landscape when compared to adult colonies, where calcification priorities are diverted between extension rates vs. skeletal densities [,,].
5. Conclusions
Active coral reef restoration is becoming a key strategy to rehabilitate anthropogenic and climate change impacts [,,,,]. Direct outplanting of micro-fragments and coral nubbins from keystone species, as studied here at the Islas Marías archipelago, has emerged as an effective, suitable, and affordable coral reef restoration methodology, primarily (a) in remote and marginal reefs less accessible to activities and methodologies performed in easily-reached reefs, and (b) when corals of opportunity are frequently used. Yet, the use of direct outplanting of coral nubbins and micro-fragments is still in its initial stages, and more comprehensive studies in additional sites worldwide are required in order to improve and to establish this new restoration avenue [,,]. Direct transplantation of coral fragments in locations where time and conditions are limited, aiming to rehabilitate and support self-sustainable reef processes without available infrastructure, is thus an alternative practical tool for reef restoration in remote and marginal coral reef communities, such as the Islas Marías archipelago.
Author Contributions
Conceptualization: J.J.A.T.-L., A.P.R.-T., and B.R.; data curation: J.J.A.T.-L.; formal analysis: J.J.A.T.-L.; funding acquisition: A.P.R.-T. and A.L.C.-M.; methodology: J.J.A.T.-L. and A.P.R.-T.; resources: J.J.A.T.-L., A.P.R.-T., and A.L.C.-M.; software: J.J.A.T.-L.; supervision: J.J.A.T.-L. and B.R.; validation: A.P.R.-T. and B.R.; writing—original draft: J.J.A.T.-L.; writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was supported by the NGS-55349R-19 project to A.P.R.T. and the project P/PIFI-2010-14MSU0010Z-10 to A.C.M.
Acknowledgments
Thanks to the Mexican authorities of Reserva de la Biosfera Islas Marías (SEMARNAT/CONANP), Secretaría de Gobiernación (SEGOB), Secretaría de Marina (SEMAR) for the permits and facilities. We especially give thanks to the organization Protección y Restauración de Islas y Zonas Naturales (PROZONA AC), La Punta Outdoors SA de CV, and Grupo Cleofas “Marías Cleofas vessel” and their exceptional crew for accommodations and assistance during field expeditions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheppard, C.R.C.; Davy, S.K.; Pilling, G.M. The Biology of Coral Reefs; Oxford University Press: London, UK, 2009; p. 333. [Google Scholar]
- Perry, C.T.; Alvarez-Filip, L. Changing geo-ecological functions of coral reefs in the Anthropocene. Funct. Ecol. 2018, 33, 976–988. [Google Scholar] [CrossRef]
- Wilkinson, C. Status of Coral Reefs of the World: 2008; GCRMN: Townsville, Australia, 2008. [Google Scholar]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Hoegh-Guldberg, O.; Pendleton, L.; Kaup, A. People and the changing nature of coral reef. Reg. Stud. Mar. Sci. 2019, 30, 100699. [Google Scholar] [CrossRef]
- Bruno, J.F.; Selig, E.R. Regional decline of coral cover in the Indo-Pacific:Timing, extent, and subregional comparisons. PLoS ONE 2007, 2, e711. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.; Kairo, J.G.; Arstegui, J.; Guinder, V.A.; Hallberg, R.; Hilmi, N.; Jiao, N.; Karim, M.S.; Levin, L.; et al. Changing ocean, marine ecosystems, and dependent communities. In IPCC Special Report on The Ocean and Cryosphere in A changing Climate; Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; IPCC: Geneva, Switzerland, 2019; Chapter 5. [Google Scholar]
- Glynn, P.W.; Riegl, B.; Correa, A.M.S.; Baums, I.B. Rapid recovery of a coral reef at Darwin Island, Galápagos Islands. Galapagos. Res. 2009, 66, 6–13. Available online: https://nsuworks.nova.edu/occ_facarticles/347 (accessed on 7 September 2020).
