Revealing Cryptic Changes of Cyanobacterial Community Structure in Two Eutrophic Lakes Using eDNA Sequencing
Abstract
:1. Introduction
2. Materials and Methods
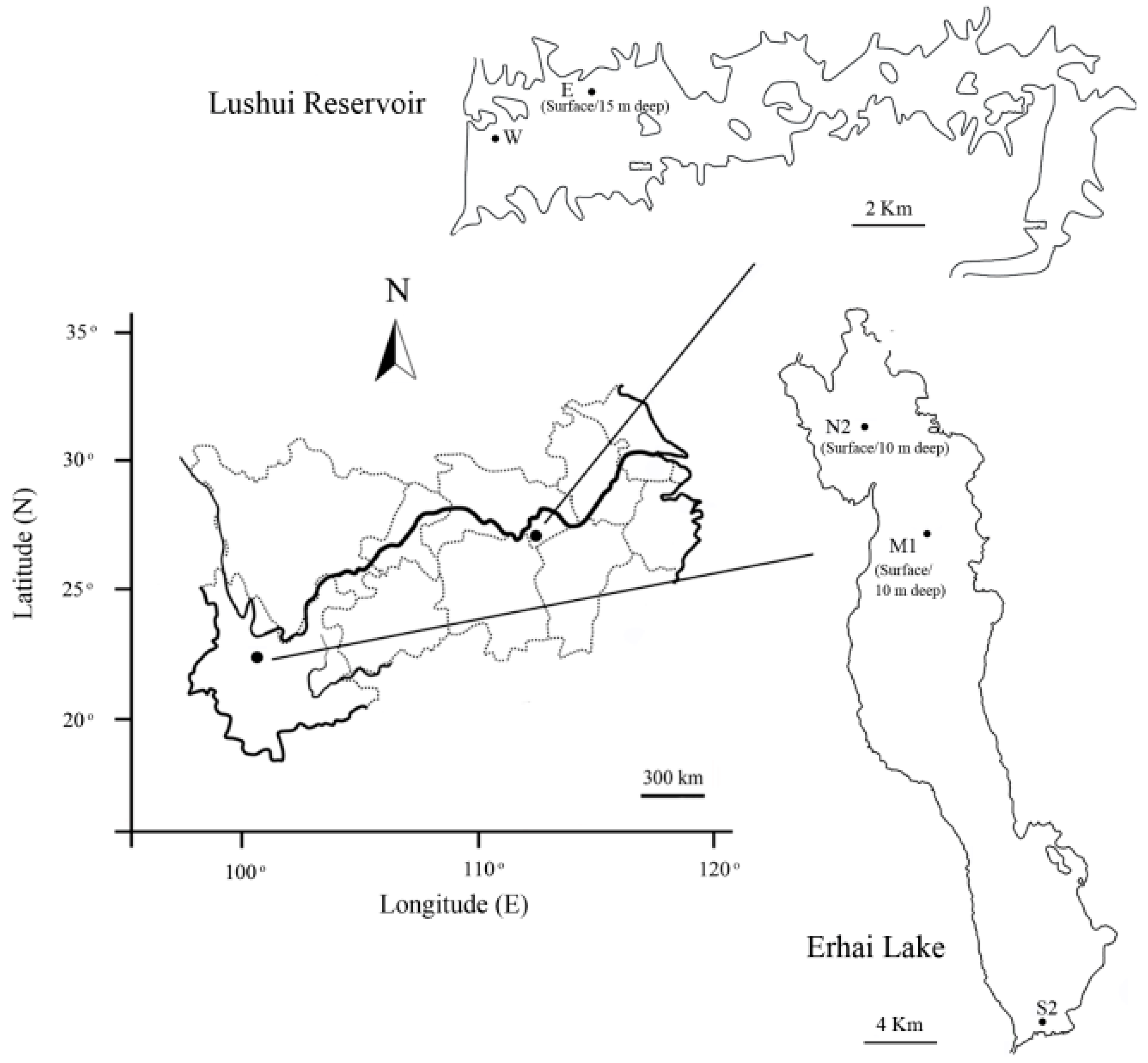
2.1. Sampling Sites and Collection of Cyanobacteria
2.2. High-Throughput Sequencing of Cyanobacterial cpcBA-IGS
2.3. Sequence Clustering
2.4. Taxonomy Assignment of OTUs
2.5. Neutral Model for Cyanobacterial Community
2.6. Statistical Analysis
3. Results
3.1. Sequence Data of cpcBA-IGS
3.2. OTU Composition and Diversity
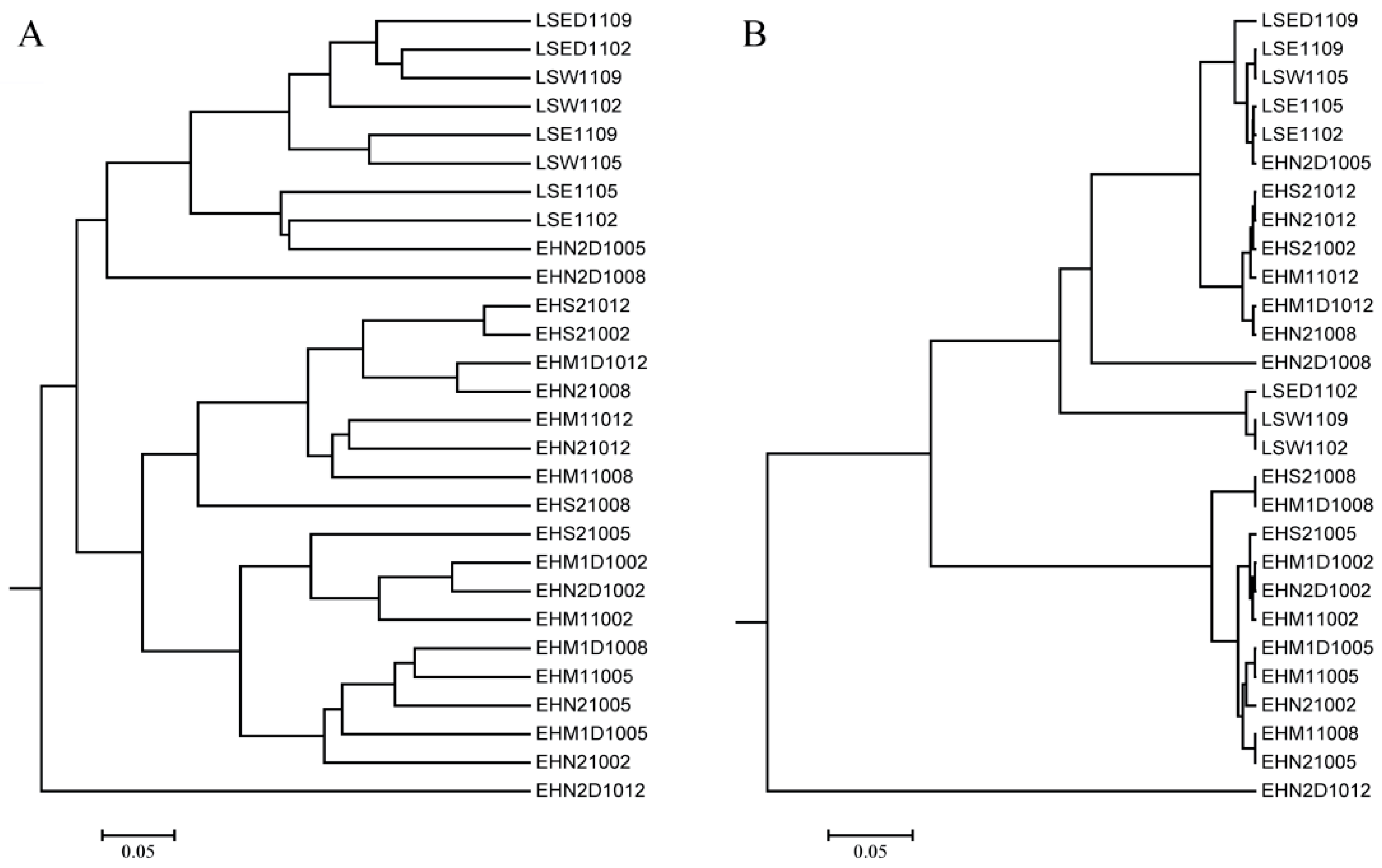
3.3. Cyanobacterial Community Structure
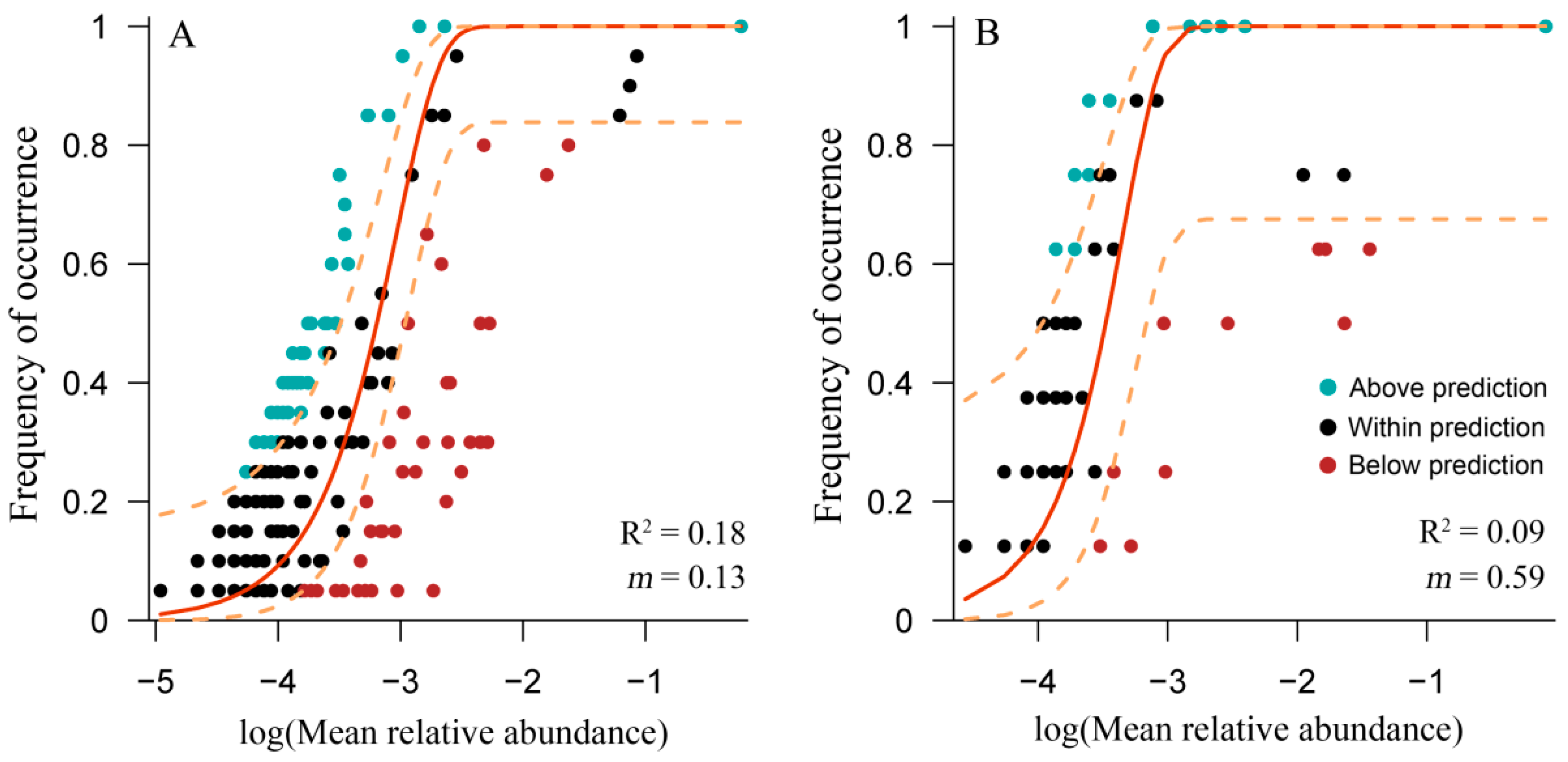
3.4. Application of the Neutral Model for Cyanobacterial Community
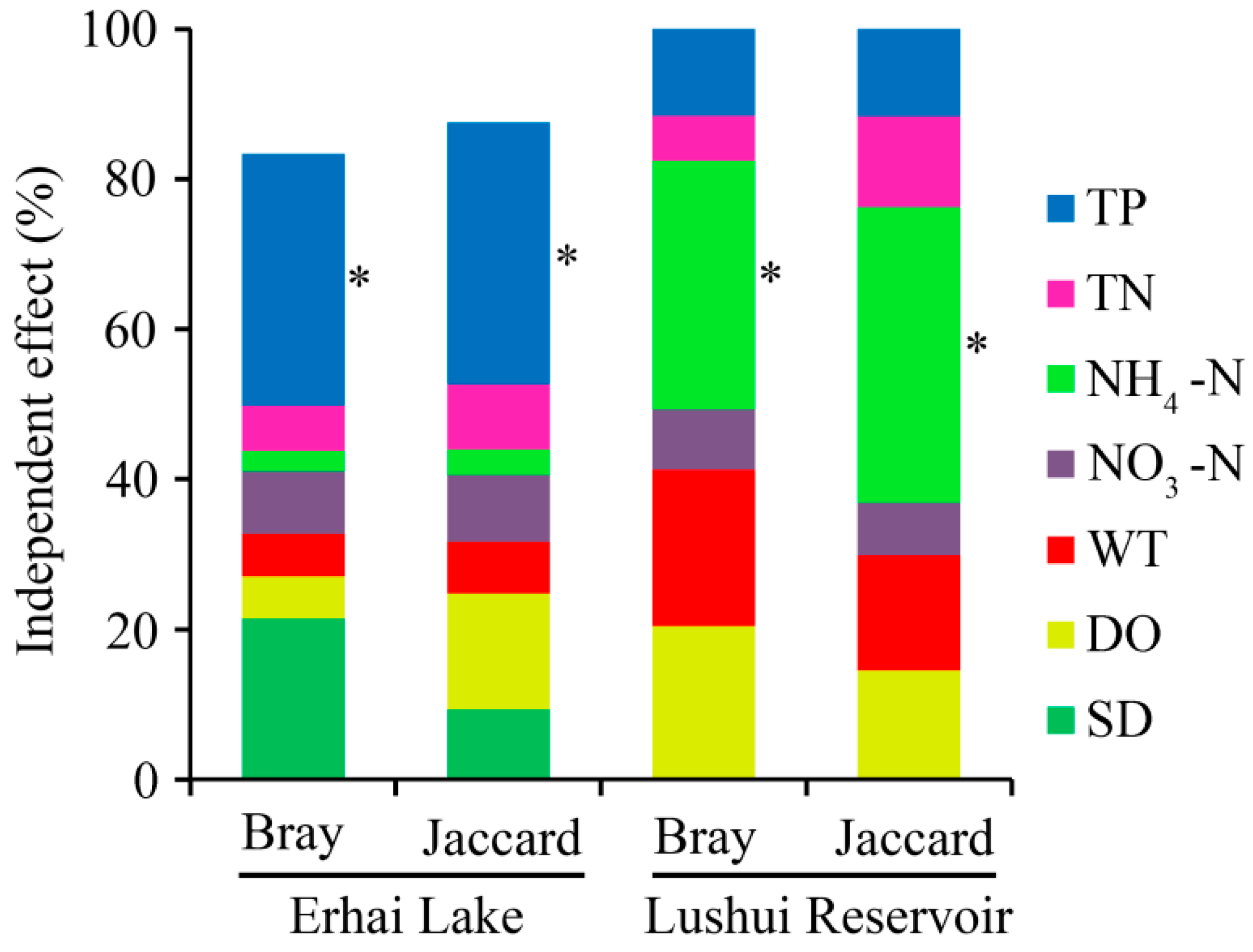
3.5. Correlations Between Environmental Factors and Cyanobacterial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Chen, C.; Ding, J.-Q.; Hou, A.; Li, Y.; Niu, Z.-B.; Su, X.-Y.; Xu, Y.-J.; Laws, E.A. The 2007 water crisis in Wuxi, China: Analysis of the origin. J. Hazard. Mater. 2010, 182, 130–135. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Niu, Y.; Xie, P.; Chen, J.; Tao, M.; Deng, X. Off-flavor compounds from decaying cyanobacterial blooms of Lake Taihu. J. Environ. Sci. 2013, 25, 495–501. [Google Scholar] [CrossRef]
- Sevrin-Reyssac, J.; Pletikosic, M. Cyanobacteria in fish ponds. Aquaculture 1990, 88, 1–20. [Google Scholar] [CrossRef]
- Dittmann, E.; Wiegand, C. Cyanobacterial toxins—Occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 2006, 50, 7–17. [Google Scholar] [CrossRef]
- Wormer, L.; Cirés, S.; Carrasco, D.; Quesada, A. Cylindrospermopsin is not degraded by co-occurring natural bacterial communities during a 40-day study. Harmful Algae 2008, 7, 206–213. [Google Scholar] [CrossRef]
