Post-Exercise Hypotension and Reduced Cardiac Baroreflex after Half-Marathon Run: In Men, but Not in Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
2.3. Heart Rate Variability, Baroreflex Sensitivity and Hemodynamic Assessment
2.4. Data Analysis
2.5. Statistical Analysis
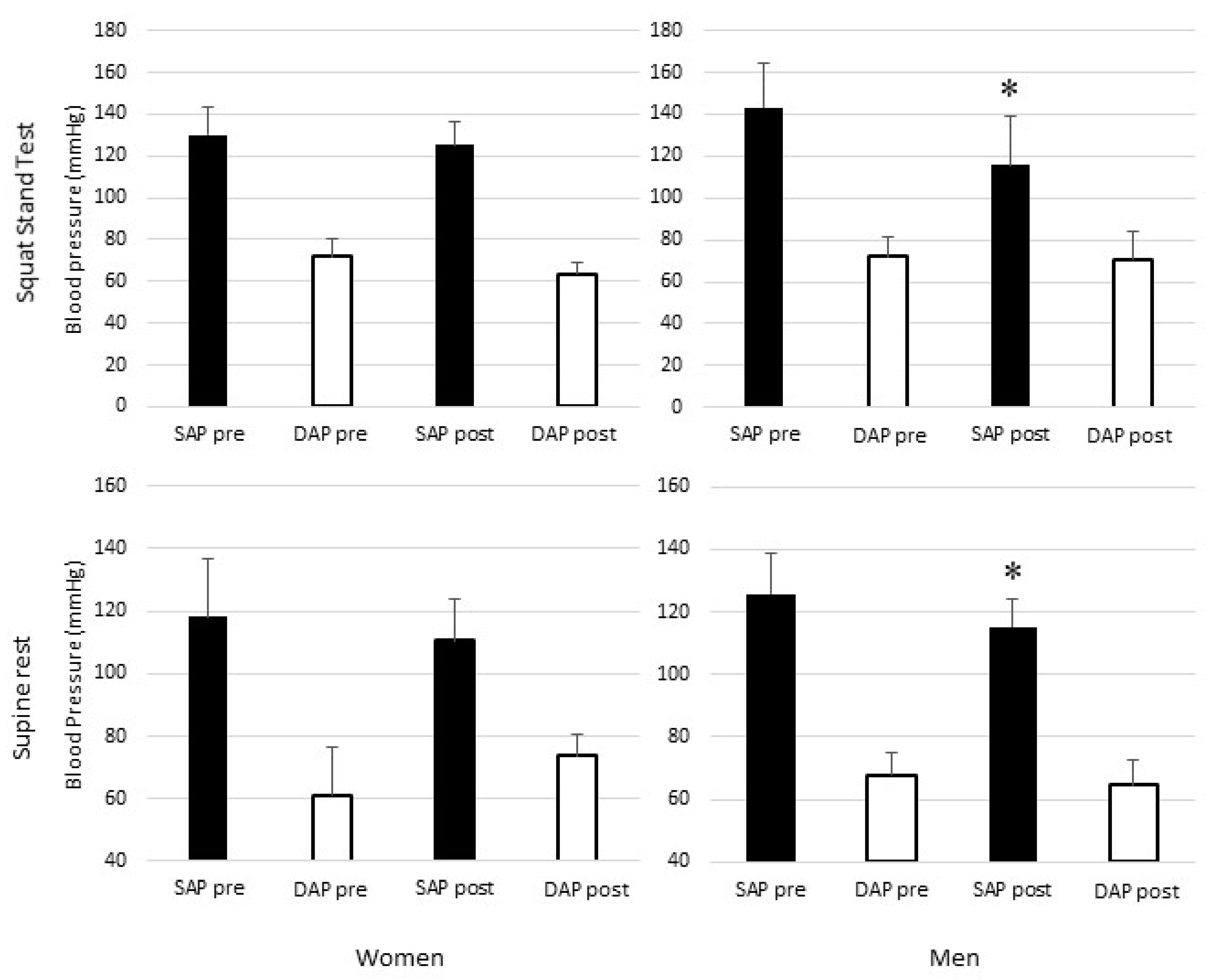
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Disease Burden and Mortality Estimates. Available online: http://www.who.int/healthinfo/global_burden_disease/estimates/en/ (accessed on 26 February 2020).
