Are There Seasonal Variations in Faecal Contamination of Exposure Pathways? An Assessment in a Low–Income Settlement in Uganda
Abstract
:1. Introduction
2. Materials and Methods
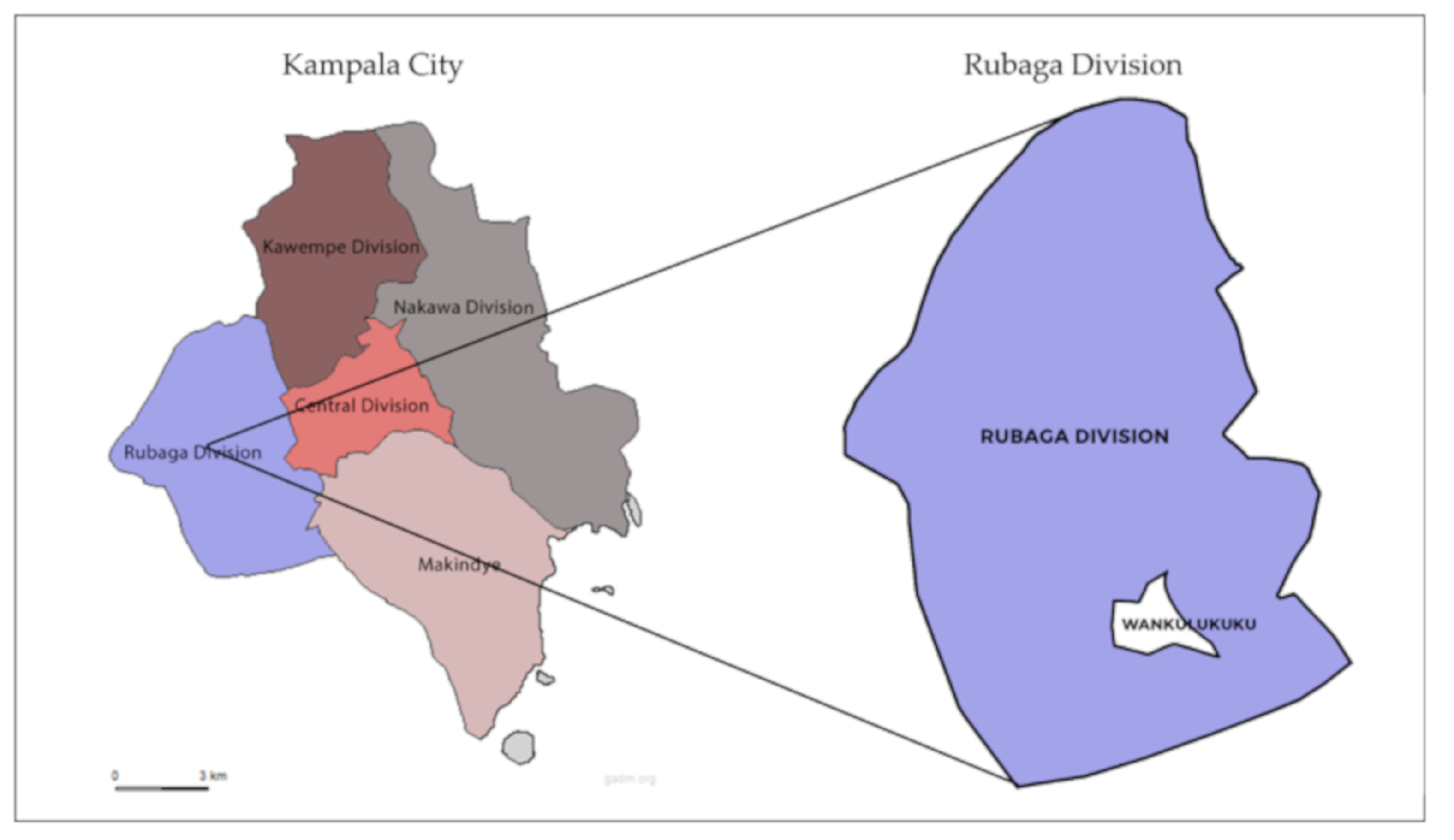
2.1. Study Location
2.2. Data Collection
2.3. Risk Analysis
2.4. Statistical Methods
2.5. Ethical Approval
3. Results and Discussion
3.1. Household, Water, Sanitation, and Hygiene Characteristics
3.2. Environmental Contamination
3.2.1. Open Drains
3.2.2. Soil
3.2.3. Compound Latrines
3.2.4. Raw Produce
3.2.5. Bathing Water (Spring Water)
3.2.6. Drinking Water
3.2.7. Street Food
3.3. Behaviour Frequency
3.3.1. Bathing Water
3.3.2. Drinking Water
3.3.3. Street Food
3.3.4. Open Drains
3.3.5. Raw Produce
3.3.6. Public Latrines
3.3.7. Surface Water
3.3.8. Seasonal Variation in Behavior
3.4. Risk Profiles and People Plots
3.5. Most Dominant Pathways
3.5.1. Flood Water
3.5.2. Open Drains
3.5.3. Street Food
3.6. Recommendations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urban Population Growth. Available online: http://www.who.int/gho/urban_health/situation_trends/urban_population_growth_text/en/ (accessed on 22 November 2018).