- Song, G.; Jiang, Y.; Yu, G.; Li, R. Genotypes of ITS region of rRNA in Microcystis (Cyanobacteria) populations in Erhai Lake (China) and their correlation with eutrophication level. J. Basic Microbiol. 2015, 55, 1203–1211. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, Y.; Wang, Z.; Xiao, P.; Yu, G.; Wang, G.; Li, R. Dominance and succession of Microcystis genotypes and morphotypes in Lake Taihu, a large and shallow freshwater lake in China. Environ. Pollut. 2016, 219, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Y.; Xu, Y.; Xiao, P.; Li, R. The divergence of cpcBA-IGS sequences between Dolichospermum and Aphanizomenon (Cyanobacteria) and the molecular detection of Dolichospermum flos-aquaein Taihu Lake, China. Phycologia 2013, 52, 447–454. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H. Mitigation of lake eutrophication: Loosen nitrogen control and focus on phosphorus abatement. Prog. Nat. Sci. 2009, 19, 1445–1451. [Google Scholar] [CrossRef]
- Cai, Y.; Kong, F. Diversity and dynamics of picocyanobacteria and the bloom-forming cyanobacteria in a large shallow eutrophic lake (lake Chaohu, China). J. Limnol. 2013, 72, 38. [Google Scholar] [CrossRef] [Green Version]
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhães, V.; Rangel, L.M.; Azevedo, S.M.F.D.O.E.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Toporowska, M.; Mazur-Marzec, H.; Pawlik-Skowrońska, B. The effects of cyanobacterial bloom extracts on the biomass, Chl-a, MC and other oligopeptides contents in a natural Planktothrix agardhii population. Int. J. Environ. Res. Public Health 2020, 17, 2881. [Google Scholar] [CrossRef]