- Johns, K.A.; Osborne, K.O.; Logan, M. Contrasting rates of coral recovery and reassembly in coral communities on the Great Barrier Reef. Coral Reefs 2014, 33, 553–563. [Google Scholar] [CrossRef]
- Gilmour, J.P.; Smith, L.D.; Heyward, A.J.; Baird, A.H.; Pratchett, M.S. Recovery of an isolated coral reef system following severe disturbance. Science 2013, 340, 69–71. [Google Scholar] [CrossRef]
- Glynn, P.W.; Riegl, B.; Purkis, S.; Kerr, J.M.; Smith, T.B. Coral reef recovery in the Galápagos Islands: The northernmost islands (Darwin and Wenman). Coral Reefs 2015, 34, 421–436. [Google Scholar] [CrossRef]
- Suding, K.; Higgs, E.; Palmer, M.; Callicott, J.B.; Anderson, C.B.; Baker, M.; Gutruch, J.J.; Hondula, K.L.; LaFevor, M.C.; Larson, B.M.H.; et al. Committing to ecological restoration. Science 2015, 348, 638–640. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Rodríguez-Troncoso, A.P.; Carricart-Ganivet, J.P.; Cupul-Magaña, A.L. Calcification and growth rate recovery of the reef-building Pocillopora species in the northeast tropical Pacific following an ENSO disturbance. PeerJ 2017, 5, e3191. [Google Scholar] [CrossRef]
- Rinkevich, B. Ecological engineering approaches in coral reef restoration. ICES J. Mar. Sci. 2020. [Google Scholar] [CrossRef]
- Romero-Torres, M.; Treml, E.A.; Acosta, A.; Paz-García, D.A. The Eastern Tropical Pacific coral population connectivity and the role of the Eastern Pacific Barrier. Sci. Rep. 2018, 8, 9352. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.J. Reef Rehabilitation Manual; Coral Reef Targeted Research and Capacity Building for Management Program: St. Lucia, Queensland, Australia, 2010; p. 166. [Google Scholar]
- Rinkevich, B. Rebuilding coral reefs: Does active reef restoration lead to sustainable reefs? Curr. Opin. Environ. Sust. 2014, 7, 28–36. [Google Scholar] [CrossRef]
- Rinkevich, B. Climate change and active reef restoration—Ways of constructing the ‘reefs of tomorrow’. J. Mar. Sci. Eng. 2015, 3, 111–127. [Google Scholar] [CrossRef]
- Horoszowski-Fridman, B.; Brêthes, J.-C.; Rahmani, N.; Rinkevich, B. Marine silviculture: Incorporating ecosystem engineering properties into reef restoration acts. Ecol. Eng. 2015, 82, 201–213. [Google Scholar] [CrossRef]
- Horoszowski-Fridman, Y.B.; Rinkevich, B. Restoring the animal forests: Harnessing silviculture biodiversity concepts for coral transplantation. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, C., Eds.; Springer: Berlin, Germany, 2017; pp. 1313–1335. [Google Scholar]
- Omori, M. Coral restoration research and technical developments: What we have learned so far. Mar. Biol. Res. 2019, 7, 377–409. [Google Scholar] [CrossRef]
- Bayraktarov, E.; Stewart-Sinclair, P.J.; Brisbane, S.; Boström-Einarsson, L.; Saunders, M.I.; Lovelock, C.E.; Possingham, H.P.; Mumby, P.J.; Wilson, K.A. Motivations, success and cost of coral reef restoration. Restor. Ecol. 2019, 27, 981–991. [Google Scholar] [CrossRef]
- Golomb, D.; Shashar, N.; Rinkevich, B. Coral carpets-a novel ecological engineering tool aimed at constructing coral communities on soft sand bottoms. Ecol. Eng. 2020, 145, 105743. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral restoration—A systematic review of current methods, successes, failures and future directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 2017, 7, 627–636. [Google Scholar] [CrossRef]
