Comparison of Ammonia Emission Estimation between Passive Sampler and Chamber System in Paddy Soil after Fertilizer Application
Abstract
:1. Introduction
2. Materials and Methods
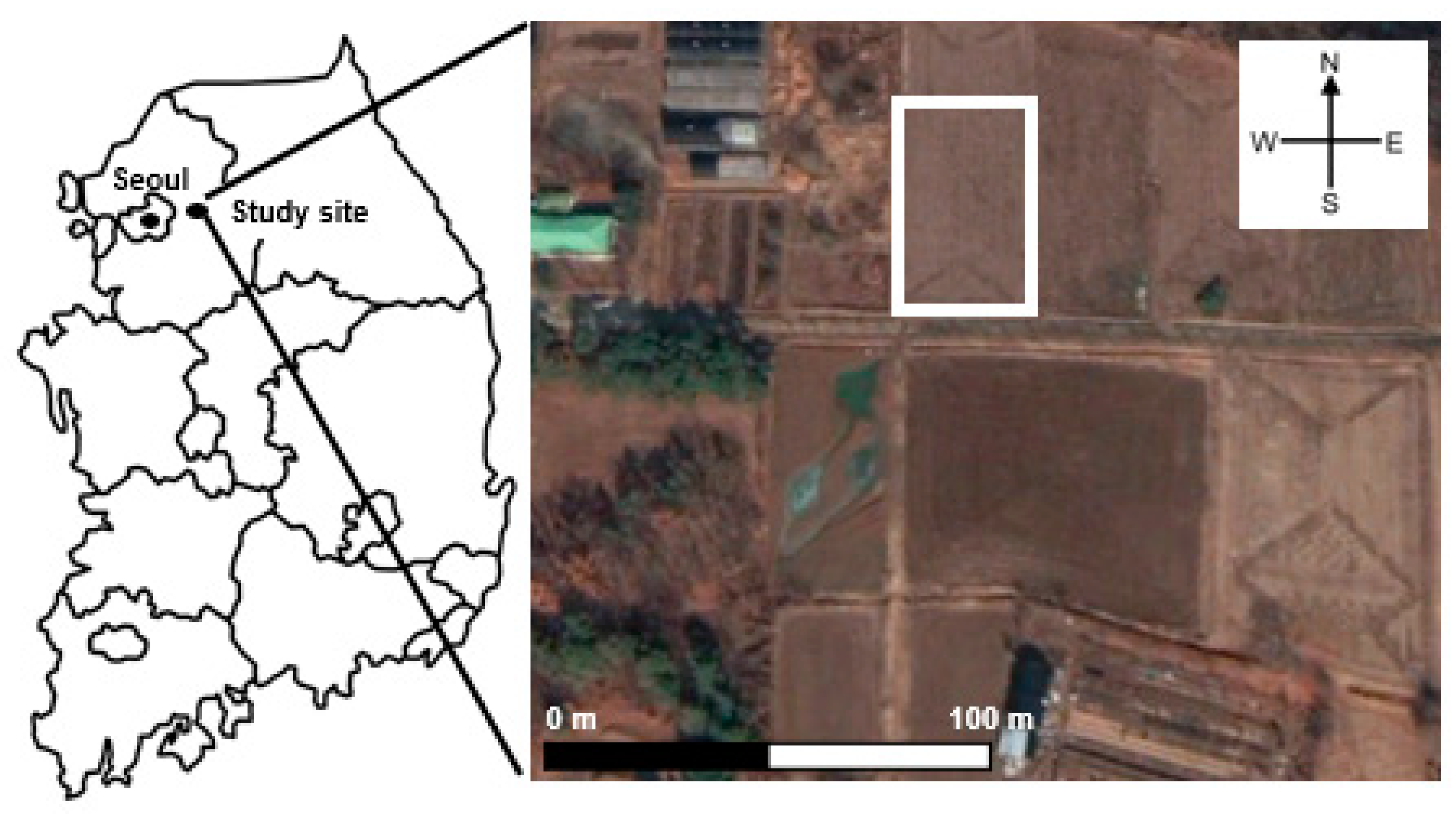
2.1. Site Description and Fertilizer Application
2.2. On-Site Passive Samper Experiement
2.3. Chamber Experiement in Laboratory
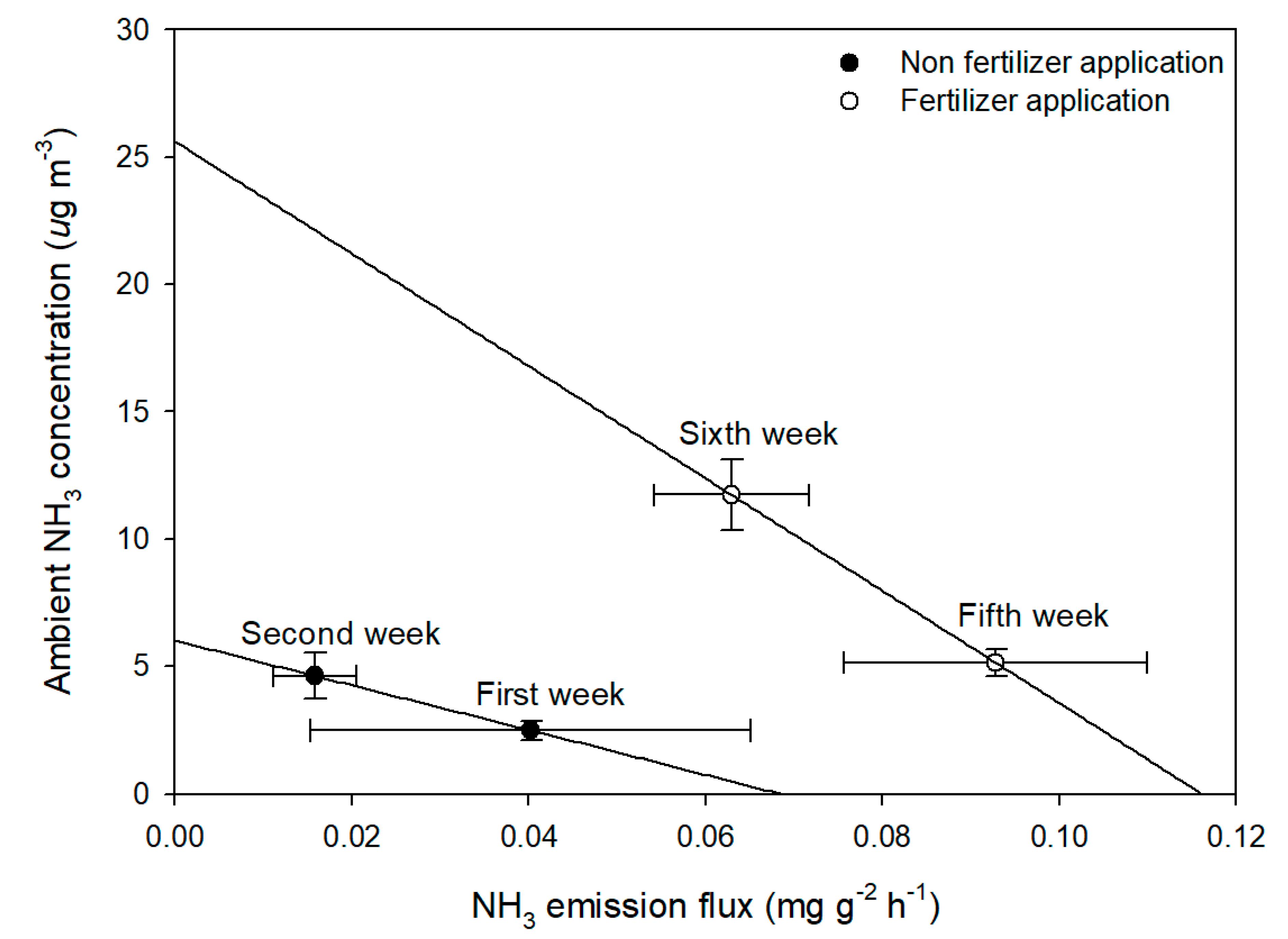
3. Results and Discussion
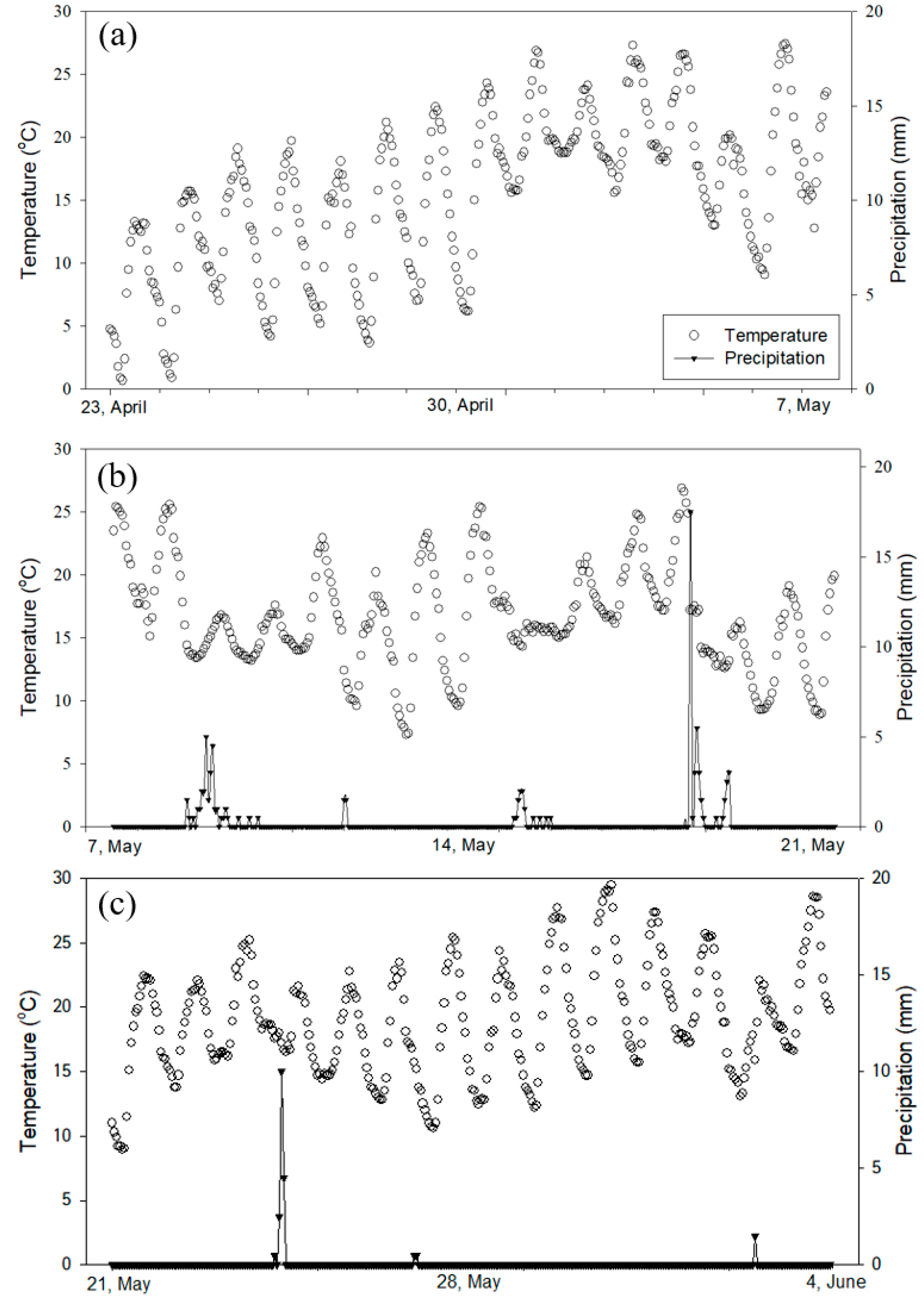
3.1. Site Weather Information during the Experiment
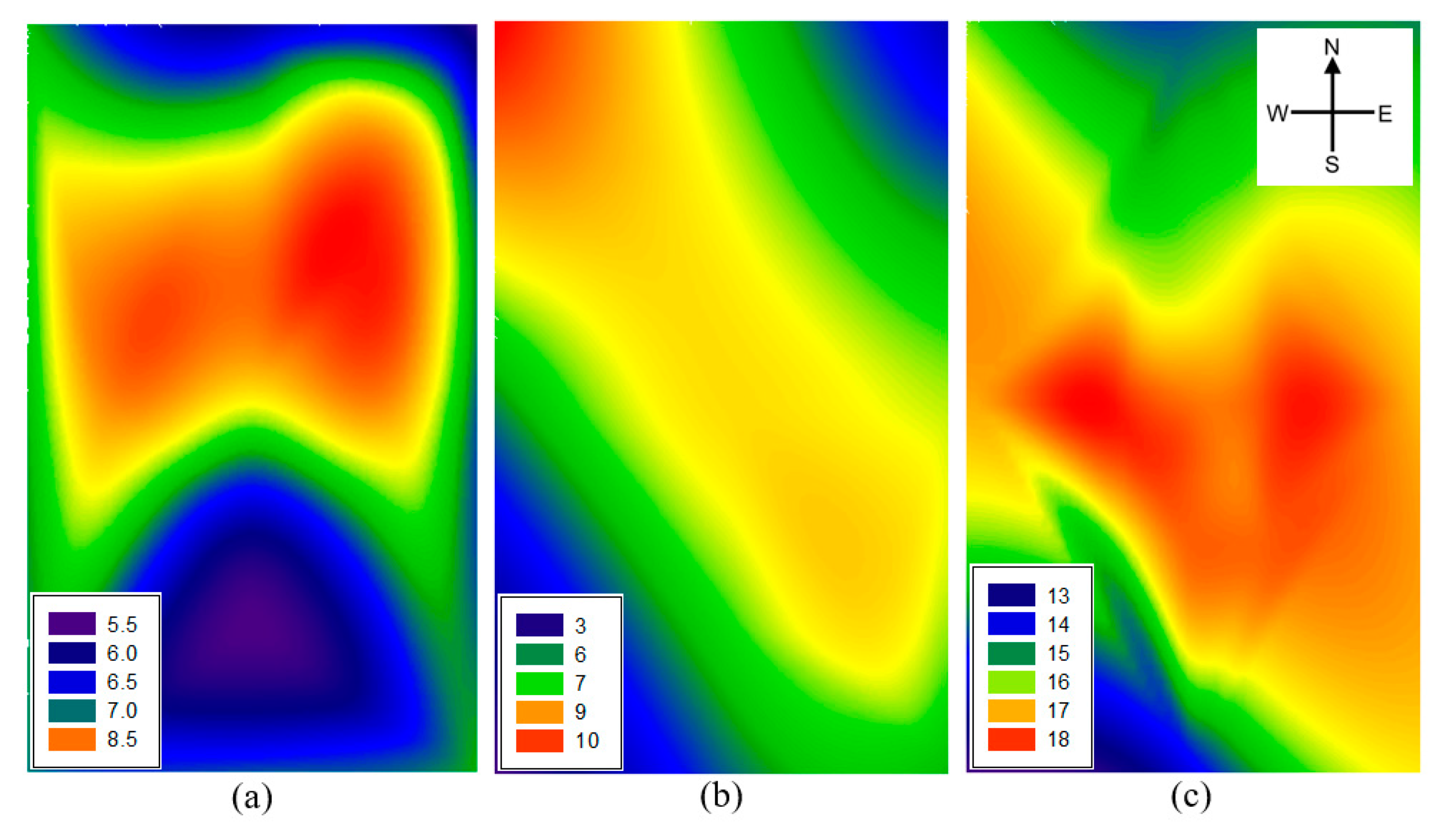
3.2. Ambient NH3 Concentration in the Study Site
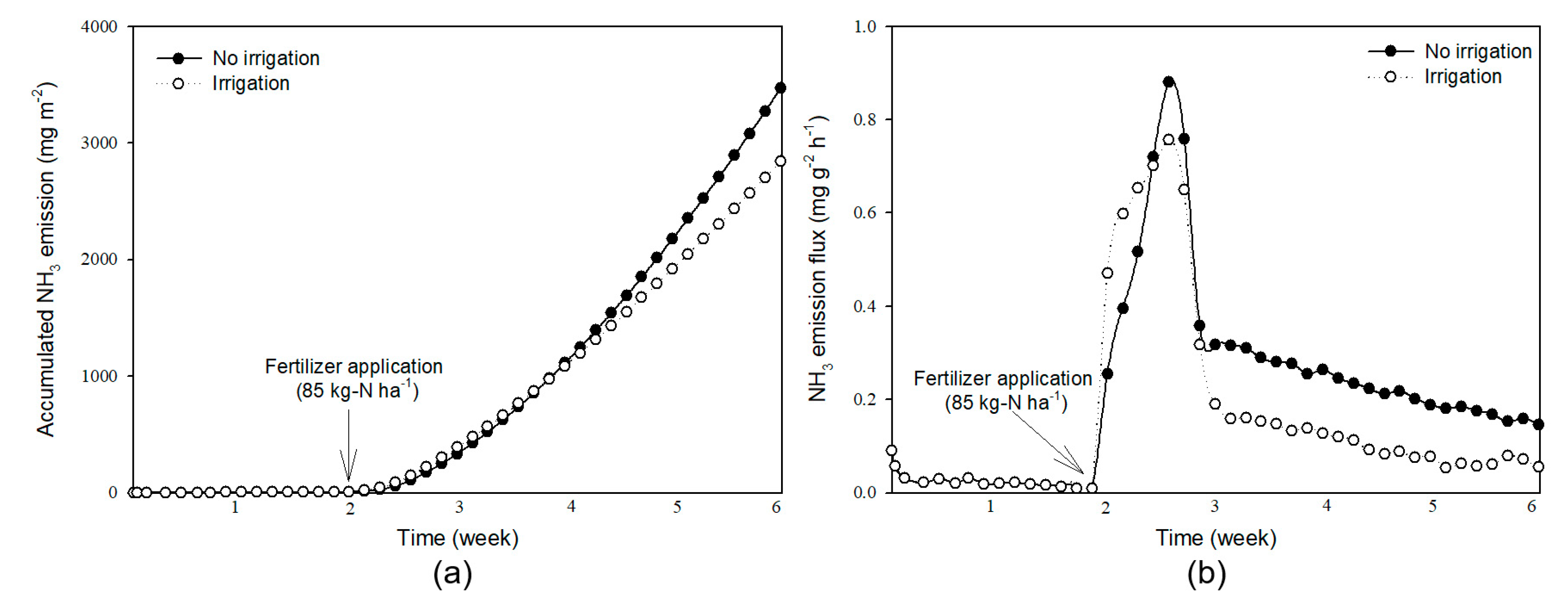
3.3. Long-Term Monitoring of NH3 Emission using the Chamber System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mukherjee, A.; Agrawal, M. World air particulate matter: Sources, distribution and health effects. Environ. Chem. Lett. 2017, 15, 283–309. [Google Scholar] [CrossRef]
- World Health Organization. 7 Million Premature Deaths Annually Linked to Air Pollution. 2014. Available online: http://www.who.int/mediacentre/news/releases/2014/air-pollution/en (accessed on 31 May 2020).
- Organization for Economic Cooperation and Development. Exposure to PM2.5 in Countries and Regions. 2014. Available online: http://stats.oecd.org/index.aspx?queryid=72722 (accessed on 31 May 2020).
- Shin, D.W.; Joo, H.S.; Seo, E.; Kim, C.Y. Management Strategies to Reduce PM-2.5 Emission: Emphasis–Ammonia; Korea Environment Institute: Sejong City, Korea, 2017. [Google Scholar]