- Wejnerowski, Ł.; Rzymski, P.; Kokociński, M.; Meriluoto, J. The structure and toxicity of winter cyanobacterial bloom in a eutrophic lake of the temperate zone. Ecotoxicology 2018, 27, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Kokociński, M.; Gągała, I.; Jasser, I.; Karosienė, J.; Kasperovičienė, J.; Kobos, J.; Koreivienė, J.; Soininen, J.; Szczurowska, A.; Woszczyk, M.; et al. Distribution of invasive Cylindrospermopsis raciborskii in the East-Central Europe is driven by climatic and local environmental variables. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, S.; Aubriot, L.; Soares, M.C.S.; González-Piana, M.; Fabre, A.; Huszar, V.L.; Lürling, M.; Antoniades, D.; Padisák, J.; Kruk, C. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol. Ecol. 2012, 79, 594–607. [Google Scholar] [CrossRef]
- Chia, M.A.; Jankowiak, J.G.; Kramer, B.J.; Goleski, J.A.; Huang, I.-S.; Zimba, P.; Bittencourt-Oliveira, M.D.C.; Gobler, C.J. Succession and toxicity of Microcystis and Anabaena (Dolichospermum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful Algae 2018, 74, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Shan, K.; Song, L.; Chen, W.; Li, L.; Liu, L.; Wu, Y.; Jia, Y.; Zhou, Q.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Kahru, M.; Elmgren, R.; Kaiser, J.; Wasmund, N.; Savchuk, O. Cyanobacterial blooms in the Baltic Sea: Correlations with environmental factors. Harmful Algae 2020, 92, 101739. [Google Scholar] [CrossRef] [PubMed]
- Briand, E.; Escoffier, N.; Straub, C.; Sabart, M.; Quiblier, C.; Humbert, J. Spatiotemporal changes in the genetic diversity of a bloom-forming Microcystis aeruginosa (cyanobacteria) population. ISME J. 2009, 3, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, Y.; Xiao, P.; Pan, Q.; Yu, G.; Li, R. Genetic analysis on Dolichospermum (Cyanobacteria; sensu Anabaena) populations based on the culture-independent clone libraries revealed the dominant genotypes existing in Lake Taihu, China. Harmful Algae 2014, 31, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Xiao, P.; Liu, Y.; Wang, J.; Li, R. Targeted deep sequencing reveals high diversity and variable dominance of bloom-forming cyanobacteria in eutrophic lakes. Harmful Algae 2017, 64, 42–50. [Google Scholar] [CrossRef]