- Rinkevich, B. The coral gardening concept and the use of underwater nurseries: Lesson learned from silvics and silviculture. In Coral Reef Restoration Handbook; Precht, W.F., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 291–301. [Google Scholar]
- Rinkevich, B. Rebutting the inclined analyses on the cost-effectiveness and feasibility of coral reef restoration. Ecol. Appl. 2017, 27, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Shaish, L.; Levi, G.; Katzir, G.; Rinkevich, B. Coral reef restoration (Bolinao, the Philippines) in the face of frequent natural catastrophes. Restor. Ecol. 2010, 18, 285–299. [Google Scholar] [CrossRef]
- Shaish, L.; Levy, G.; Katzir, G.; Rinkevich, B. Employing a highly fragmented, weedy coral species in reef restoration. Ecolo. Eng. 2010, 3836, 1424–1432. [Google Scholar] [CrossRef]
- Rachmilovitz, E.N.; Rinkevich, B. Tiling the reef—Exploring the first step of an ecological engineering tool that may promote phase-shift reversals in coral reefs. Ecol. Eng. 2017, 105, 150–161. [Google Scholar] [CrossRef]
- Fine, M.; Cinar, M.; Voolstra, C.R.; Safa, A.; Rinkevich, B.; Laffoley, D.; Hilmi, N.; Allemand, D. Coral reefs of the Red Sea—Challenges and potential solutions. Reg. Stud. Mar. Sci. 2019, 25, 100498. [Google Scholar] [CrossRef]
- Rinkevich, B. The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 2019, 7, 201. [Google Scholar] [CrossRef]
- Wang, J.T.; Wang, Y.T.; Keshavmurthy, S.; Meng, P.J.; Chen, C.A. The coral Platygyra verweyi exhibits local adaptation to long-term thermal stress through host-specific physiological and enzymatic response. Sci. Rep. 2019, 9, 13492. [Google Scholar] [CrossRef]
- Rinkevich, B. Coral chimerism as an evolutionary rescue mechanism to mitigate global climate change impacts. Glob. Chang. Biol. 2019, 25, 1198–1206. [Google Scholar] [CrossRef]
- Forrester, G.E.; Chan, M.; Conetta, D.; Dauksis, R.; Nikles, K.; Sivaro, A. Comparing the Efficiency of Nursery and Direct Transplanting Methods for Restoring Endangered Corals. Ecol. Res. 2019, 37, 81–89. Available online: https://muse.jhu.edu/article/725208 (accessed on 7 September 2020). [CrossRef]
- Glynn, P.W. Coral mortality and disturbances to coral reefs in the tropical eastern Pacific. In Global ecological consequences of the 1982-83 El Nino-Southern Oscillation; Glynn, P.W., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 55–126. [Google Scholar]
- Glynn, P.W. Effects of the 1997-98 El Niño Southern-oscillation on Eastern Pacific corals and coral reefs: An overview. In Proceedings of the 9th International Coral Reefs Symposium, Bali, Indonesia, 23–27 October 2000; Volume 2, pp. 169–1174. [Google Scholar]
- Carriquiry, J.D.; Cupul-Magaña, A.; Rodríguez-Zaragoza, F.; Medina-Rosas, P. Coral bleaching and mortality in the Mexican Pacific during the 1997-98 El Niño, and prediction from a remote sensing approach. Bull. Mar. Sci. 2001, 69, 237–249. [Google Scholar]
- Reyes-Bonilla, H.; Carriquiry, J.D.; Morales, G.E.; Cupul-Magaña, A.L. Effects of the 1997-99 El Niño and anti El Niño events on coral communities of the Pacific coast of México. Coral Reefs 2002, 21, 368–372. [Google Scholar] [CrossRef]