- Zhang, R.; Khalizov, A.; Wang, L.; Hu, M.; Xu, W. Nucleation and growth of nanoparticles in the atmosphere. Chem. Rev. 2012, 112, 1957–2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef]
- Huan, L.; Kebin, H. Traffic optimization: A new way for air pollution control in China’s urban areas. Environ. Sci. Technol. 2012, 46, 5660–5661. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Ogink, N.W.M.; Aarnink, A.J.A.; Koerkamp, P.W.G. Livestock farms ad major emissions source of atmospheric particles in the Netherlands: Lessons learned and future perspectives. In Proceedings of the International Symposium on Animal Environment and Welfare, Chongqing, China, 23–25 October 2017; pp. 23–31. [Google Scholar]
- Kim, M.S.; Koo, N.; Kim, J.G. A comparative study on ammonia emission inventory in livestock manure compost application through a foreign case study. Korean J. Environ. Biol. 2020, 38, 71–81. [Google Scholar] [CrossRef]
- National Institute of Environmental Research. 2016 National Air Pollutants Emissions; National Institute of Environmental Research: Incheon, Korea, 2019. [Google Scholar]
- Thöni, L.; Seitler, E.; Blatter, A.; Neftel, A. A passive sampling method to determine ammonia in ambient air. J. Environ. Monit. 2003, 5, 96–99. [Google Scholar] [CrossRef]