- Li, H.; Alsanea, A.; Barber, M.; Goel, R. High-throughput DNA sequencing reveals the dominance of pico- and other filamentous cyanobacteria in an urban freshwater Lake. Sci. Total. Environ. 2019, 661, 465–480. [Google Scholar] [CrossRef]
- Batista, A.M.M.; Figueredo, C.C.; Giani, A. Variability in a permanent cyanobacterial bloom: Species-specific responses to environmental drivers. FEMS Microbiol. Ecol. 2018, 94, fiy197. [Google Scholar] [CrossRef]
- Willis, A.; Chuang, A.W.; Orr, P.T.; Beardall, J.; Burford, M.A. Subtropical freshwater phytoplankton show a greater response to increased temperature than to increased pCO2. Harmful Algae 2019, 90, 101705. [Google Scholar] [CrossRef]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Jiang, Y.; Song, G.; Tan, W.; Zhu, M.; Li, R. Variation of Microcystis and microcystins coupling nitrogen and phosphorus nutrients in Lake Erhai, a drinking-water source in Southwest Plateau, China. Environ. Sci. Pollut. Res. 2014, 21, 9887–9898. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.K.; Xie, P.; Liang, G.D.; Wang, S.B.; Liang, X.M. Relationships between microcystins and environmental parameters in 30 subtropical shallow lakes along the Yangtze River, China. Freshw. Biol. 2006, 51, 2309–2319. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant. Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Neilan, B.A.; Jacobs, D.; Goodman, A.E. Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 1995, 61, 3875–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Boil. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Sloan, W.