- Alirol, E.; Getaz, L.; Stoll, B.; Chappuis, F.; Loutan, L. Urbanisation and infectious diseases in a globalised world. Lancet Infect. Dis. 2011, 11, 131–141. [Google Scholar] [CrossRef]
- UN-Habitat. State of the World’s Cities Report (SWCR) 2006/2007; United Nations Human Settlements Programme: Nairobi, Kenya, 2006. [Google Scholar]
- UN-Habitat. The Challenge of Slums: Global Report on Human Settlements 2003; United Nations Human Settlements Programme: London, UK, 2003. [Google Scholar]
- Cohen, B. Urbanization in developing countries: Current trends, future projections, and key challenges for sustainability. Technol. Soc. 2006, 28, 63–80. [Google Scholar] [CrossRef]
- Hawkins, P.; Blackett, I.; Heymans, C. Poor-Inclusive Urban Sanitation: An Overview; Water and Sanitation Program: Washington, DC, USA, 2013. [Google Scholar]
- Robb, K.; Null, C.; Teunis, P.; Yakubu, H.; Armah, G.; Moe, C.L. Assessment of Fecal Exposure Pathways in Low-Income Urban Neighborhoods in Accra, Ghana: Rationale, Design, Methods, and Key Findings of the SaniPath Study. Am. J. Trop. Med. Hyg. 2017, 97, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.C.; Ross, P.; Nasir, Z.A.; Taylor, H.; Parkinson, J. Development and application of a methodology to assess sanitary risks in Maputo, Mozambique. Environ. Urban. 2015, 27. [Google Scholar] [CrossRef]
- Labite, H.; Lunani, I.; van der Steen, P.; Vairavamoorthy, K.; Drechsel, P.; Lens, P. Quantitative Microbial Risk Analysis to evaluate health effects of interventions in the urban water system of Accra, Ghana. J. Water Health 2010, 8, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Stenström, T.A.; Seidu, R.; Ekane, N.; Zurbrügg, C. Microbial Exposure and Health Assessments in Sanitation Technologies and Systems; Stockholm Environment Institute: Stockholm, Sweden, 2011; ISBN 978-91-86125-36-3. [Google Scholar]
- WHO. Quantitative Microbial Risk Assessment: Application for Water Safety Management; WHO: Geneve, Switzerland, 2016. [Google Scholar]
- Daniels, M.E.; Smith, W.A.; Jenkins, M.W. Estimating Cryptosporidium and Giardia disease burdens for children drinking untreated groundwater in a rural population in India. PLoS Negl. Trop. Dis. 2018, 12, e0006231. [Google Scholar] [CrossRef]
- Kulinkina, A.V.; Mohan, V.R.; Francis, M.R.; Kattula, D.; Sarkar, R.; Plummer, J.D.; Ward, H.; Kang, G.; Balraj, V.; Naumova, E.N. Seasonality of water quality and diarrheal disease counts in urban and rural settings in south India. Sci. Rep. 2016, 6, 20521. [Google Scholar] [CrossRef] [Green Version]
- Kostyla, C.; Bain, R.; Cronk, R.; Bartram, J. Seasonal variation of fecal contamination in drinking water sources in developing countries: A systematic review. Sci. Total Environ. 2015, 514, 333–343. [Google Scholar] [CrossRef]