- Butler, T.; Vermeylen, F.; Lehmann, C.M.; Likens, G.E.; Puchalski, M. Increasing ammonia concentration trends in large regions of the USA derived from the NADP/AMoN network. Atmos. Environ. 2016, 146, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Zbieranowski, A.L.; Aherne, J. Spatial and temporal concentration of ambient atmosphere ammonia in southern Ontario, Canada. Atmos. Environ. 2012, 62, 441–450. [Google Scholar] [CrossRef]
- Rabaud, N.E.; James, T.A.; Ashbaugh, L.L.; Flocchini, R.G. A passive sampler for the determination of airborne ammonia concentrations near large-scale animal facilities. Environ. Sci. Technol. 2001, 35, 1190–1196. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wen, Z.; Wang, D.; Hao, T.; Tang, A.; Liu, X. Atmospheric deposition of inorganic nitrogen in a semi-arid grassland of inner Mongnolia, China. J. Arid Land 2017, 9, 810–822. [Google Scholar] [CrossRef] [Green Version]
- Hojito, M.; Hayashi, K.; Matsuura, S.; Kohyama, K. Two-year monitoring of the vertical distrivution of ammonia in the atmosphere above an intensive dairy farming area in central Japan. J. Jpn. Soc. Atmos. Environ. 2010, 45, 166–170. [Google Scholar]
- Hayashi, K.; Koga, N.; Yanai, Y. Effects of field applied composted cattle manure and chemical fertilizer on ammonia and particulate ammonium exchanges at an upland field. Atmos. Environ. 2009, 43, 5702–5707. [Google Scholar] [CrossRef]