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J.R. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, R.K.; Luoto, M.; Virkkala, R.; Rainio, K. Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural-forest mosaic. J. Appl. Ecol. 2004, 41, 824–835. [Google Scholar] [CrossRef]
- Mantel, N.; Valand, R.S. A technique of nonparametric multivariate analysis. Biometrics 1970, 26, 547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qin, B.; Gao, G.; Paerl, H.W. Nutrient enrichment and selective predation by zooplankton promote Microcystis (Cyanobacteria) bloom formation. J. Plankton Res. 2010, 32, 457–470. [Google Scholar] [CrossRef]
- Epp, G.T. Grazing on filamentous cyanobacteria by Daphnia pulicaria. Limnol. Oceanogr. 1996, 41, 560–567. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Gan, N.; Zheng, L.; Li, T.; Song, L. Growth inhibitory effect of Microcystis on Aphanizomenon flos-aquae isolated from cyanobacteria bloom in Lake Dianchi, China. Harmful Algae 2015, 42, 43–51. [Google Scholar] [CrossRef]
- Millette, N.; Kelble, C.; Linhoss, A.; Ashby, S.; Visser, L. Shift in baseline chlorophyll a concentration following a three-year Synechococcus bloom in southeastern Florida. Bull. Mar. Sci. 2018, 94, 3–19. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nakahara, H.; Horiya, K. Relationship between the occurrence of Phormidium tenue bloom and water quality or weather factors in the south basin of Lake Biwa. Nippon Suisan Gakkaishi 1996, 62, 872–877. [Google Scholar] [CrossRef]
- Evans, S.; Saleh, M.T. Cyanobacteria diversity in blooms from the Greater Sudbury Area. J. Water Resour. Prot. 2015, 7, 871–882. [Google Scholar] [CrossRef] [Green Version]
- McAllister, T.G.; Wood, S.A.; Hawes, I. The rise of toxic benthic Phormidium proliferations: A review of their taxonomy, distribution, toxin content and factors regulating prevalence and increased severity. Harmful Algae 2016, 55, 282–294. [Google Scholar] [CrossRef]