- Bhavnani, D.; Goldstick, J.E.; Cevallos, W.; Trueba, G.; Eisenberg, J.N. Impact of rainfall on diarrheal disease risk associated with unimproved water and sanitation. Am. J. Trop. Med. Hyg. 2014, 90, 705–711. [Google Scholar] [CrossRef]
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef] [Green Version]
- Kumpel, E.; Cock-Esteb, A.; Duret, M.; de Waal, D.; Khush, R. Seasonal Variation in Drinking and Domestic Water Sources and Quality in Port Harcourt, Nigeria. Am. J. Trop. Med. Hyg. 2017, 96, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Lwasa, S.; Owens, K. Kampala: Rebuilding Public Sector Legitimacy with a New Approach to Sanitation Services; World Resource Institute: Washington, DC, USA, 2018. [Google Scholar]
- Lars, S.; Niwagaba, C.; Strande, L. SFD Promotion Initiative: Kampala Uganda—Final Report; Eawag/Sandec: Dubendorf, Switzerland, 2016. [Google Scholar]
- MWE. Economic Assessment of the Impacts of Climate Change in Uganda; MWE: Kampala, Uganda, 2015. [Google Scholar]
- UBOS. National Population and Housing Census 2014: Census Results; Uganda Bureau of Statistics: Kampala, Uganda, 2014. [Google Scholar]
- CoHS. Unpublished SaniPath Report for the Rainy Season; Makerere School of Public Health: Gainesville, FL, USA, 2019. [Google Scholar]
- SaniPath Manual and Protocols. Available online: https://sites.google.com/view/sanipathwiki (accessed on 13 September 2019).
- Raj, S.J.; Wang, Y.; Yakubu, H.; Robb, K.; Siesel, C.; Green, J.; Kirby, A.; Mairinger, W.; Michiel, J.; Null, C.; et al. The SaniPath Exposure Assessment Tool: A quantitative approach for assessing exposure to fecal contamination through multiple pathways in low resource urban settlements. PLoS ONE 2020, 15, e0234364. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]
- SaniPath Rapid Assessment Tool Manual; Emory University: Atlanta, GA, USA, 2014.
- Bukenya, J. Household perceptions of the quality of drinking water in Uganda. In Proceedings of the Annual Meeting, Orlando, FL, USA, 5–8 February 2006. [Google Scholar]
- MOH. The National Sanitation Policy for Uganda; MOH: Kampala, Uganda, 2000. [Google Scholar]
- Katukiza, A.Y.; Ronteltap, M.; Oleja, A.; Niwagaba, C.B.; Kansiime, F.; Lens, P.N. Selection of sustainable sanitation technologies for urban slums–A case of Bwaise III in Kampala, Uganda. Sci. Total Environ. 2010, 409, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Nakagiri, A.; Kulabako, R.N.; Nyenje, P.M.; Tumuhairwe, J.B.; Niwagaba, C.B.; Kansiime, F. Performance of pit latrines in urban poor areas: A case of Kampala, Uganda. Habitat Int. 2015, 49, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Nyenje, P.M.; Foppen, J.W.; Kulabako, R.; Muwanga, A.; Uhlenbrook, S. Nutrient pollution in shallow aquifers underlying pit latrines and domestic solid waste dumps in urban slums. J. Environ. Manag. 2013, 122, 15–24. [Google Scholar] [CrossRef]
