The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review
Abstract
1. Introduction
2. E-Cigarettes
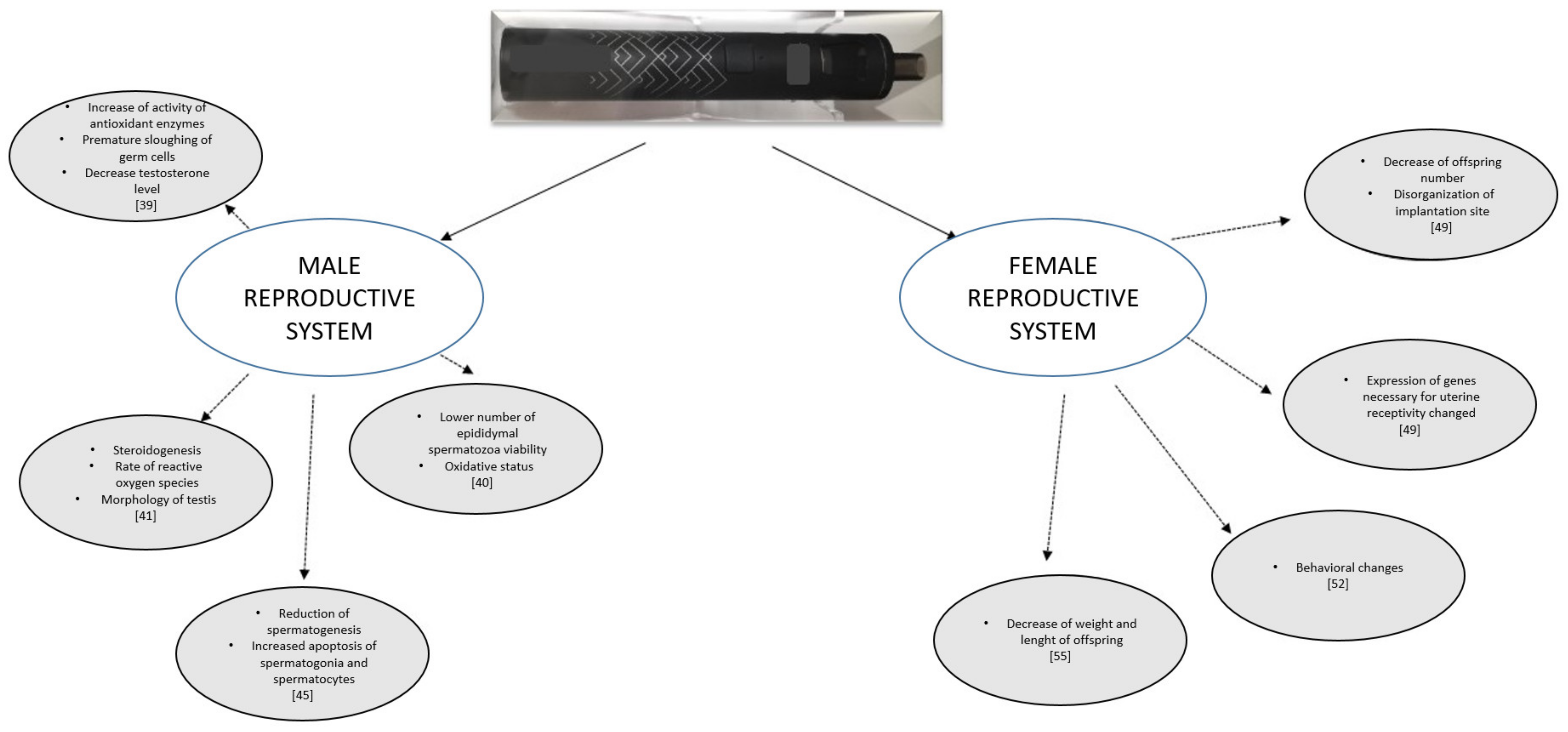
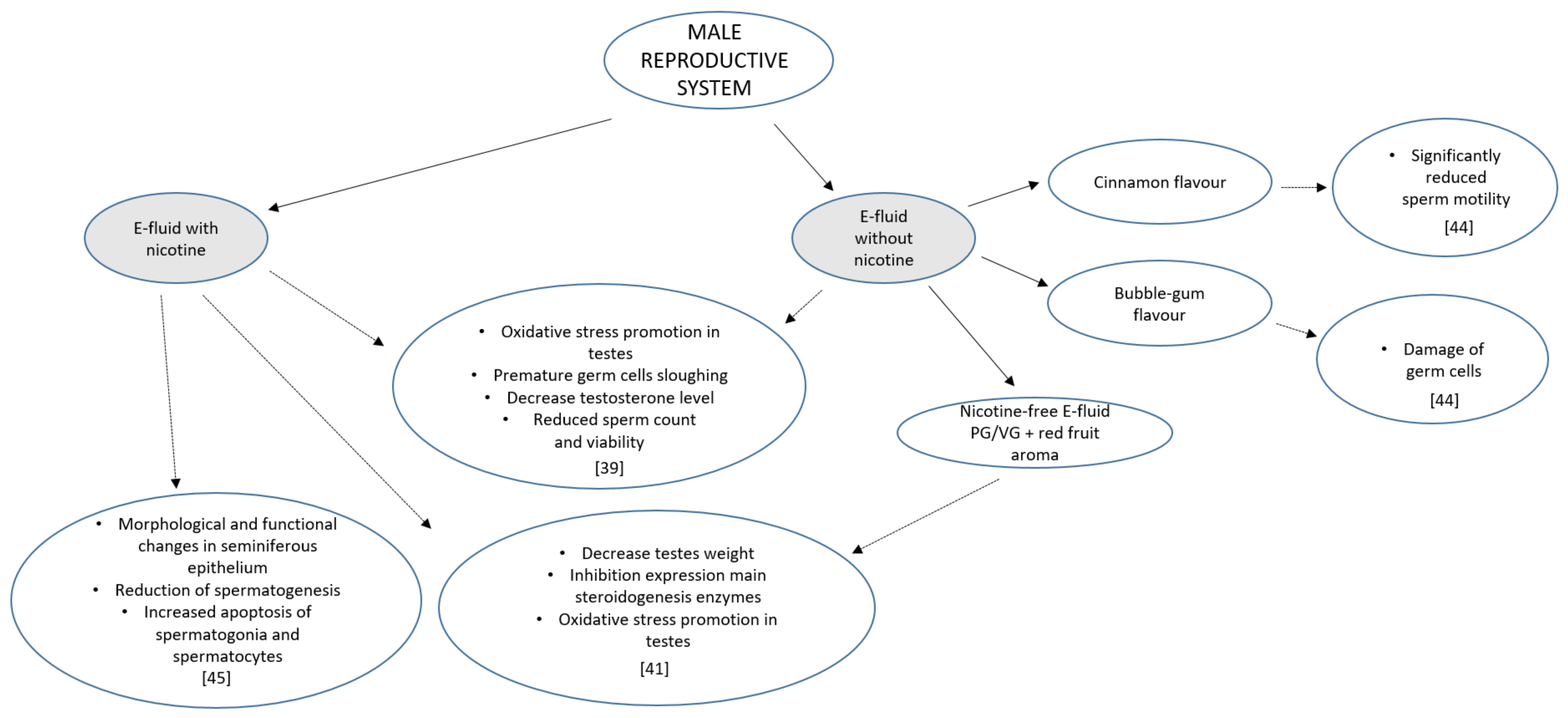
3. Male Reproductive System
4. Female Reproductive System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harris, C.C. Tobacco smoking, E-cigarettes, and nicotine harm. Proc. Natl. Acad. Sci. USA 2018, 115, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jandikova, H.; Duskova, M.; Starka, L. The influence of smoking and cessation on the human reproductive hormonal balance. Physiol. Res. 2017, 66 (Suppl. 3), S323–S331. [Google Scholar] [CrossRef] [PubMed]
- Adamcova, K.; Kolatorova, L.; Chlupacova, T.; Simkova, M.; Jandikova, H.; Parizek, A.; Starka, L.; Duskova, M. Changes to fetal steroidogenesis caused by maternal smoking. Physiol. Res. 2017, 66 (Suppl. 3), S375–S386. [Google Scholar] [CrossRef]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef]
- Tweed, J.O.; Hsia, S.H.; Lutfy, K.; Friedman, T.C. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metab. 2012, 23, 334–342. [Google Scholar] [CrossRef]
- Ito, S.; Ishimori, K.; Ishikawa, S. Effects of repeated cigarette smoke extract exposure over one month on human bronchial epithelial organotypic culture. Toxicol. Rep. 2018, 5, 864–870. [Google Scholar] [CrossRef]
- Kovac, J.R.; Khanna, A.; Lipshultz, L.I. The effects of cigarette smoking on male fertility. Postgrad. Med. 2015, 127, 338–341. [Google Scholar] [CrossRef]
