Acute Myocardial Infarction among Young Adult Men in a Region with Warm Climate: Clinical Characteristics and Seasonal Distribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cases
2.2. Data Collection of Clinical Characteristics
2.3. Weather Data and Season
2.4. Statistical Analysis
3. Results
3.1. Comparison of Clinical Characteristics between the Young and the Older Groups
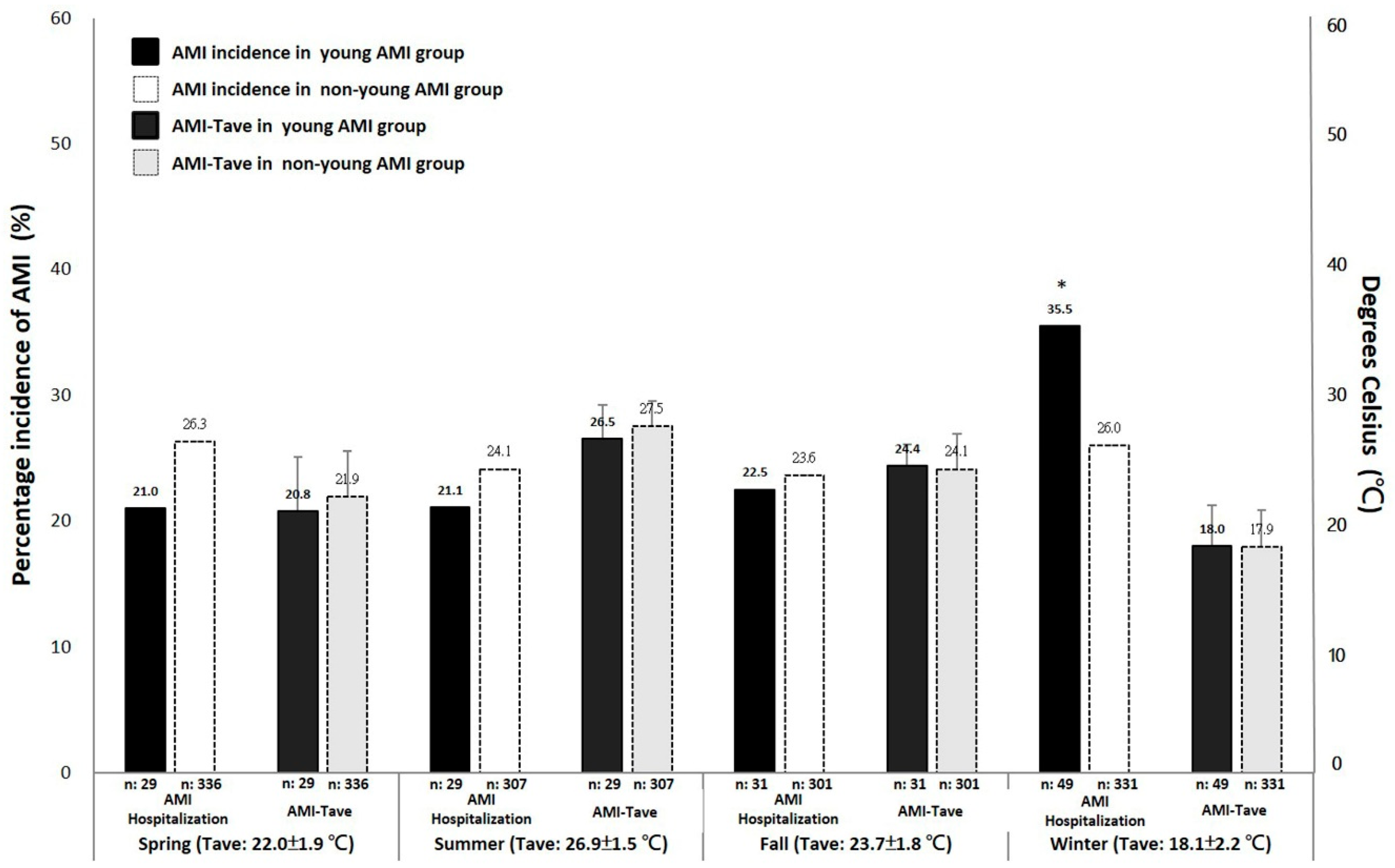
3.2. Comparison of AMI Hospitalizations between the Seasonal Distribution
3.3. Association of the Seasons with AMI Hospitalization among the Age Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2017, 15, 230–240. [Google Scholar] [CrossRef]
- Rubin, J.B.; Borden, W.B. Coronary Heart Disease in Young Adults. Curr. Atheroscler. Rep. 2012, 14, 140–149. [Google Scholar] [CrossRef]
- Chua, S.-K.; Hung, H.-F.; Shyu, K.-G.; Cheng, J.-J.; Chiu, C.-Z.; Chang, C.-M.; Lin, S.-C.; Liou, J.-Y.; Lo, H.-M.; Kuan, P.; et al. Acute ST-elevation Myocardial Infarction in Young Patients: 15 Years of Experience in a Single Center. Clin. Cardiol. 2010, 33, 140–148. [Google Scholar] [CrossRef]
- Leifheit-Limson, E.C.; D’Onofrio, G.; Daneshvar, M.; Geda, M.; Bueno, H.; Spertus, J.A.; Krumholz, H.M.; Lichtman, J.H. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients with Acute Myocardial Infarction: The VIRGO Study. J. Am. Coll. Cardiol. 2015, 66, 1949–1957. [Google Scholar] [CrossRef]
- Nishiyama, S.; Watanabe, T.; Arimoto, T.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; Nitobe, J.; Shibata, Y.; Konta, T.; et al. Trends in coronary risk factors among patients with acute myocardial infarction over the last decade: The Yamagata AMI registry. J. Atheroscler. Thromb. 2010, 17, 989–998. [Google Scholar] [CrossRef]
- Lee, C.H.; Fang, C.C.; Tsai, L.M.; Gan, S.T.; Lin, S.H.; Li, Y.H. Patterns of Acute Myocardial Infarction in Taiwan from 2009 to 2015. Am. J. Cardiol. 2018, 122, 1996–2004. [Google Scholar] [CrossRef]
- Tofler, G.; Muller, J.E. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation 2006, 114, 1863–1872. [Google Scholar] [CrossRef]
- Bhatnagar, A. Environmental Determinants of Cardiovascular Disease. Circ. Res. 2017, 121, 162–180. [Google Scholar] [CrossRef]
- Stewart, S.; Keates, A.K.; Redfern, A.; McMurray, J.J.V. Seasonal variations in cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 654–664. [Google Scholar] [CrossRef]