- Katukiza, A.Y.; Ronteltap, M.; van der Steen, P.; Foppen, J.W.; Lens, P.N. Quantification of microbial risks to human health caused by waterborne viruses and bacteria in an urban slum. J. Appl. Microbiol. 2014, 116, 447–463. [Google Scholar] [CrossRef]
- Berendes, D.M.; Kirby, A.E.; Clennon, J.A.; Agbemabiese, C.; Ampofo, J.A.; Armah, G.E.; Baker, K.K.; Liu, P.; Reese, H.E.; Robb, K.A.; et al. Urban sanitation coverage and environmental fecal contamination: Links between the household and public environments of Accra, Ghana. PLoS ONE 2018, 13, e0199304. [Google Scholar] [CrossRef]
- Ercumen, A.; Pickering, A.J.; Kwong, L.H.; Arnold, B.F.; Parvez, S.M.; Alam, M.; Sen, D.; Islam, S.; Kullmann, C.; Chase, C.; et al. Animal Feces Contribute to Domestic Fecal Contamination: Evidence from E. coli Measured in Water, Hands, Food, Flies, and Soil in Bangladesh. Environ. Sci. Technol. 2017, 51, 8725–8734. [Google Scholar] [CrossRef] [Green Version]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, R.; Sian-Denton, C.; Borja, M.; Castro, J.; Morphew, K. Soil: The environmental source of Escherichia coli and Enterococci in Guam’s streams. J. Appl. Microbiol. Symp. Suppl. 1998. [Google Scholar] [CrossRef]
- Tumwebaze, I.K. Prevalence and determinants of the cleanliness of shared toilets in Kampala slums, Uganda. J. Public Health 2013, 22, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Moore, G.; Griffith, C. Problems associated with traditional hygiene swabbing: The need for in-house standardization. J. Appl. Microbiol. 2007, 103, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- Amoah, P.; Drechsel, P.; Abaidoo, R.C. Irrigated urban vegetable production in Ghana: Sources of pathogen contamination and health risk elimination. Irrig. Drain. 2005, 54, S49–S61. [Google Scholar] [CrossRef]
- Nsubuga, F.B.; Kansiime, F.; Okot-Okumu, J. Pollution of protected springs in relation to high and low density settlements in Kampala—Uganda. Phys. Chem. Earth Parts A B C 2004, 29, 1153–1159. [Google Scholar] [CrossRef]
- Annan, S.T.; Adjibolosoo, S.V.; Adarkwah, F.; Frimpong, B.; Ampofo, J.A.; Santaigo, P.K. Assessment of Bacteriological Quality of Sources of Drinking Water in some Selected Communities in the Akuapem South District of the Eastern Region, Ghana. Appl. Ecol. Environ. Sci. 2018, 6, 153–159. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; Fourth Edition Incorporating the First Addendum, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Rusca, M.; Alda-Vidal, C.; Hordijk, M.; Kral, N. Bathing without water, and other stories of everyday hygiene practices and risk perception in urban low-income areas: The case of Lilongwe, Malawi. Environ. Urban. 2017, 29, 533–550. [Google Scholar] [CrossRef]