- Hofhuis, W.; de Jongste, J.C.; Merkus, P.J. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch. Dis. Child. 2003, 88, 1086–1090. [Google Scholar] [CrossRef]
- Mund, M.; Louwen, F.; Klingelhoefer, D.; Gerber, A. Smoking and pregnancy-a review on the first major environmental risk factor of the unborn. Int. J. Environ. Res. Public Health 2013, 10, 6485–6499. [Google Scholar] [CrossRef]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and Male Infertility: An Evidence-Based Review. World J. Men’s Health 2015, 33, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Dechanet, C.; Anahory, T.; Mathieu Daude, J.C.; Quantin, X.; Reyftmann, L.; Hamamah, S.; Hedon, B.; Dechaud, H. Effects of cigarette smoking on reproduction. Hum. Reprod. Update 2011, 17, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Han, Y.F.; Song, T.; Dai, Y.T. Updated application of penile electrophysiological examination in the diagnosis and treatment of male sexual dysfunction. Zhonghua Nan Ke Xue Natl. J. Androl. 2019, 25, 559–565. [Google Scholar] [PubMed]
- De Mateo, S.; Gazquez, C.; Guimera, M.; Balasch, J.; Meistrich, M.L.; Ballesca, J.L.; Oliva, R. Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil. Steril. 2009, 91, 715–722. [Google Scholar] [CrossRef]
- Hammadeh, M.E.; Hamad, M.F.; Montenarh, M.; Fischer-Hammadeh, C. Protamine contents and P1/P2 ratio in human spermatozoa from smokers and non-smokers. Hum. Reprod. 2010, 25, 2708–2720. [Google Scholar] [CrossRef]