- Cruz-García, R.; Rodríguez-Troncoso, A.P.; Rodríguez-Zaragoza, F.A.; Mayfield, A.; Cupul-Magaña, A.L. Ephemeral effects of El Niño southern oscillation events on an eastern tropical Pacific coral community. Mar. Freshw. Res. 2020. [Google Scholar] [CrossRef]
- Alvarado, J.J.; Sánchez-Noguera Arias-Godínez, C.G.; Araya, T.; Fernández-García, C.; Guzmán, A.G. Impact of El Niño 2015-2016 on the coral reefs of the Pacific of Costa Rica: The potential role of marine protection. Rev. Biol. Trop. 2020, 68 (Suppl. 1), S271–S282. [Google Scholar] [CrossRef]
- Hueerkamp, C.; Glynn, P.W.; D’Croz, L.; Maté, J.L.; Colley, S.B. Bleaching and recovery of five eastern Pacific corals in an el Niño-related temperature. Exp. Bull. Mar. Sci. 2001, 69, 215–236. [Google Scholar]
- Guzmán, H.M.; Cortés, J. Reef recovery 20 years after the 1982-1983 El Niño massive mortality. Mar. Biol. 2007, 15, 401–411. [Google Scholar] [CrossRef]
- Manzello, D.P.; Enochs, I.C.; Bruckner, A.; Renaud, P.G.; Kolodziej, G.; Budd, D.A.; Carlton, R.; Glynn, P.W. Galápagos coral reef persistence after ENSO warming across an acidification gradient. Geophys. Res. Lett. 2014, 41, 9001–9008. [Google Scholar] [CrossRef]
- Glynn, P.W.; Feingold, J.S.; Baker, A.; Banks, S.; Baums, I.B.; Cole, J.; Colgan, M.W.; Fong, P.; Glynn, P.J.; Keith, I.; et al. Status of corals and coral reefs of the Galápagos Islands (Ecuador): Past, present, and future. Mar. Pollut. Bull. 2018, 133, 717–733. [Google Scholar] [CrossRef]
- Rodríguez-Troncoso, A.P.; Carpizo-Ituarte, E.; Cupul-Magaña, A.L. Differential response to cold and warm water conditions in Pocillopora colonies from the Central Mexican Pacific. J. Exp. Mar. Biol. Ecol. 2010, 391, 57–64. [Google Scholar] [CrossRef]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting Patterns of Coral Bleaching Susceptibility in 2010 Suggest an Adaptive Response to Thermal Stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Rodríguez-Troncoso, A.P.; Carricart-Ganivet JP y Cupul- Magaña, A.L. Historical insights on growth rates of the reef-building corals Pavona gigantea and Porites panamensis from the Northeastern tropical Pacific in the last two decades. Mar. Environ. Res. 2017, 132, 23–32. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Smith, R.; Walther, M.; Pinzón, J.; Pettay, D.T.; McGinley, M.; Aschaffenburg, M.; Medina-Rosas, P.; Cupul-Magaña, A.L.; López Pérez, A.; et al. Host–symbiont recombination versus natural selection in the response of coral–dinoflagellate symbioses to environmental disturbance. Proc. R. Soc. 2010, 277, 2925–2934. [Google Scholar] [CrossRef]
- Liñán-Cabello, M.A.; Flores-Ramírez, L.A.; Laurel-Sandoval, M.A.; Mendoza, E.; García, S.; Olinda, S.; Delgadillo-Nuño, M.A. Acclimation in Pocillopora spp. during a coral restoration program in Carrizales Bay, Colima, Mexico. Mar. Fresh. Behav. Physiol. 2010, 44, 1–12. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.J.A.; Cupul-Magaña, A.L.; Rodríguez-Troncoso, A.P. Restoration of a degraded coral reef using a natural remediation process: A case study from a Central Mexican Pacific National Park. Ocean. Coast. Manag. 2014, 96, 12–19. [Google Scholar] [CrossRef]
- Nava, H.; Figueroa-Camacho, A.G. Rehabilitation of damaged reefs: Outcome of the use of recently broken coral fragments and healed coral fragments of pocilloporid corals on rocky boulders. Mar. Ecol. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Muñiz-Anguiano, D.; Verduzco-Zapata, M.; Liñan-Cabello, M.A. Factors associated with response Pocillopora spp. (Anthozoa: Scleractinia) during a restoration processon the Mexican Pacific coast. Rev. Biol. Mar. Oceanogr. 2017, 52, 299–310. [Google Scholar] [CrossRef]