- Steyn, N.P.; McHiza, Z.; Hill, J.; Davids, Y.D.; Venter, I.; Hinrichsen, E.; Opperman, M.; Rumbelow, J.; Jacobs, P. Nutritional contribution of street foods to the diet of people in developing countries: A systematic review. Public Health Nutr. 2014, 17, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Gretsch, S.R.; Ampofo, J.A.; Baker, K.K.; Clennon, J.; Null, C.A.; Peprah, D.; Reese, H.; Robb, K.; Teunis, P.; Wellington, N.; et al. Quantification of exposure to fecal contamination in open drains in four neighborhoods in Accra, Ghana. J. Water Health 2016, 14, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Tumwebaze, I.K.; Lüthi, C. Households’ access and use of water and sanitation facilities in poor urban areas of Kampala, Uganda. J. Water Sanit. Hyg. Dev. 2013, 3, 96–105. [Google Scholar] [CrossRef]
- Tumwebaze, I.K.; Orach, C.G.; Niwagaba, C.; Luthi, C.; Mosler, H.J. Sanitation facilities in Kampala slums, Uganda: Users’ satisfaction and determinant factors. Int. J. Environ. Health Res. 2013, 23, 191–204. [Google Scholar] [CrossRef]
- Funari, E.; Manganelli, M.; Sinisi, L. Impact of climate change on waterborne diseases. Ann. Ist. Super Sanit. 2012, 48, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Batréau, Q.; Bonnet, F. Managed Informality: Regulating Street Vendors in Bangkok. City Commun. 2016, 15, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Dudeja, P.; Kaushal, N.; Mukherji, S. Impact of health education intervention on food safety and hygiene of street vendors: A pilot study. Med. J. Armed Forces India 2016, 72, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holy, A.; Makhoane, F.M. Improving street food vending in South Africa: Achievements and lessons learned. Int. J. Food Microbiol. 2006, 111, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Armitage, N.; Beauclair, R.; Ashipala, N.; Spiege, A. Draining the Shantytowns; Lessons from Kosovo Informal Settlement; University of Cape Town: Cape Town, South Africa, 2010. [Google Scholar]
- McFarlane, C.; Desai, R.; Graham, S. Informal Urban Sanitation: Everyday Life, Poverty, and Comparison. Annal. Assoc. Am. Geogr. 2014, 104, 989–1011. [Google Scholar] [CrossRef] [Green Version]
- Head, B.W. Community Engagement: Participation on Whose Terms? Aust. J. Polit. Sci. 2007, 42, 441–454. [Google Scholar] [CrossRef]
| Sample Type | No Dilution | Dilution 1 | Dilution 2 | Dilution 3 | Dilution 4 |
|---|---|---|---|---|---|
| Drinking Water (Municipal Water) | ✔ | - | - | - | - |
| Bathing Water (Spring Water) | ✔ | - | - | - | - |
| Drain Water | - | 1:10 | 1:100 | 1:1000 | 1:10,000 |
| Produce | - | 1:10 | - | - | - |
| Street Food | - | 1:10 | - | - | - |
| Latrine Swabs | - | 1:10 | - | - | - |
| Soil | - | 1:10 | 1:100 | - | - |
| E. coli Geometric Mean | WSR | |||||
|---|---|---|---|---|---|---|
| Pathway | Unit | Rainy [22] | SD | Dry | SD | p-Value |
| Open Drains | Log10CFU/100 mL | 6.52 (n = 7) | 0.17 | 6.80 (n = 10) | 0.38 | 0.010 |
| Soil | Log10CFU/g | 3.79 (n = 6) | 0.27 | 3.65 (n = 10) | 0.36 | 0.419 |
| Produce | Log10CFU/g | 3.26 (n = 7) | 0.93 | 2.38 (n = 10) | 0.03 | 0.371 |