- Hamad, M.; Shelko, N.; Montenarh, M.; Hammadeh, M.E. The impact of cigarette smoking on protamines 1 and 2 transcripts in human spermatozoa. Hum. Fertil. (Camb.) 2019, 22, 104–110. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: A committee opinion. Fertil. Steril. 2012, 98, 1400–1406. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: A committee opinion. Fertil. Steril. 2018, 110, 611–618. [Google Scholar] [CrossRef]
- Hull, M.G.; North, K.; Taylor, H.; Farrow, A.; Ford, W.C. Delayed conception and active and passive smoking. The Avon Longitudinal Study of Pregnancy and Childhood Study Team. Fertil. Steril. 2000, 74, 725–733. [Google Scholar] [CrossRef]
- Whitcomb, B.W.; Purdue-Smithe, A.C.; Szegda, K.L.; Boutot, M.E.; Hankinson, S.E.; Manson, J.E.; Rosner, B.; Willett, W.C.; Eliassen, A.H.; Bertone-Johnson, E.R. Cigarette Smoking and Risk of Early Natural Menopause. Am. J. Epidemiol. 2018, 187, 696–704. [Google Scholar] [CrossRef]
- Plante, B.J.; Cooper, G.S.; Baird, D.D.; Steiner, A.Z. The impact of smoking on antimullerian hormone levels in women aged 38 to 50 years. Menopause 2010, 17, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Van Dulken, S. The Patent for E-Cigarettes. Available online: http://stephenvandulken.blogspot.com/2014/01/the-patents-for-e-cigarettes.html (accessed on 26 January 2014).