- Marti-Soler, H.; Gubelmann, C.; Aeschbacher, S.; Alves, L.; Bobak, M.; Bongard, V.; Clays, E.; De Gaetano, G.; Di Castelnuovo, A.; Elosua, R.; et al. Seasonality of cardiovascular risk factors: An analysis including over 230 000 participants in 15 countries. Heart 2014, 100, 1517–1523. [Google Scholar] [CrossRef]
- Shahar, D.; Yerushalmi, N.; Lubin, F.; Froom, P.; Shahar, A.; Kristal-Boneh, E. Seasonal variations in dietary intake affect the consistency of dietary assessment. Eur. J. Epidemiol. 2001, 17, 129–133. [Google Scholar] [CrossRef]
- Matthews, C.E.; Freedson, P.S.; Hebert, J.R.; Stanek, E.J.; Merriam, P.A.; Rosal, M.C.; Ebbeling, C.B.; Ockene, I.S. Seasonal variation in household, occupational, and leisure time physical activity: Longitudinal analyses from the seasonal variation of blood cholesterol study. Am. J. Epidemiol. 2001, 153, 172–183. [Google Scholar] [CrossRef]
- Lee, J.H.; Chae, S.C.; Yang, D.H.; Park, H.-S.; Cho, Y.; Jun, J.E.; Park, W.-H.; Kam, S.; Lee, W.K.; Kim, Y.-J.; et al. Influence of weather on daily hospital admissions for acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Int. J. Cardiol. 2010, 144, 16–21. [Google Scholar] [CrossRef]
- Heyer, H.E.; Teng, H.C.; Barris, W. The increased frequency of acute myocardial infarction during summer months in a warm climate; a study of 1,386 cases from Dallas, Texas. Am. Heart J. 1953, 45, 741–748. [Google Scholar] [CrossRef]
- Ku, C.-S.; Yang, C.-Y.; Lee, W.-J.; Chiang, H.-T.; Liu, C.-P.; Lin, S.-L. Absence of a seasonal variation in myocardial infarction onset in a region without temperature extremes. Cardiology 1998, 89, 277–282. [Google Scholar] [CrossRef]
- Lam, H.C.; Chan, J.C.; Luk, A.O.Y.; Chan, E.Y.Y.; Goggins, W. Short-term association between ambient temperature and acute myocardial infarction hospitalizations for diabetes mellitus patients: A time series study. PLoS Med. 2018, 15, e1002612. [Google Scholar] [CrossRef]
- Chu, M.-L.; Shih, C.-Y.; Hsieh, T.-C.; Chen, H.-L.; Lee, C.-W.; Hsieh, J.-C. Acute Myocardial Infarction Hospitalizations between Cold and Hot Seasons in an Island across Tropical and Subtropical Climate Zones-A Population-Based Study. Int. J. Environ. Res. Public Health 2019, 16, 2769. [Google Scholar] [CrossRef]
- Teo, K.K.; Ôunpuu, S.; Hawken, S.; Pandey, M.R.; Valentin, V.; Hunt, D.; Diaz, R.; Rashed, W.; Freeman, R.; Jiang, L.; et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: A case-control study. Lancet 2006, 368, 647–658. [Google Scholar] [CrossRef]