| Bathing Water(Spring Water) | Log10CFU/100 mL | 2.81 (n = 9) | 0.20 | 2.84 (n = 10) | 0.39 | 0.100 |
| Street Food | Log10CFU/g | 2.57 (n = 6) | 0.00 | 3.55 (n = 10) | 0.49 | 0.002 |
| Drinking Water(Municipal Water) | Log10CFU/100 mL | TFTC * (n = 10) | - | TFTC * (n = 10) | - | - |
| Rainy Season [22] | Dry Season | MW-U | ||||
|---|---|---|---|---|---|---|
| Adults% | Children% | Adults% | Children% | |||
| Pathway | Frequency of Exposure | % | % | % | % | p-Value |
| Surface Water | More than 10 times in the past month | 8 | 4 | 2 | 12 | adults p = 0.002 |
| 6–10 times in the past month | 4 | 2 | 2 | 5 | children p = 0.016 | |
| 5 times or less in the past month | 7 | 6 | 12 | 14 | - | |
| Never | 80 | 86 | 82 | 68 | - | |
| Do not know | 1 | 2 | 2 | 1 | - | |
| Open Drains | More than 10 times in the past month | 36 | 25 | 32 | 43 | adults p = 0.075 |
| 6–10 times in the past month | 36 | 8 | 7 | 10 | children p = 0.001 | |
| 5 times or less in the past month | 12 | 20 | 10 | 15 | - | |
| Never | 44 | 39 | 43 | 32 | - | |
| Do not know | 0 | 8 | 1 | 0 | - | |
| Drinking Water * | Every day | 61 | 60 | 72 | 76 | adults p = 0.109 |
| 4–6 days within the past week | 3 | 8 | 9 | 12 | children p = 0.074 | |
| 3 days or less within the past week | 5 | 9 | 5 | 1 | - | |
| Never | 29 | 22 | 12 | 11 | - | |
| Do not know | 2 | 1 | 2 | 0 | - | |
| Bathing Water (Spring Water) | More than 10 times in the past week | 91 | 70 | 93 | 71 | adults p = 0.236 |
| 6–10 times in the past week | 7 | 28 | 4 | 26 | children p = 0.865 | |
| 5 times or less in the past week | 0 | 1 | 1 | 2 | - | |
| Never within the past week | 0 | 0 | 0 | 0 | - | |
| Do not know | 2 | 1 | 2 | 1 | - | |
| Raw Produce | More than 10 times in the past week | 10 | 8 | 13 | 15 | adults p = 0.040 |
| 6–10 times in the past week | 13 | 10 | 20 | 23 | children p = 0.001 | |
| 5 times or less in the past week | 38 | 39 | 28 | 26 | - | |
| Never within the past week | 34 | 41 | 34 | 35 | - | |
| Do not know | 5 | 2 | 5 | 1 | - | |
| Street Food | More than 10 times in the past week | 21 | 39 | 48 | 66 | adults p = 0.000 |
| 6–10 times in the past week | 37 | 34 | 13 | 11 | children p = 0.000 | |
| 5 times or less in the past week | 29 | 21 | 15 | 18 | - | |
| Never within the past week | 11 | 5 | 18 | 4 | - | |
| Do not know | 2 | 1 | 6 | 1 | - | |
| Public Latrines | More than 10 times in the past week | 21 | 34 | 24 | 22 | adults p = 0.006 |
| 6–10 times in the past week | 19 | 15 | 3 | 2 | children p = 0.000 | |
| 5 times or less in the past week | 10 | 8 | 6 | 8 | - | |
| Never within the past week | 48 | 33 | 62 | 67 | - | |
| Do not know | 2 | 10 | 5 | 1 | - | |
| Food type | Ingredients | Cooking Method | Serving |
|---|---|---|---|
| Rolex | Eggs, chapati, onions, cabbage and tomatoes | The chapati is usually prepared in advance, it is a flat bread made from wheat flour fried on a pan. The onions, tomatoes, and cabbage are mixed with one egg and fried to make an omelette. The omelette is then wrapped in the chapati | Chopped on a board and served by hands. Usually put in a polythene bag |