- Lizcano-Sandoval, L.D.; Londoño-Cruz, E.; Zapata, F. Growth and survival of Pocillopora damicornis (Scleractinia: Pocilloporidae) coral fragments and their potential for coral reef restoration in the Tropical Eastern Pacific. Mar. Biol. Res. 2018, 14, 887–897. [Google Scholar] [CrossRef]
- Ishida-Castañeda, J.; Pizarro, V.; López-Victoria, M.; Zapata, F.A. Coral reef restoration in the Eastern Tropical Pacific: Feasibility of the coral nursery approach. Res. Ecol. 2019, 1–7. [Google Scholar] [CrossRef]
- Tortolero-Langarica, J.A.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Alarcón-Ortega, L.C.; Santiago-Valentín, J.D. Accelerated recovery of calcium carbonate production in coral reefs using low-tech ecological restoration. Ecol. Eng. 2019, 128, 89–97. [Google Scholar] [CrossRef]
- Nakamura, R.; Ando, W.; Yamamoto, H.; Kitano, M.; Sato, A.; Nakamura, M.; Kayanne, H.; Omori, M. Corals mass-cultured from eggs and transplanted as juveniles to their native, remote coral reef. Mar. Ecol. Prog. Ser. 2011, 436, 161–168. [Google Scholar] [CrossRef]
- Beger, M.; Sommer, B.; Harrison, P.L.; Smith, S.D.A.; Pandolfi, J.M. Conserving potential coral reef refugia at high latitudes. Divers. Distrib. 2013. [Google Scholar] [CrossRef]
- CONANP. Programa de Conservación y Manejo Reserva de la Biosfera Islas Marias, Mexico. Secretaria de Medio Ambiente y Recursos Naturales; CONANP: Mexico D.F., Mexico, 2010; p. 216. [Google Scholar]
- López-Pérez, A.; Cupul-Magaña, A.; Ahumada-Sempoal, M.A.; Medina-Rosas, P.; Reyes-Bonilla, H.; Herrero-Pérezrul, M.D.; Reyes-Hernández, C.; Lara-Hernández, J. The coral communities of the Islas Marias archipelago, Mexico: Structure and biogeographic relevance to the Eastern Pacific. Mar. Ecol. 2015, 37, 679–690. [Google Scholar] [CrossRef]
- Santiago-Valentín, J.D.; Rodríguez-Troncoso, A.P.; Bautista-Guerrero, E.; López-Pérez, A.; Cupul-Magaña, L. Settlement ecology of scleractinian corals of the Northeastern Tropical Pacific. Coral Reefs 2019, 39, 133–146. [Google Scholar] [CrossRef]
- Pérez-Vivar, T.L.; Reyes-Bonilla, H.; Padilla, C. Corales petreos (Scleractinia) de las Islas Marías, Pacífico de Mexico. Cien. Mar. 2006, 32, 259–270. [Google Scholar] [CrossRef]
- Pantoja, D.A.; Marinone, S.G.; Parés-Sierra, A.; Gómez-Valdivia, F. Numerical modeling of seasonal and mesoscale hydrography and circulation in the Mexican Central Pacific. Cien Mar. 2012, 38, 363–379. [Google Scholar] [CrossRef]
- Kessler, W.S. The circulation of the Eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 181–217. [Google Scholar] [CrossRef]
- Palacios-Hernández, E.; Carrillo, L.E.; Filonov, A.; Brito-Castillo, L.; Cabrera-Ramos, C.E. Seasonality and anomalies of surface temperature off the coast of Nayarit, Mexico. Ocean Dyn. 2010, 60, 81–91. [Google Scholar] [CrossRef]
- Cai, W.; Wang, G.; Dewitte, B.; Wu, L.; Santoso, A.; Takahashi, K.; Yang, Y.; Carreric, A.; McPhaden, M.J. Increased variability of eastern Pacific El Niño under greenhouse warming. Nature 2018, 564, 201–209. [Google Scholar] [CrossRef]