- Gholizadeh, L.; Davidson, P. More similarities than differences: An international comparison of CVD mortality and risk factors in women. Health Care Women Int. 2008, 29, 3–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattioli, A.V.; Sciomer, S.; Moscucci, F.; Maiello, M.; Cugusi, L.; Gallina, S.; Dei Cas, A.; Lombardi, C.; Pengo, M.; Parati, G.; et al. Cardiovascular prevention in women: A narrative review from the Italian Society of Cardiology working groups on “Cardiovascular Prevention, Hypertension and peripheral circulation” and on “Women Disease”. J. Cardiovasc. Med. 2019, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Kurth, J.; Malik, S. Reducing women’s cardiovascular disease risk profile. Women’s Health Lond. Engl. 2015, 11, 385–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merghani, A.; Malhotra, A.; Sharma, S. The U-shaped relationship between exercise and cardiac morbidity. Trends Cardiovasc. Med. 2016, 26, 232–240. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Ong, J.C.; Wang, G. Historical analysis of participation in 161 km ultramarathons in North America. Int. J. Hist. Sport 2010, 27, 1877–1891. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Cuk, I.; Clemente-Suárez, V.J.; Villiger, E.; Knechtle, B. Number of finishers and performance of age group women and men in long-distance running: Comparison among 10 km, half-marathon and marathon races in Oslo. Res. Sports Med. Print 2020, 1–11. [Google Scholar] [CrossRef]
- Baldwin, B. The State of Running 2019; IIRM: Hyderabad, India, 2019. [Google Scholar]
- Knechtle, B.; Nikolaidis, P.T.; Zingg, M.A.; Rosemann, T.; Rüst, C.A. Half-marathoners are younger and slower than marathoners. SpringerPlus 2016, 5. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Merghani, A.; Mont, L. Exercise and the heart: The good, the bad, and the ugly. Eur. Heart J. 2015, 36, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Favaloro, E.J.; Sanchis-Gomar, F. Sudden Cardiac and Noncardiac Death in Sports: Epidemiology, Causes, Pathogenesis, and Prevention. Semin. Thromb. Hemost. 2018, 44, 780–786. [Google Scholar] [CrossRef]
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited: Autonomic neural control of heart rate. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- Mourot, L.; Bouhaddi, M.; Perrey, S.; Rouillon, J.-D.; Regnard, J. Quantitative Poincare plot analysis of heart rate variability: Effect of endurance training. Eur. J. Appl. Physiol. 2004, 91, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Mourot, L.; Bouhaddi, M.; Tordi, N.; Rouillon, J.-D.; Regnard, J. Short- and long-term effects of a single bout of exercise on heart rate variability: Comparison between constant and interval training exercises. Eur. J. Appl. Physiol. 2004, 92. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.; Zimmermann, M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation 2002, 105, 2753–2759. [Google Scholar] [CrossRef] [Green Version]
- Neves, V.R.; Kiviniemi, A.M.; Hautala, A.J.; Karjalainen, J.; Piira, O.-P.; Catai, A.M.; Mäkikallio, T.H.; Huikuri, H.V.; Tulppo, M.P. Heart Rate Dynamics after Exercise in Cardiac Patients with and without Type 2 Diabetes. Front. Physiol. 2011, 2, 57. [Google Scholar] [CrossRef] [Green Version]