- Hwang, W.; Park, M.; Cho, K.; Kim, J.G.; Hyun, S.H. Mitigation of CO2 and N2O emission from cabbage field in Korea by optimizing tillage depth and N-fertilizer level: DNDC model simulation under RCP 8.5 scenario. Sustainability 2019, 11, 6158. [Google Scholar] [CrossRef] [Green Version]
- NIAST. Method of Soil and Plant Analysis; National Institute of Agricultural Science and Technology, Rural Development Administration: Suwon, Korea, 2000. [Google Scholar]
- Currie, L. Nomenclature in evaluation of analytical methods including detection and quantification capabilities: (IUPAC Recommendations 1995). Anal. Chim. Acta. 1999, 391, 105–126. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, Y.S.; Min, H.G.; Kim, J.G. Applicability of the dynamic chamber-capture system (DCS) for estimating the flux of ammonia emission during liquid fertilizer spreading. Atmo. Pollut. Res. 2020, 11, 723–729. [Google Scholar] [CrossRef]
- Schjoerring, J.K.; Sommer, S.G.; Ferm, M. A simple passive sampler for measuring ammonia emission in the field. Water Air Soil Pollut. 1992, 62, 13–24. [Google Scholar] [CrossRef]
- McGinn, S.M.; Sommer, S.G. Ammonia emissions from land-applied beef cattle manure. Can. J. Soil Sci. 2007, 87, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhou, C.; Li, N.; Han, K.; Meng, Y.; Tian, X.; Wang, L. Effects of conservation tillage practices on ammonia emissions from loess plateau rain-fed winter wheat field. Atmos. Environ. 2015, 104, 59–68. [Google Scholar] [CrossRef]