- Paradise, J. Electronic cigarettes: Smoke-free laws, sale restrictions, and the public health. Am. J. Public Health 2014, 104, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Goniewicz, M.L.; Kuma, T.; Gawron, M.; Knysak, J.; Kosmider, L. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. 2013, 15, 158–166. [Google Scholar] [CrossRef]
- Grana, R.; Benowitz, N.; Glantz, S.A. E-cigarettes: A scientific review. Circulation 2014, 129, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Flora, J.W.; Meruva, N.; Huang, C.B.; Wilkinson, C.T.; Ballentine, R.; Smith, D.C.; Werley, M.S.; McKinney, W.J. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul. Toxicol. Pharmacol. 2016, 74, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Margham, J.; McAdam, K.; Forster, M.; Liu, C.; Wright, C.; Mariner, D.; Proctor, C. Chemical Composition of Aerosol from an E-Cigarette: A Quantitative Comparison with Cigarette Smoke. Chem. Res. Toxicol. 2016, 29, 1662–1678. [Google Scholar] [CrossRef]
- Tayyarah, R.; Long, G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharmacol. 2014, 70, 704–710. [Google Scholar] [CrossRef]
- Forster, M.; Fiebelkorn, S.; Yurteri, C.; Mariner, D.; Liu, C.; Wright, C.; McAdam, K.; Murphy, J.; Proctor, C. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018, 93, 14–33. [Google Scholar] [CrossRef]
- Beauval, N.; Antherieu, S.; Soyez, M.; Gengler, N.; Grova, N.; Howsam, M.; Hardy, E.M.; Fischer, M.; Appenzeller, B.M.R.; Goossens, J.F.; et al. Chemical Evaluation of Electronic Cigarettes: Multicomponent Analysis of Liquid Refills and their Corresponding Aerosols. J. Anal. Toxicol. 2017, 41, 670–678. [Google Scholar] [CrossRef]
- Ooi, B.G.; Dutta, D.; Kazipeta, K.; Chong, N.S. Influence of the E-Cigarette Emission Profile by the Ratio of Glycerol to Propylene Glycol in E-Liquid Composition. ACS Omega 2019, 4, 13338–13348. [Google Scholar] [CrossRef]
- Zhu, S.H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 2014, 23 (Suppl. 3), iii3–iii9. [Google Scholar] [CrossRef] [PubMed]
- Krusemann, E.J.Z.; Boesveldt, S.; de Graaf, K.; Talhout, R. An E-Liquid Flavor Wheel: A Shared Vocabulary Based on Systematically Reviewing E-Liquid Flavor Classifications in Literature. Nicotine Tob. Res. 2019, 21, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Spyrou, A.; Stefopoulos, C.; Tsimopoulou, K.; Kourkoveli, P.; Tsiapras, D.; Kyrzopoulos, S.; Poulas, K.; Voudris, V. Nicotine absorption from electronic cigarette use: Comparison between experienced consumers (vapers) and naive users (smokers). Sci. Rep. 2015, 5, 11269. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T. Chemical evaluation of electronic cigarettes. Tob. Control 2014, 23 (Suppl. 2), ii11–ii17. [Google Scholar] [CrossRef]
- Pisinger, C.; Dossing, M. A systematic review of health effects of electronic cigarettes. Prev. Med. 2014, 69, 248–260. [Google Scholar] [CrossRef]
- Hutzler, C.; Paschke, M.; Kruschinski, S.; Henkler, F.; Hahn, J.; Luch, A. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol. 2014, 88, 1295–1308. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2016, 25, e10–e15. [Google Scholar] [CrossRef]
- El Golli, N.; Rahali, D.; Jrad-Lamine, A.; Dallagi, Y.; Jallouli, M.; Bdiri, Y.; Ba, N.; Lebret, M.; Rosa, J.P.; El May, M.; et al. Impact of electronic-cigarette refill liquid on rat testis. Toxicol. Mech. Methods 2016, 26, 427–434. [Google Scholar] [CrossRef]