- Vanoli, E.; De Ferrari, G.M.; Stramba-Badiale, M.; Hull, S.S.; Foreman, R.D.; Schwartz, P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 1991, 68, 1471–1481. [Google Scholar] [CrossRef] [Green Version]
- Seiler, S.; Haugen, O.; Kuffel, E. Autonomic Recovery after Exercise in Trained Athletes: Intensity and Duration Effects. Med. Sci. Sports Exerc. 2007, 39, 1366–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stergiou, D.; Duncan, E. Atrial Fibrillation (AF) in Endurance Athletes: A Complicated Affair. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 98. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Ogoh, S. Sex differences in baroreflex function in health and disease. J. Physiol. Sci. JPS 2019, 69, 851–859. [Google Scholar] [CrossRef]
- Dutra, S.G.V.; Pereira, A.P.M.; Tezini, G.C.S.V.; Mazon, J.H.; Martins-Pinge, M.C.; Souza, H.C.D. Cardiac autonomic modulation is determined by gender and is independent of aerobic physical capacity in healthy subjects. PLoS ONE 2013, 8, e77092. [Google Scholar] [CrossRef]
- Joyner, M.J.; Wallin, B.G.; Charkoudian, N. Sex differences and blood pressure regulation in humans. Exp. Physiol. 2016, 101, 349–355. [Google Scholar] [CrossRef]
- Yoshino, K.; Adachi, K.; Ihochi, K.; Matsuoka, K. Modeling effects of age and sex on cardiovascular variability responses to aerobic ergometer exercise. Med. Biol. Eng. Comput. 2007, 45, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Gratas-Delamarche, A.; Le Cam, R.; Delamarche, P.; Monnier, M.; Koubi, H. Lactate and catecholamine responses in male and female sprinters during a Wingate test. Eur. J. Appl. Physiol. 1994, 68, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.V.; Heffernan, K.S.; Rossow, L.; Guerra, M.; Pereira, F.D.; Fernhall, B. Sex differences in linear and nonlinear heart rate variability during early recovery from supramaximal exercise. Appl. Physiol. Nutr. Metab. 2010, 35, 439–446. [Google Scholar] [CrossRef]
- Brito, L.C.; Queiroz, A.C.C.; Forjaz, C.L.M. Influence of population and exercise protocol characteristics on hemodynamic determinants of post-aerobic exercise hypotension. Braz. J. Med. Biol. Res. 2014, 47, 626–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senitko, A.N.; Charkoudian, N.; Halliwill, J.R. Influence of endurance exercise training status and gender on postexercise hypotension. J. Appl. Physiol. 2002, 92, 2368–2374. [Google Scholar] [CrossRef] [Green Version]
- Stachenfeld, N.S. Including women in research. It’s necessary, and really not so hard to do. Exp. Physiol. 2018, 103, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.M.; Ziegler, M.G.; Patwardhan, A.R.; Ott, J.B.; Kim, C.S.; Leonelli, F.M.; Knapp, C.F. Gender differences in autonomic cardiovascular regulation: Spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. 2001, 91, 2611–2618. [Google Scholar] [CrossRef]
- Cote, A.T.; Phillips, A.A.; Foulds, H.J.; Charlesworth, S.A.; Bredin, S.S.D.; Burr, J.F.; Koehle, M.S.; Warburton, D.E.R. Sex differences in cardiac function after prolonged strenuous exercise. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2015, 25, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Mourot, L.; Fornasiero, A.; Rakobowchuk, M.; Skafidas, S.; Brighenti, A.; Stella, F.; Zignoli, A.; Savoldelli, A.; Pellegrini, B.; Danese, E.; et al. Similar cardiovascular and autonomic responses in trained type 1 diabetes mellitus and healthy participants in response to half marathon. Diabetes Res. Clin. Pract. 2020, 160, 107995. [Google Scholar] [CrossRef]