- Hayashi, K.; Koga, N.; Fueki, N. Limited ammonia volatilization loss from upland fields of andosols following fertilizer application. Agric. Ecosys. Environ. 2011, 140, 534–538. [Google Scholar] [CrossRef]
- Craswell, E.T.; Vlek, P.L.G. Fate of fertilizer nitrogen applied to wetland rice. In Gaseous Loss of Nitrogen from Plant-Soil System; Freney, J.R., Simpson, J.R., Eds.; Springer: Dordrecht, The Netherlands, 1983; Volume 9, pp. 237–264. [Google Scholar]
- Su, C.; Yin, B.; Zhu, Z.; Shen, Q. Ammonia volatilization loss of nitrogen fertilizer from rice field and wet deposition of atmospheric nitrogen in rice growing season. J. Appl. Ecol. 2003, 14, 1884–1888. [Google Scholar]
- Randy, J.D.; Nathan, A.S.; Richard, J.N.; Trenton, L.R. Ammonia volatilization, rice yield, and nitrogen uptake responses to simulated rainfall and urease inhibitor. Agron. J. 2017, 109, 363–377. [Google Scholar]
- Hayashi, K.; Nishimura, S.; Yagi, K. Ammonia volatilization from the surface a Japanese paddy field during rice cultivation. Soil Sci. Plant Nut. 2006, 52, 545–555. [Google Scholar] [CrossRef]
- Dewes, T. Ammonia emission during the initial phase of microbial degradation of solid and liquid cattle manure. Bioresour. Technol. 1999, 70, 245–248. [Google Scholar] [CrossRef]
- Siman, F.C.; Andrade, F.V.; Passos, R.R. Nitrogen fertilizers and NH3 volatilization: Effect of temperature and soil moisture. Comm. Soil Sci. Plant Anal. 2020, in press. [Google Scholar] [CrossRef]