- Rahali, D.; Jrad-Lamine, A.; Dallagi, Y.; Bdiri, Y.; Ba, N.; El May, M.; El Fazaa, S.; El Golli, N. Semen Parameter Alteration, Histological Changes and Role of Oxidative Stress in Adult Rat Epididymis on Exposure to Electronic Cigarette Refill Liquid. Chin. J. Physiol. 2018, 61, 75–84. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-free conditions. Life Sci. 2019, 228, 53–65. [Google Scholar] [CrossRef]
- Khalil, S.R.; Awad, A.; Ali, S.A. Melamine and/or formaldehyde exposures affect steroidogenesis via alteration of StAR protein and testosterone synthetic enzyme expression in male mice. Environ. Toxicol. Pharmacol. 2017, 50, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.J.; Fang, Y.Q.; Ji, S.Y.; Gao, Y.; Zhu, Y.Q.; Xia, T.T.; Jiang, M.H.; Zhang, Y.N. Formaldehyde Inhibits Sexual Behavior and Expression of Steroidogenic Enzymes in the Testes of Mice. J. Sex. Med. 2017, 14, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, H. Effect of Electronic-Cigarette Flavourings on (I) Human Sperm Motility, Chromatin Integrity in Vitro and (II) Mice Testicular Function in Vivo. Available online: http://srf-reproduction.org/wp-content/uploads/2017/01/Fertility-2017-Final-Programme-and-Abstracts.pdf (accessed on 7 January 2017).
- Wawryk-Gawda, E.; Zarobkiewicz, M.K.; Chłapek, K.; Chylińska-Wrzos, P.; Jodłowska-Jędrych, B. Histological changes in the reproductive system of male rats exposed to cigarette smoke or electronic cigarette vapor. Toxicol. Environ. Chem. 2019, 101, 404–419. [Google Scholar] [CrossRef]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Aschengrau, A.; Wise, L.A. Prospective study of cigarette smoking and fecundability. Hum. Reprod. 2019, 34, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.A.; Anders, A.M.; Aagaard, K.M. Maternal smoking as a model for environmental epigenetic changes affecting birthweight and fetal programming. Mol. Hum. Reprod. 2013, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mark, K.S.; Farquhar, B.; Chisolm, M.S.; Coleman-Cowger, V.H.; Terplan, M. Knowledge, Attitudes, and Practice of Electronic Cigarette Use Among Pregnant Women. J. Addict. Med. 2015, 9, 266–272. [Google Scholar] [CrossRef]
- Wetendorf, M.; Randall, L.T.; Lemma, M.T.; Hurr, S.H.; Pawlak, J.B.; Tarran, R.; Doerschuk, C.M.; Caron, K.M. E-Cigarette Exposure Delays Implantation and Causes Reduced Weight Gain in Female Offspring Exposed In Utero. J. Endocr. Soc. 2019, 3, 1907–1916. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Roadmap to embryo implantation: Clues from mouse models. Nat. Rev. Genet. 2006, 7, 185–199. [Google Scholar] [CrossRef]
- Matsumoto, H. Molecular and cellular events during blastocyst implantation in the receptive uterus: Clues from mouse models. J. Reprod. Dev. 2017, 63, 445–454. [Google Scholar] [CrossRef]
- Smith, D.; Aherrera, A.; Lopez, A.; Neptune, E.; Winickoff, J.P.; Klein, J.D.; Chen, G.; Lazarus, P.; Collaco, J.M.; McGrath-Morrow, S.A. Adult Behavior in Male Mice Exposed to E-Cigarette Nicotine Vapors during Late Prenatal and Early Postnatal Life. PLoS ONE 2015, 10, e0137953. [Google Scholar] [CrossRef]