- Chen, C.-C.; Li, T.-C.; Chang, P.-C.; Liu, C.-S.; Lin, W.-Y.; Wu, M.-T.; Li, C.-I.; Lai, M.-M.; Lin, C.-C. Association among cigarette smoking, metabolic syndrome, and its individual components: The metabolic syndrome study in Taiwan. Metabolism 2008, 57, 544–548. [Google Scholar] [CrossRef]
- Slagter, S.N.; Van Vliet-Ostaptchouk, J.V.; Vonk, J.M.; Boezen, H.M.; Dullaart, R.P.F.; Kobold, A.C.M.; Feskens, E.J.; Van Beek, A.P.; Van Der Klauw, M.M.; Wolffenbuttel, B.H. Associations between smoking, components of metabolic syndrome and lipoprotein particle size. BMC Med. 2013, 11, 195. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143. [CrossRef]
- Choi, S.; Kim, K.; Kim, S.M.; Lee, G.; Jeong, S.-M.; Park, S.Y.; Kim, Y.-Y.; Son, J.S.; Yun, J.-M.; Park, S.M. Association of Obesity or Weight Change with Coronary Heart Disease Among Young Adults in South Korea. JAMA Intern. Med. 2018, 178, 1060–1068. [Google Scholar] [CrossRef]
- Danet, S.; Richard, F.; Montaye, M.; Beauchant, S.; Lemaire, B.; Graux, C.; Cottel, D.; Marécaux, N.; Amouyel, P. Unhealthy effects of atmospheric temperature and pressure on the occurrence of myocardial infarction and coronary deaths. A 10-year survey: The Lille-World Health Organization MONICA project (Monitoring trends and determinants in cardiovascular disease). Circulation 1999, 100, E1–E7. [Google Scholar] [CrossRef]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef]
- Ma, Y.; Olendzki, B.; Li, W.; Hafner, A.R.; Chiriboga, D.; Hebert, J.R.; Campbell, M.; Sarnie, M.; Ockene, I.S. Seasonal variation in food intake, physical activity, and body weight in a predominantly overweight population. Eur. J. Clin. Nutr. 2005, 60, 519–528. [Google Scholar] [CrossRef]
- McCormack, G.R.; Friedenreich, C.M.; Shiell, A.; Corti, B.; Doyle-Baker, P.K. Sex- and age-specific seasonal variations in physical activity among adults. J. Epidemiol. Community Health 2009, 64, 1010–1016. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Maahs, D.M.; Daniels, S.R.; Eckel, R.H. Childhood obesity and cardiovascular disease: Links and prevention strategies. Nat. Rev. Cardiol. 2011, 8, 513–525. [Google Scholar] [CrossRef]
- Bjerregaard, L.G.; Adelborg, K.; Baker, J.L. Change in body mass index from childhood onwards and risk of adult cardiovascular disease. Trends Cardiovasc. Med. 2020, 30, 39–45. [Google Scholar] [CrossRef]
- Magnus, K.; Matroos, A.; Strackee, J. Walking, cycling, or gardening, with or without seasonal interruption, in relation to acute coronary events. Am. J. Epidemiol. 1979, 110, 724–733. [Google Scholar] [CrossRef]