- Hayashi, K.; Nishimura, S.; Yagi, K. Ammonia volatilization from a paddy field following applications of urea: Rice plants are both an absorber and an emitter for atmospheric ammonia. Sci. Total Environ. 2008, 390, 485–494. [Google Scholar] [CrossRef]
- Fenn, L.B.; Hossner, L.R. Ammonia volatilization from ammonium or ammonium-forming nitrogen fertilizers. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1985; Volume 1, pp. 123–169. [Google Scholar]
- Gao, H.; Bai, J.; Deng, X.; Lu, Q.; Ye, X. Short-term effects of tidal flooding on soil nitrogen mineralization in Chinese tidal salt marsh. Phys. Chem. Erath. 2018, 103, 3–10. [Google Scholar] [CrossRef]
- DeLaune, P.B.; Moore, P.A.; Daniel, T.C., Jr.; Lemunyon, J.L. Effect of chemical and microbial amendments on ammonia volatilization composting poultry litter. J. Environ. Qual. 2004, 33, 728–734. [Google Scholar] [CrossRef] [Green Version]
- Chastain, J.P. Ammonia volatilization losses during irrigation of liquid animal manure. Sustainability 2019, 11, 6168. [Google Scholar] [CrossRef] [Green Version]
- Sommer, S.G.; Génermont, S.; Cellier, P.; Hutchings, N.J.; Olesen, J.E.; Morvan, T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Bouwmeester, R.J.B.; Vlek, P.L.G.; Stumpe, J.M. Effect of environmental factors on ammonia volatilization from a urea-fertilizer soil. Soil Sci. Soc. Am. J. 1985, 49, 376–381. [Google Scholar] [CrossRef]