- Teneva, I.; Dzhambazov, B.; KolevaValkova, L.; Mladenov, R.; Schirmer, K. Toxic potential of five freshwater Phormidium species (Cyanoprokaryota). Toxicon 2005, 45, 711–725. [Google Scholar] [CrossRef]
- Forsberg, C.; Ryding, S.O. Eutrophication parameters and trophic state indices in 30 swedish waste receiving lakes. Arch. Hydrobiol. 1980, 89, 189–207. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Hall, N.S.; Shi, K.; Zhou, Y.; Guo, J.-S.; Li, Z.; Xu, H.; et al. The persistence of cyanobacterial (Microcystis spp.) blooms throughout winter in Lake Taihu, China. Limnol. Oceanogr. 2016, 61, 711–722. [Google Scholar] [CrossRef] [Green Version]
- Ye, R.; Shan, K.; Gao, H.; Zhang, R.; Xiong, W.; Wang, Y.; Qian, X. Spatio-temporal distribution patterns in environmental factors, chlorophyll-a and microcystins in a large shallow lake, Lake Taihu, China. Int. J. Environ. Res. Public Health 2014, 11, 5155–5169. [Google Scholar] [CrossRef]
- Preston, T.; Stewart, W.D.P.; Reynolds, C.S. Bloom-forming cyanobacterium Microcystis aeruginosa overwinters on sediment surface. Nature 1980, 288, 365–367. [Google Scholar] [CrossRef]
- Verspagen, J.M.H.; Snelder, E.O.F.M.; Visser, P.M.; Huisman, J.; Mur, L.R.; Ibelings, B.W. Recruitment of benthic Microcystis (Cyanophyceae) to the water column: Internal buoyancy changes or resuspension? J. Phycol. 2004, 40, 260–270. [Google Scholar] [CrossRef]
- Latour, D.; Sabido, O.; Salençon, M.-J.; Giraudet, H. Dynamics and metabolic activity of the benthic cyanobacterium Microcystis aeruginosa in the Grangent reservoir (France). J. Plankton Res. 2004, 26, 719–726. [Google Scholar] [CrossRef]