- Festa, L.; Tarperi, C.; Skroce, K.; Boccia, G.; Lippi, G.; La Torre, A.; Schena, F. Effects of Flywheel Strength Training on the Running Economy of Recreational Endurance Runners. J. Strength Cond. Res. 2019, 33, 684–690. [Google Scholar] [CrossRef]
- Bogert, L.W.J.; van Lieshout, J.J. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger: Noninvasive pressure and flow. Exp. Physiol. 2005, 90, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.; Deo, S.H.; Vianna, L.C.; Balanos, G.M.; Hartwich, D.; Fisher, J.P.; Fadel, P.J. Sex differences in carotid baroreflex control of arterial blood pressure in humans: Relative contribution of cardiac output and total vascular conductance. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2454–H2465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zuckerman, J.H.; Giller, C.A.; Levine, B.D. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 1998, 274, H233–H241. [Google Scholar] [CrossRef] [Green Version]
- Pinna, G.D.; Maestri, R.; La Rovere, M.T. Assessment of baroreflex sensitivity from spontaneous oscillations of blood pressure and heart rate: Proven clinical value? Physiol. Meas. 2015, 36, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Martelli, D.; Silvani, A.; McAllen, R.M.; May, C.N.; Ramchandra, R. The low frequency power of heart rate variability is neither a measure of cardiac sympathetic tone nor of baroreflex sensitivity. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1005–H1012. [Google Scholar] [CrossRef] [PubMed]
- Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Burt, V.L.; Whelton, P.; Roccella, E.J.; Brown, C.; Cutler, J.A.; Higgins, M.; Horan, M.J.; Labarthe, D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995, 25, 305–313. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Narkiewicz, K.; Phillips, B.G.; Kato, M.; Hering, D.; Bieniaszewski, L.; Somers, V.K. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005, 45, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.; Thayer, J.F. Sex differences in healthy human heart rate variability: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 64, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, A.R.; Merrill, R.H.; Wooles, W.R. Gender-related differences in the baroreceptor reflex control of heart rate in normotensive humans. J. Appl. Physiol. 1994, 77, 606–613. [Google Scholar] [CrossRef]
- Beske, S.D.; Alvarez, G.E.; Ballard, T.P.; Davy, K.P. Gender difference in cardiovagal baroreflex gain in humans. J. Appl. Physiol. 2001, 91, 2088–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, T.; Hartikainen, J.; Vanninen, E.; Niskanen, L.; Geelen, G.; Länsimies, E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J. Appl. Physiol. 1998, 84, 576–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasti, L.; Grimoldi, P.; Mainardi, L.T.; Petrozzino, M.R.; Piantanida, E.; Garganico, D.; Diolisi, A.; Zanotta, D.; Bertolini, A.; Ageno, W.; et al. Autonomic function and baroreflex sensitivity during a normal ovulatory cycle in humans. Acta Cardiol. 1999, 54, 209–213. [Google Scholar] [PubMed]