- Sommer, S.G.; Schjoerring, J.K.; Denmead, O.T. Ammonia emission from mineral fertilizers and fertilized crops. Adv. Agron. 2004, 82, 558–622. [Google Scholar]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Koo, N.; Hyun, S.; Kim, J.-G. Comparison of Ammonia Emission Estimation between Passive Sampler and Chamber System in Paddy Soil after Fertilizer Application. Int. J. Environ. Res. Public Health 2020, 17, 6387. https://doi.org/10.3390/ijerph17176387
Kim M-S, Koo N, Hyun S, Kim J-G. Comparison of Ammonia Emission Estimation between Passive Sampler and Chamber System in Paddy Soil after Fertilizer Application. International Journal of Environmental Research and Public Health. 2020; 17(17):6387. https://doi.org/10.3390/ijerph17176387
Chicago/Turabian StyleKim, Min-Suk, Namin Koo, Seunghun Hyun, and Jeong-Gyu Kim. 2020. "Comparison of Ammonia Emission Estimation between Passive Sampler and Chamber System in Paddy Soil after Fertilizer Application" International Journal of Environmental Research and Public Health 17, no. 17: 6387. https://doi.org/10.3390/ijerph17176387
APA StyleKim, M.-S., Koo, N., Hyun, S., & Kim, J.-G. (2020). Comparison of Ammonia Emission Estimation between Passive Sampler and Chamber System in Paddy Soil after Fertilizer Application. International Journal of Environmental Research and Public Health, 17(17), 6387. https://doi.org/10.3390/ijerph17176387