- Zhao, H.; Raga, B.G. On the distinct interannual variability of tropical cyclone activity over the eastern North Pacific. Atmósfera 2015, 28, 161–178. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Rinkenvich, B.; Hunter, C.L. Investigating fragment size for culturing reef-building corals (Porites lobata and P. compressa) in ex situ nurseries. Aquaculture 2006, 261, 89–97. [Google Scholar] [CrossRef]
- Duprey, N.; Boucher, H.; Jiménez, C. Digital correction of computed X-radiographs for coral densitometry. J. Exp. Mar. Biol. Ecol. 2012, 438, 84–92. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Barnes, D.J. Densitometry from digitized images of X-radiographs: Methodology for measurement of coral skeletal density. J. Exp. Mar. Biol. Ecol. 2007, 344, 67–72. [Google Scholar] [CrossRef]
- Lough, J.M.; Cooper, T.F. New insights from coral growth band studies in an era of rapid environmental change. Earth Sci. Rev. 2011, 108, 170–184. [Google Scholar] [CrossRef]
- Bucher, D.J.; Harriott, V.J.; Roberts, L.G. Microdensity, bulk density and porosity of acroporid corals. J. Exp. Mar. Biol. Ecol. 1998, 228, 117–135. [Google Scholar] [CrossRef]
- Lee, E.T. Nonparametric methods of estimating survival functions. In Statistical Methods for Survival Analysis; Lee, E.T., Ed.; Wiley: New York, NY, USA, 1992; pp. 66–130. [Google Scholar]
- Wood, K.M.; Klotzbach, P.J.; Collins, J.M.; Schreck, C.J. The record-setting 2018 eastern North Pacific hurricane season. Geophys. Res. Lett. 2019, 46, 10072–10081. [Google Scholar] [CrossRef]
- Dela Cruz, D.W.; Rinkevich, B.; Gomez, E.D.; Yap, H.T. Assessing an abridged nursery phase for slow growing corals used in coral restoration. Ecol. Eng. 2015, 84, 408–415. [Google Scholar] [CrossRef]
- Page, C.P.; Muller, E.M.; Vaughan, D.E. Microfragmenting for the successful restoration of slow growing massive corals. Ecol. Eng. 2018, 123, 86–94. [Google Scholar] [CrossRef]
- Rinkevich, B. Restoration strategies for coral reefs damaged by recreational activities: The use of sexual and asexual recruits. Restor. Ecol. 1995, 3, 241–251. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. Steps in the construction of underwater coral nursery, an essential component in reef restoration acts. Mar. Biol. 2006, 149, 679–687. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.; Mercado-Molina, A.; Suleimán-Ramos, S. Multi-disciplinary lessons learned from low-tech coral farming and reef rehabilitation practices. I. Best management practices. In Corals in a Changing World; Duque-Beltrán, C., Tello-Camacho, E., Eds.; InTech Publ.: London, UK, 2018; pp. 213–243. [Google Scholar] [CrossRef]
- Forrester, G.E.; Maynard, A.; Schofield, S.; Taylor, K. Evaluating causes of transplant stress in fragments of Acropora palmata used for coral reef restoration. Bull. Mar. Sci. 2012, 88, 1099–1113. [Google Scholar] [CrossRef]
- Cabaitan, P.C.; Yap, H.T.; Gomez, E.D. Performance of single versus mixed coral species for transplantation to restore degraded reefs. Restor. Ecol. 2015, 23, 349–356. [Google Scholar] [CrossRef]
- Mbije, N.E.; Spanier, E.; Rinkevich, B. Testing the first phase of the gardening concept as an applicable tool in restoring denuded reefs in Tanzania. Ecol. Eng. 2010, 36, 713–721. [Google Scholar] [CrossRef]