| Samosa | Wheat flour, rice and minced meat | The wheat flour is used to make a shell which is stuffed with either minced meat or rice and then deep fried | Packaged into a polythene bag by hands |
| Kikomando | Chapati and beans | Boiled beans served with a chapati | Beans served with a spoon in plate, and chapati is first chopped and served by hand. |
| Rainy Season [22] | Dry Season | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Children | Adults | Children | |||||||||
| Pathway | Exposure | Dose (Log10 CFU/month) | E (Risk) | Exposure | Dose (Log10 CFU/month) | E (Risk) | Exposure | Dose (Log10 CFU/month) | E (Risk) | Exposure | Dose (Log10 CFU/month) | E (Risk) |
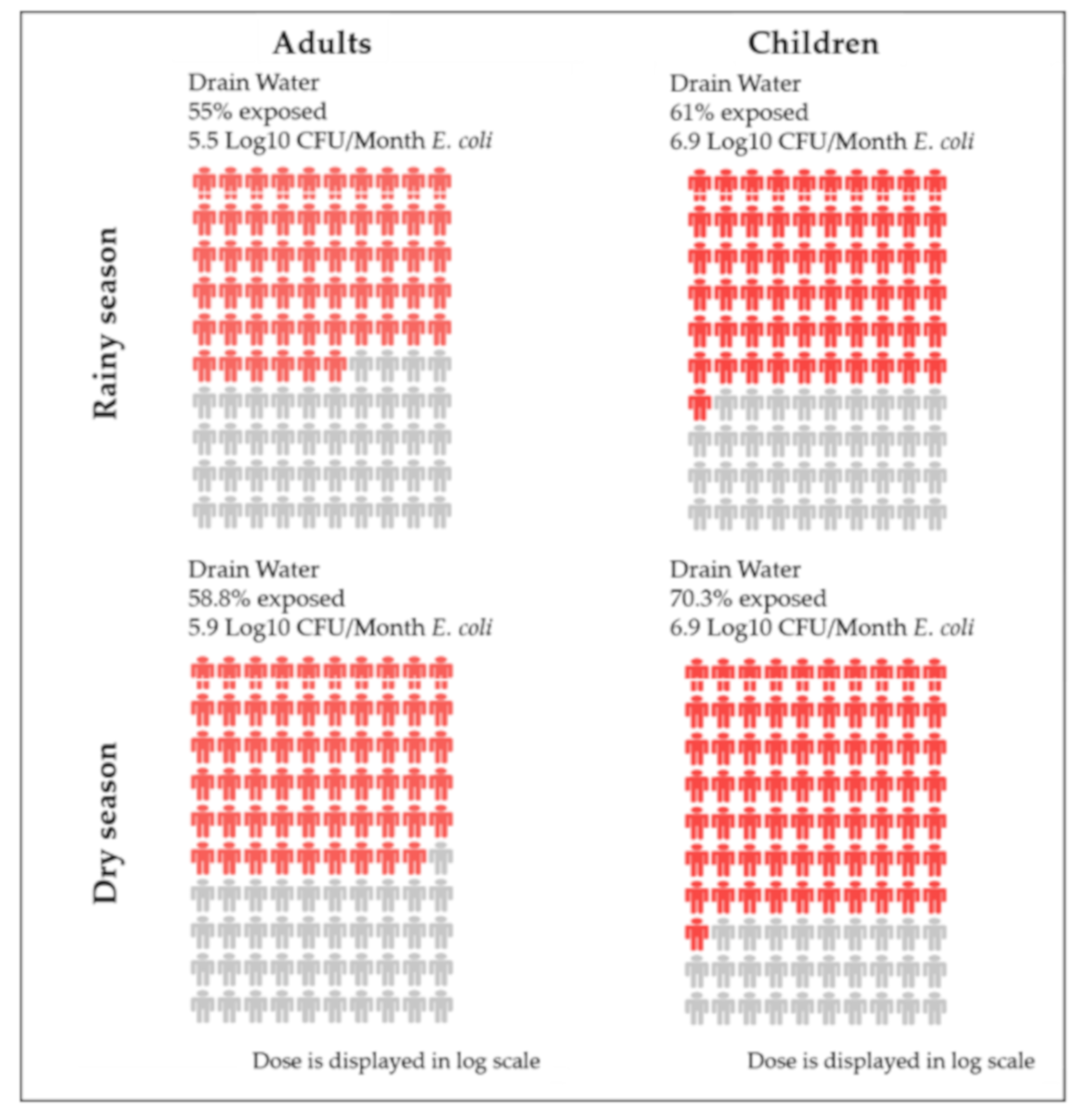
| Open Drains | 55% | 5.5 | 5.2 | 61% | 6.9 | 6.6 | 58.8% | 5.9 | 5.7 | 70.3% | 6.9 | 6.8 |
| Street Food * | 93% | 6.2 * | 6.2 | 98% | 5.8 * | 5.8 | 83.9% | 6.9 | 6.8 | 96.6% | 6.5 | 6.4 |
| Raw Produce | 67% | 5.6 | 5.4 | 60% | 5.4 | 5.2 | 66.9% | 3.4 | 3.2 | 69.6% | 3 | 2.8 |
| Bathing Water ** | 100% | 4.4 ** | 4.4 | 100% | 5.2 ** | 5.2 | 100% | 5.5 ** | 5.5 | 100% | 5.5 ** | 5.5 |
| Drinking Water ** | 73% | 2.1 ** | 1.9 | 78 | 1.7 ** | 1.5 | 90.7% | 2.4 ** | 2.3 | 91.1% | 2 ** | 1.9 |
| Public/Shared Latrines ** | 53% ** | 0.3 | 0.0 | 64% ** | 0.6 | 0.4 | 32.3% ** | 0.5 | 0.0 | 32.9% ** | 0.4 | 0.0 |
| Flood Water | 40% | 8.8 | 8.4 | 46% | 8.8 | 8.4 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronoh, P.; Furlong, C.; Kansiime, F.; Mugambe, R.; Brdjanovic, D. Are There Seasonal Variations in Faecal Contamination of Exposure Pathways? An Assessment in a Low–Income Settlement in Uganda. Int. J. Environ. Res. Public Health 2020, 17, 6355. https://doi.org/10.3390/ijerph17176355
Ronoh P, Furlong C, Kansiime F, Mugambe R, Brdjanovic D. Are There Seasonal Variations in Faecal Contamination of Exposure Pathways? An Assessment in a Low–Income Settlement in Uganda. International Journal of Environmental Research and Public Health. 2020; 17(17):6355. https://doi.org/10.3390/ijerph17176355
Chicago/Turabian StyleRonoh, Patrick, Claire Furlong, Frank Kansiime, Richard Mugambe, and Damir Brdjanovic. 2020. "Are There Seasonal Variations in Faecal Contamination of Exposure Pathways? An Assessment in a Low–Income Settlement in Uganda" International Journal of Environmental Research and Public Health 17, no. 17: 6355. https://doi.org/10.3390/ijerph17176355
APA StyleRonoh, P., Furlong, C., Kansiime, F., Mugambe, R., & Brdjanovic, D. (2020). Are There Seasonal Variations in Faecal Contamination of Exposure Pathways? An Assessment in a Low–Income Settlement in Uganda. International Journal of Environmental Research and Public Health, 17(17), 6355. https://doi.org/10.3390/ijerph17176355