- Tank, J.; Diedrich, A.; Szczech, E.; Luft, F.C.; Jordan, J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension 2005, 45, 1159–1164. [Google Scholar] [CrossRef] [Green Version]
- Samora, M.; Teixeira, A.L.; Sabino-Carvalho, J.L.; Vianna, L.C. Spontaneous cardiac baroreflex sensitivity is enhanced during post-exercise ischemia in men but not in women. Eur. J. Appl. Physiol. 2019, 119, 103–111. [Google Scholar] [CrossRef]
- Sandbakk, Ø.; Solli, G.S.; Holmberg, H.-C. Sex Differences in World-Record Performance: The Influence of Sport Discipline and Competition Duration. Int. J. Sports Physiol. Perform. 2018, 13, 2–8. [Google Scholar] [CrossRef]
- Boccia, G.; Dardanello, D.; Tarperi, C.; Festa, L.; La Torre, A.; Pellegrini, B.; Schena, F.; Rainoldi, A. Women show similar central and peripheral fatigue to men after half-marathon. Eur. J. Sport Sci. 2018, 18, 695–704. [Google Scholar] [CrossRef]
- Ramaekers, D.; Ector, H.; Aubert, A.E.; Rubens, A.; Van de Werf, F. Heart rate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur. Heart J. 1998, 19, 1334–1341. [Google Scholar] [CrossRef]
- Halliwill, J.R.; Buck, T.M.; Lacewell, A.N.; Romero, S.A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 2013, 98, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Raczak, G.; Pinna, G.D.; La Rovere, M.T.; Maestri, R.; Danilowicz-Szymanowicz, L.; Ratkowski, W.; Figura-Chmielewska, M.; Szwoch, M.; Ambroch-Dorniak, K. Cardiovagal response to acute mild exercise in young healthy subjects. Circ. J. Off. J. Jpn. Circ. Soc. 2005, 69, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Terziotti, P.; Schena, F.; Gulli, G.; Cevese, A. Post-exercise recovery of autonomic cardiovascular control: A study by spectrum and cross-spectrum analysis in humans. Eur. J. Appl. Physiol. 2001, 84, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.J.; De Ste Croix, M.B.A.; James, D.V.B. The Influence of Exercise Intensity on Postexercise Baroreflex Sensitivity. Res. Q. Exerc. Sport 2017, 88, 36–43. [Google Scholar] [CrossRef]
- Hoppel, F.; Calabria, E.; Pesta, D.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Physiological and Pathophysiological Responses to Ultramarathon Running in Non-elite Runners. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, A.L.; Ritti-Dias, R.; Antonino, D.; Bottaro, M.; Millar, P.J.; Vianna, L.C. Sex Differences in Cardiac Baroreflex Sensitivity after Isometric Handgrip Exercise. Med. Sci. Sports Exerc. 2018, 50, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Schena, F. Run for Science (R4S): The history of a successful project of precision and laboratory medicine in sport and exercise. J. Lab. Precis. Med. 2017, 2, 11. [Google Scholar] [CrossRef]
- Hackney, A.C.; Kallman, A.L.; Ağgön, E. Female sex hormones and the recovery from exercise: Menstrual cycle phase affects responses. Biomed. Hum. Kinet. 2019, 11, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef] [Green Version]
- Way, K.L.; Sultana, R.N.; Sabag, A.; Baker, M.K.; Johnson, N.A. The effect of high Intensity interval training versus moderate intensity continuous training on arterial stiffness and 24h blood pressure responses: A systematic review and meta-analysis. J. Sci. Med. Sport 2019, 22, 385–391. [Google Scholar] [CrossRef]