- Gomez, E.D.; Yap, H.T.; Cabaitan, P.C.; Dizon, R.M. Successful transplantation of a fragmenting coral, Montipora digitata for reef rehabilitation. Coast. Manag. 2011, 39, 556–574. [Google Scholar] [CrossRef]
- Boch, C.A.; Morse, A.N.C. Testing the effectiveness of direct propagation techniques for coral restoration of Acropora spp. Ecol. Eng. 2012, 40, 11–17. [Google Scholar] [CrossRef]
- Hernández-Delgado, E.A.; Mercado-Molina, A.E.; Alejandro-Camis, P.J.; Candelas-Sánchez, F.; Fonseca-Miranda, J.S.; González-Ramos, C.M.; Guzmán-Rodríguez, R.; Mège, P.; Montañez-Acuña, A.A.; Olivo-Maldonado, I.; et al. Community-based coral reef rehabilitation in a changing climate: Lessons learned from hurricanes, extreme rainfall, and changing land use impacts. Open J. Ecol. 2014, 4, 918–944. [Google Scholar] [CrossRef]
- Wellington, G.M.; Glynn, P.W. Environmental influences on skeletal banding in eastern Pacific (Panamá) corals. Coral Reefs 1983, 1, 215–222. [Google Scholar] [CrossRef]
- Manzello, D.P. Coral growth with thermal stress and ocean acidification: Lessons from the eastern tropical Pacific. Coral Reefs 2010, 29, 749–758. [Google Scholar] [CrossRef]
- Highsmith, R.C. Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. 1982, 7, 207–226. [Google Scholar] [CrossRef]
- Lirman, D.; Thyberg, T.; Herlan, J.; Hill, C.; Young-Lahiff, C.; Schopmeyer, S.; Huntington, B.; Santos, R.; Drury, C. Propagation of the threatened staghorn coral Acropora cervicornis: Methods to minimize the impacts of fragment collection and maximize production. Coral Reefs 2010, 29, 729–735. [Google Scholar] [CrossRef]
- Denis, V.; Debreuil, J.; De Palmas, S.; Richardm, J.; Guillaume, M.M.M.; Bruggemann, J.H. Lesion regeneration capacities in populations of the massive coral Porites lutea at R’eunion Island: Environmental correlates. Mar. Ecol. Prog. Ser. 2011, 428, 105–117. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rjin, J.; Rinkevich, B. The use of coral nubbins in coral reef ecotoxicology testing. Biomol. Eng. 2003, 20, 401–406. [Google Scholar] [CrossRef]
- Shafir, S.; Van Rijn, J.; Rinkevich, B. Coral nubbins as a source material for coral biological research: A prospectus. Aquaculture 2006, 259, 444–448. [Google Scholar] [CrossRef]
- Guzmán, H.M.; Cortés, J. Growth rates of eight species of scleractinian corals in the eastern Pacific (Costa Rica). Bull. Mar. Sci. 1989, 44, 1186–1194. [Google Scholar]
- Jiménez, C.; Cortés, J. Growth of seven species of scleractinian corals in an upwelling environment of the eastern Pacific (Golfo de Papagayo, Costa Rica). Bull. Mar. Sci. 2003, 72, 187–198. [Google Scholar]
- Medellín-Maldonado, F.; Cabral-Tena, R.F.; López-Pérez, A.; Calderón-Aguilera, L.E.; Norzagaray-López, C.O.; Chapa-Balcorta, C.; Zepeda-Vilchis, R.C. Calcification of the main reef-building coral species on the Pacific coast of southern Mexico. Cien. Mar. 2016, 42, 209–225. [Google Scholar] [CrossRef]
- Reyes-Bonilla, H.; Calderón-Aguilar, L.E. Growth and mortality rates of the reef coral Pavona gigantea in Cabo Pulmo reef, Gulf of California. Bull. Mar. Sci. 2019, 95, 105–112. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Merino, M. Growth responses of the reefbuilding coral Montastraea annularis along a gradient of continental influence in the southern Gulf of Mexico. Bull. Mar. Sci. 2001, 68, 133–146. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).