| The Young (n = 138) | The Older (n = 1275) | p Value | |
|---|---|---|---|
| Age (SD) | 39.1 (5.2) | 66.7 (11.1) | <0.001 * |
| CAG (%) | 128 (92.8) | 1093 (85.7) | 0.023 * |
| Number of stenosed vessels | 0.001 * | ||
| ≤1 (%) | 77 (60.2) | 489 (44.7) | |
| ≥2 (%) | 51 (39.8) | 604 (55.3) | |
| Conventional risk factors | |||
| Smoking (%) | 99 (71.7) | 651 (51.1) | <0.001 * |
| DM (%) | 28 (20.3) | 469 (36.8) | <0.001 * |
| Hypertension (%) | 52 (37.7) | 841 (66.0) | <0.001 * |
| T C (mg/dL) | 183.0(62.0) | 172.0(55.0) | 0.002 * |
| HDL-C (mg/dL) | 38.0(17.3) | 39.0(14.0) | 0.119 |
| TG (mg/dL) | 140.0(139.5) | 106.0(91.0) | <0.001 * |
| LDL-C (mg/dL) | 107.5(58.4) | 106.8(48.0) | 0.505 |
| BMI (kg/m2) | 26.8(5.4) | 24.7(4.8) | <0.001 * |
| Multivariate Logistic Regression | ||
|---|---|---|
| Main Predictor Variables | Adjusted Odd Ratio (OR) (95% CI) | p Value |
| Winter vs. Non-winter | 1.750 (1.151–2.259) | 0.009 * |
| Spring vs. Non-spring | 0.727 (0.450–1.174) | 0.129 |
| Summer vs. Non-summer | 0.685 (0.424–1.107) | 0.123 |
| Fall vs. Non-fall | 1.043 (0.656–1.660) | 0.857 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.-Y.; Chu, M.-L.; Hsieh, T.-C.; Chen, H.-L.; Lee, C.-W. Acute Myocardial Infarction among Young Adult Men in a Region with Warm Climate: Clinical Characteristics and Seasonal Distribution. Int. J. Environ. Res. Public Health 2020, 17, 6140. https://doi.org/10.3390/ijerph17176140
Shih C-Y, Chu M-L, Hsieh T-C, Chen H-L, Lee C-W. Acute Myocardial Infarction among Young Adult Men in a Region with Warm Climate: Clinical Characteristics and Seasonal Distribution. International Journal of Environmental Research and Public Health. 2020; 17(17):6140. https://doi.org/10.3390/ijerph17176140
Chicago/Turabian StyleShih, Chiao-Yu, Min-Liang Chu, Tsung-Cheng Hsieh, Han-Lin Chen, and Chih-Wei Lee. 2020. "Acute Myocardial Infarction among Young Adult Men in a Region with Warm Climate: Clinical Characteristics and Seasonal Distribution" International Journal of Environmental Research and Public Health 17, no. 17: 6140. https://doi.org/10.3390/ijerph17176140
APA StyleShih, C.-Y., Chu, M.-L., Hsieh, T.-C., Chen, H.-L., & Lee, C.-W. (2020). Acute Myocardial Infarction among Young Adult Men in a Region with Warm Climate: Clinical Characteristics and Seasonal Distribution. International Journal of Environmental Research and Public Health, 17(17), 6140. https://doi.org/10.3390/ijerph17176140