- Edwards, S. High performance training and racing. In The Heart Rate Monitor Book; Feet Fleet Press: Sacramento, CA, USA, 1993; pp. 113–123. [Google Scholar]
- Billat, V.L.; Petot, H.; Landrain, M.; Meilland, R.; Koralsztein, J.P.; Mille-Hamard, L. Cardiac output and performance during a marathon race in middle-aged recreational runners. Sci. World J. 2012, 2012, 810859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.R.C.; Schreuder, J.J.; Mulier, J.P.; Smith, N.T.; Settels, J.J.; Wesseling, K.H. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br. J. Anaesth. 2001, 87, 212–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| REST | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||||||||||
| Pre | Post | Pre | Post | |||||||||||||
| Hemodynamics | ||||||||||||||||
| HR | bpm | 60.2 | ± | 10.5 | 68.3 | ± | 10.9 | * | 60.4 | ± | 7.8 | 74.4 | ± | 9.0 | * | |
| SV | mL | 78 | ± | 21 | 75 | ± | 21 | 97 | ± | 18 | # | 75 | ± | 20 | * | |
| CO | L/min | 4.7 | ± | 1.6 | 5.1 | ± | 1.8 | 5.9 | ± | 0.9 | # | 5.7 | ± | 1.7 | ||
| SAP | mmHg | 118 | ± | 12 | 110 | ± | 6 | 126 | ± | 13 | # | 115 | ± | 9 | * | |
| DAP | mmHg | 61 | ± | 13 | 59 | ± | 7 | 68 | ± | 7 | 65 | ± | 8 | |||
| MAP | mmHg | 79 | ± | 12 | 76 | ± | 5 | 87 | ± | 6 | # | 81 | ± | 9 | * | |
| TPR | mmHg per L·min−1 | 1.19 | ± | 0.51 | 1.06 | ± | 0.54 | 0.90 | ± | 0.17 | 0.91 | ± | 0.42 | |||
| Heart Rate Variability | ||||||||||||||||
| IBI | ms | 1029 | ± | 179 | 904 | ± | 152 | * | 1010 | ± | 127 | 819 | ± | 103 | * | |
| Ln-RMSSD | ms | 3.94 | ± | 0.64 | 3.61 | ± | 0.64 | * | 3.43 | ± | 0.34 | # | 2.91 | ± | 0.34 | *,# |
| Ln-HF | ms2 | 6.51 | ± | 1.14 | 5.99 | ± | 1.53 | * | 5.61 | ± | 0.78 | # | 4.40 | ± | 0.94 | *,# |
| HFnu | 52 | ± | 14 | 41 | ± | 14 | * | 27 | ± | 12 | # | 24 | ± | 13 | *,# | |
| Transfer Function Analysis | ||||||||||||||||
| Gain-VLF | ms mmHg−1 | 5.1 | ± | 1.5 | 6.0 | ± | 2.9 | 4.7 | ± | 1.5 | 4.8 | ± | 1.7 | |||
| Gain-LF | ms mmHg−1 | 8.7 | ± | 6.1 | 9.8 | ± | 8.8 | 8.1 | ± | 2.7 | 5.7 | ± | 2.5 | * | ||
| Gain-HF | ms mmHg−1 | 2.43 | ± | 0.49 | 2.35 | ± | 0.85 | 2.11 | ± | 0.26 | 1.77 | ± | 0.46 | # | ||
| Phase-VLF | rads | 0.10 | ± | 1.10 | 0.03 | ± | 0.71 | −0.17 | ± | 0.67 | 0.06 | ± | 0.71 | |||
| Phase-LF | rads | −0.59 | ± | 0.38 | −0.49 | ± | 0.56 | −0.75 | ± | 0.28 | −0.61 | ± | 0.25 | |||
| Phase-HF | rads | 0.08 | ± | 0.48 | −0.06 | ± | 0.36 | −0.16 | ± | 0.27 | −0.31 | ± | 0.19 | # | ||
| Coh-VLF | 0.47 | ± | 0.08 | 0.53 | ± | 0.16 | 0.47 | ± | 0.10 | 0.53 | ± | 0.05 | ||||
| Coh-LF | 0.44 | ± | 0.16 | 0.46 | ± | 0.17 | 0.58 | ± | 0.09 | # | 0.54 | ± | 0.15 | |||
| Coh-HF | 0.40 | ± | 0.18 | 0.44 | ± | 0.17 | 0.46 | ± | 0.13 | 0.45 | ± | 0.15 | ||||
| Sequence Method | ||||||||||||||||
| n seq+ | 4 | ± | 2 | 4 | ± | 3 | 5 | ± | 3 | 8 | ± | 5 | *,# | |||
| n seq− | 6 | ± | 3 | 4 | ± | 2 | 6 | ± | 3 | 9 | ± | 5 | *,# | |||
| BRS-seq+ | ms mmHg−1 | 12.6 | ± | 10.1 | 16.3 | ± | 10.1 | 11.2 | ± | 5.7 | 8.3 | ± | 3.6 | # | ||
| BRS-seq− | ms mmHg−1 | 13.7 | ± | 10.6 | 19.1 | ± | 15.4 | 11.7 | ± | 7.9 | 9.2 | ± | 5.5 | # | ||
| BRS-seq | ms mmHg−1 | 13.3 | ± | 9.6 | 16.5 | ± | 10.1 | 11.5 | ± | 4.7 | 9.2 | ± | 4.7 | # | ||
| Squat Stand Test | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||||||||||
| Pre | Post | Pre | Post | |||||||||||||
| Hemodynamics | ||||||||||||||||
| HR | bpm | 84 | ± | 10 | 95 | ± | 11 | * | 81 | ± | 11 | 101 | ± | 8 | * | |