- Benowitz, N.L. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol. Rev. 1996, 18, 188–204. [Google Scholar] [CrossRef] [PubMed]
- McGrath-Morrow, S.A.; Hayashi, M.; Aherrera, A.; Lopez, A.; Malinina, A.; Collaco, J.M.; Neptune, E.; Klein, J.D.; Winickoff, J.P.; Breysse, P.; et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS ONE 2015, 10, e0118344. [Google Scholar] [CrossRef] [PubMed]
- Orzabal, M.R.; Lunde-Young, E.R.; Ramirez, J.I.; Howe, S.Y.F.; Naik, V.D.; Lee, J.; Heaps, C.L.; Threadgill, D.W.; Ramadoss, J. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl. Res. 2019, 207, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, V.M.; Fischbach, L.A.; Chowdhury, P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: A systematic review of the literature. Tob. Induc. Dis. 2019, 17, 52. [Google Scholar] [CrossRef]
- St Helen, G.; Havel, C.; Dempsey, D.A.; Jacob, P., III; Benowitz, N.L. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016, 111, 535–544. [Google Scholar] [CrossRef]
- Whittington, J.R.; Simmons, P.M.; Phillips, A.M.; Gammill, S.K.; Cen, R.; Magann, E.F.; Cardenas, V.M. The Use of Electronic Cigarettes in Pregnancy: A Review of the Literature. Obstet. Gynecol. Surv. 2018, 73, 544–549. [Google Scholar] [CrossRef]
- Bowker, K.; Orton, S.; Cooper, S.; Naughton, F.; Whitemore, R.; Lewis, S.; Bauld, L.; Sinclair, L.; Coleman, T.; Dickinson, A.; et al. Views on and experiences of electronic cigarettes: A qualitative study of women who are pregnant or have recently given birth. BMC Pregnancy Childbirth 2018, 18, 233. [Google Scholar] [CrossRef]
- Bryce, R.; Robson, S.J. E-cigarettes and pregnancy. Is a closer look appropriate? Aust. N. Zeal. J. Obstet. Gynaecol. 2015, 55, 218–221. [Google Scholar] [CrossRef]
- Orzabal, M.; Ramadoss, J. Impact of Electronic Cigarette Aerosols on Pregnancy and Early Development. Curr. Opin. Toxicol. 2019, 14, 14–20. [Google Scholar] [CrossRef]
- Bhandari, N.R.; Day, K.D.; Payakachat, N.; Franks, A.M.; McCain, K.R.; Ragland, D. Use and Risk Perception of Electronic Nicotine Delivery Systems and Tobacco in Pregnancy. Womens Health Issues 2018, 28, 251–257. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, S.H.; Weng, M.W.; Wang, H.T.; Huang, W.C.; Lepor, H.; Wu, X.R.; Chen, L.C.; Tang, M.S. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1560–E1569. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szumilas, K.; Szumilas, P.; Grzywacz, A.; Wilk, A. The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6152. https://doi.org/10.3390/ijerph17176152
Szumilas K, Szumilas P, Grzywacz A, Wilk A. The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(17):6152. https://doi.org/10.3390/ijerph17176152
Chicago/Turabian StyleSzumilas, Kamila, Paweł Szumilas, Anna Grzywacz, and Aleksandra Wilk. 2020. "The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review" International Journal of Environmental Research and Public Health 17, no. 17: 6152. https://doi.org/10.3390/ijerph17176152
APA StyleSzumilas, K., Szumilas, P., Grzywacz, A., & Wilk, A. (2020). The Effects of E-Cigarette Vapor Components on the Morphology and Function of the Male and Female Reproductive Systems: A Systematic Review. International Journal of Environmental Research and Public Health, 17(17), 6152. https://doi.org/10.3390/ijerph17176152