- Ji, X.; Verspagen, J.M.H.; Van De Waal, D.B.; Rost, B.; Huisman, J. Phenotypic plasticity of carbon fixation stimulates cyanobacterial blooms at elevated CO2. Sci. Adv. 2020, 6, eaax2926. [Google Scholar] [CrossRef] [Green Version]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L.-A. Climate warming and heat waves alter harmful cyanobacterial blooms along the benthic-pelagic interface. Ecology 2020, e03025. [Google Scholar] [CrossRef] [Green Version]

| Sample | OTU Richness | Coverage | Chao 1 Index | H′ | D−1 | E |
|---|---|---|---|---|---|---|
| EHN21002 | 247 | 0.95 | 891 | 2.05 | 3.56 | 0.01 |
| EHN21005 | 254 | 0.94 | 1087 | 2.06 | 3.63 | 0.01 |
| EHN21008 | 188 | 0.96 | 737 | 1.06 | 1.57 | 0.01 |
| EHN21012 | 188 | 0.96 | 933 | 1.05 | 1.55 | 0.01 |
| EHN2D1002 | 252 | 0.94 | 1380 | 2.29 | 5.63 | 0.02 |
| EHN2D1005 | 179 | 0.96 | 1209 | 0.55 | 1.15 | 0.01 |
| EHN2D1008 | 258 | 0.94 | 1386 | 1.82 | 2.33 | 0.01 |
| EHN2D1012 | 260 | 0.94 | 948 | 3.00 | 8.90 | 0.03 |
| EHM11002 | 252 | 0.94 | 1060 | 2.21 | 4.28 | 0.02 |
| EHM11005 | 259 | 0.94 | 859 | 1.92 | 2.68 | 0.01 |
| EHM11008 | 210 | 0.95 | 1857 | 0.82 | 1.29 | 0.01 |
| EHM11012 | 162 | 0.96 | 1416 | 0.67 | 1.21 | 0.01 |
| EHM1D1002 | 224 | 0.95 | 924 | 1.95 | 3.13 | 0.01 |
| EHM1D1005 | 208 | 0.95 | 1023 | 1.89 | 3.56 | 0.02 |
| EHM1D1008 | 193 | 0.96 | 918 | 1.98 | 3.63 | 0.02 |
| EHM1D1012 | 143 | 0.97 | 514 | 0.94 | 1.44 | 0.01 |
| EHS21002 | 146 | 0.97 | 848 | 0.72 | 1.27 | 0.01 |
| EHS21005 | 186 | 0.96 | 963 | 1.79 | 3.20 | 0.02 |
| EHS21008 | 197 | 0.96 | 817 | 1.97 | 3.65 | 0.02 |
| EHS21012 | 154 | 0.97 | 929 | 0.74 | 1.30 | 0.01 |
| LSW1102 | 167 | 0.96 | 735 | 1.86 | 3.44 | 0.02 |
| LSW1105 | 118 | 0.97 | 910 | 0.40 | 1.11 | 0.01 |
| LSW1109 | 120 | 0.97 | 509 | 0.58 | 1.19 | 0.01 |
| LSE1102 | 158 | 0.97 | 651 | 0.70 | 1.29 | 0.01 |