| SV | mL | 61 | ± | 23 | 66 | ± | 16 | 96 | ± | 18 | # | 58 | ± | 20 | * | |
| CO | L/min | 5.1 | ± | 2.2 | 6.0 | ± | 1.4 | 7.6 | ± | 1.8 | # | 5.6 | ± | 2.1 | * | |
| SAP | mmHg | 130 | ± | 16 | 125 | ± | 23 | 143 | ± | 28 | 116 | ± | 17 | * | ||
| DAP | mmHg | 72 | ± | 14 | 64 | ± | 21 | 72 | ± | 13 | 71 | ± | 18 | |||
| MAP | mmHg | 91 | ± | 14 | 84 | ± | 19 | 95 | ± | 17 | 85 | ± | 19 | |||
| TPR | mmHg per L.min−1 | 1.18 | ± | 0.63 | 1.10 | ± | 0.52 | 0.84 | ± | 0.24 | 0.93 | ± | 0.45 | |||
| Heart Rate Variability | ||||||||||||||||
| IBI | ms | 737 | ± | 87 | 655 | ± | 85 | * | 766 | ± | 97 | 605 | ± | 50 | * | |
| Ln-RMSSD | ms | 3.92 | ± | 0.56 | 3.53 | ± | 0.51 | * | 3.80 | ± | 0.39 | 3.14 | ± | 0.33 | *,# | |
| Ln-HF | ms2 | 6.47 | ± | 1.10 | 5.45 | ± | 1.11 | 6.22 | ± | 1.06 | * | 4.96 | ± | 0.78 | * | |
| HFnu | 9 | ± | 8 | 5 | ± | 3 | 9 | ± | 13 | 6 | ± | 3 | ||||
| Transfer Function Analysis | ||||||||||||||||
| Gain-SF | ms mmHg−1 | 3.72 | ± | 2.43 | 3.76 | ± | 2.49 | 3.82 | ± | 1.98 | 2.36 | ± | 1.00 | * | ||
| Phase-SF | rads | −0.69 | ± | 0.39 | −0.97 | ± | 0.32 | * | −0.93 | ± | 0.39 | −0.96 | ± | 0.37 | ||
| Coh-SF | 0.62 | ± | 0.20 | 0.64 | ± | 0.10 | 0.70 | ± | 0.10 | 0.72 | ± | 0.11 | ||||
| Sequence Method | ||||||||||||||||
| n seq+ | 12 | ± | 2 | 13 | ± | 3 | 11 | ± | 6 | 9 | ± | 5 | ||||
| n seq− | 15 | ± | 3 | 14 | ± | 3 | 13 | ± | 4 | 14 | ± | 3 | ||||
| BRS-seq+ | ms mmHg−1 | 7.03 | ± | 5.11 | 8.09 | ± | 7.58 | 4.79 | ± | 1.24 | 5.11 | ± | 2.80 | |||
| BRS-seq− | ms mmHg−1 | 6.13 | ± | 4.32 | 5.74 | ± | 3.65 | 3.61 | ± | 1.12 | 4.25 | ± | 2.15 | |||
| BRS-seq | ms mmHg−1 | 6.65 | ± | 4.31 | 6.83 | ± | 5.34 | 4.00 | ± | 1.09 | 4.66 | ± | 2.04 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourot, L.; Fornasiero, A.; Rakobowchuk, M.; Isacco, L.; Brighenti, A.; Stella, F.; Zignoli, A.; Pellegrini, B.; Tarperi, C.; Schena, F. Post-Exercise Hypotension and Reduced Cardiac Baroreflex after Half-Marathon Run: In Men, but Not in Women. Int. J. Environ. Res. Public Health 2020, 17, 6337. https://doi.org/10.3390/ijerph17176337
Mourot L, Fornasiero A, Rakobowchuk M, Isacco L, Brighenti A, Stella F, Zignoli A, Pellegrini B, Tarperi C, Schena F. Post-Exercise Hypotension and Reduced Cardiac Baroreflex after Half-Marathon Run: In Men, but Not in Women. International Journal of Environmental Research and Public Health. 2020; 17(17):6337. https://doi.org/10.3390/ijerph17176337
Chicago/Turabian StyleMourot, Laurent, Alessandro Fornasiero, Mark Rakobowchuk, Laurie Isacco, Alfredo Brighenti, Federico Stella, Andrea Zignoli, Barbara Pellegrini, Cantor Tarperi, and Federico Schena. 2020. "Post-Exercise Hypotension and Reduced Cardiac Baroreflex after Half-Marathon Run: In Men, but Not in Women" International Journal of Environmental Research and Public Health 17, no. 17: 6337. https://doi.org/10.3390/ijerph17176337
APA StyleMourot, L., Fornasiero, A., Rakobowchuk, M., Isacco, L., Brighenti, A., Stella, F., Zignoli, A., Pellegrini, B., Tarperi, C., & Schena, F. (2020). Post-Exercise Hypotension and Reduced Cardiac Baroreflex after Half-Marathon Run: In Men, but Not in Women. International Journal of Environmental Research and Public Health, 17(17), 6337. https://doi.org/10.3390/ijerph17176337