| LSE1105 | 133 | 0.97 | 620 | 0.46 | 1.14 | 0.01 |
| LSE1109 | 142 | 0.97 | 531 | 0.55 | 1.16 | 0.01 |
| LSED1102 | 171 | 0.96 | 774 | 1.74 | 2.95 | 0.02 |
| LSED1109 | 131 | 0.97 | 688 | 0.48 | 1.13 | 0.01 |
| Erhai Lake | Lushui Reservoir | |||||||
|---|---|---|---|---|---|---|---|---|
| Bray–Curtis | Jaccard | Bray–Curtis | Jaccard | |||||
| r | p | r | p | r | p | r | p | |
| TP | 0.350 | 0.060 | 0.363 | 0.051 | −0.227 | 0.667 | −0.210 | 0.667 |
| TN | −0.016 | 0.513 | −0.016 | 0.510 | 0.154 | 0.336 | 0.172 | 0.336 |
| TN/TP | 0.495 | 0.009 | 0.505 | 0.007 | −0.358 | 0.935 | −0.354 | 0.942 |
| NH4-N | −0.256 | 0.892 | −0.264 | 0.903 | 0.201 | 0.233 | 0.231 | 0.233 |
| NO3-N | 0.142 | 0.195 | 0.138 | 0.206 | −0.012 | 0.374 | −0.003 | 0.371 |
| WT | 0.197 | 0.118 | 0.205 | 0.101 | 0.439 | 0.050 | 0.470 | 0.049 |
| DO | 0.287 | 0.100 | 0.28 | 0.096 | 0.559 | 0.061 | 0.590 | 0.060 |
| SD | −0.063 | 0.573 | −0.044 | 0.526 | NA | NA | NA | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Xiao, P.; Yu, G.; Song, G.; Li, R. Revealing Cryptic Changes of Cyanobacterial Community Structure in Two Eutrophic Lakes Using eDNA Sequencing. Int. J. Environ. Res. Public Health 2020, 17, 6356. https://doi.org/10.3390/ijerph17176356
Jiang Y, Xiao P, Yu G, Song G, Li R. Revealing Cryptic Changes of Cyanobacterial Community Structure in Two Eutrophic Lakes Using eDNA Sequencing. International Journal of Environmental Research and Public Health. 2020; 17(17):6356. https://doi.org/10.3390/ijerph17176356
Chicago/Turabian StyleJiang, Yongguang, Peng Xiao, Gongliang Yu, Gaofei Song, and Renhui Li. 2020. "Revealing Cryptic Changes of Cyanobacterial Community Structure in Two Eutrophic Lakes Using eDNA Sequencing" International Journal of Environmental Research and Public Health 17, no. 17: 6356. https://doi.org/10.3390/ijerph17176356




