Risk of Mitral Valve Prolapse in Patients with Keratoconus in Taiwan: A Population-Based Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Database
2.2. Selection of Patients and Variables
2.3. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Incidence Rates for MVP
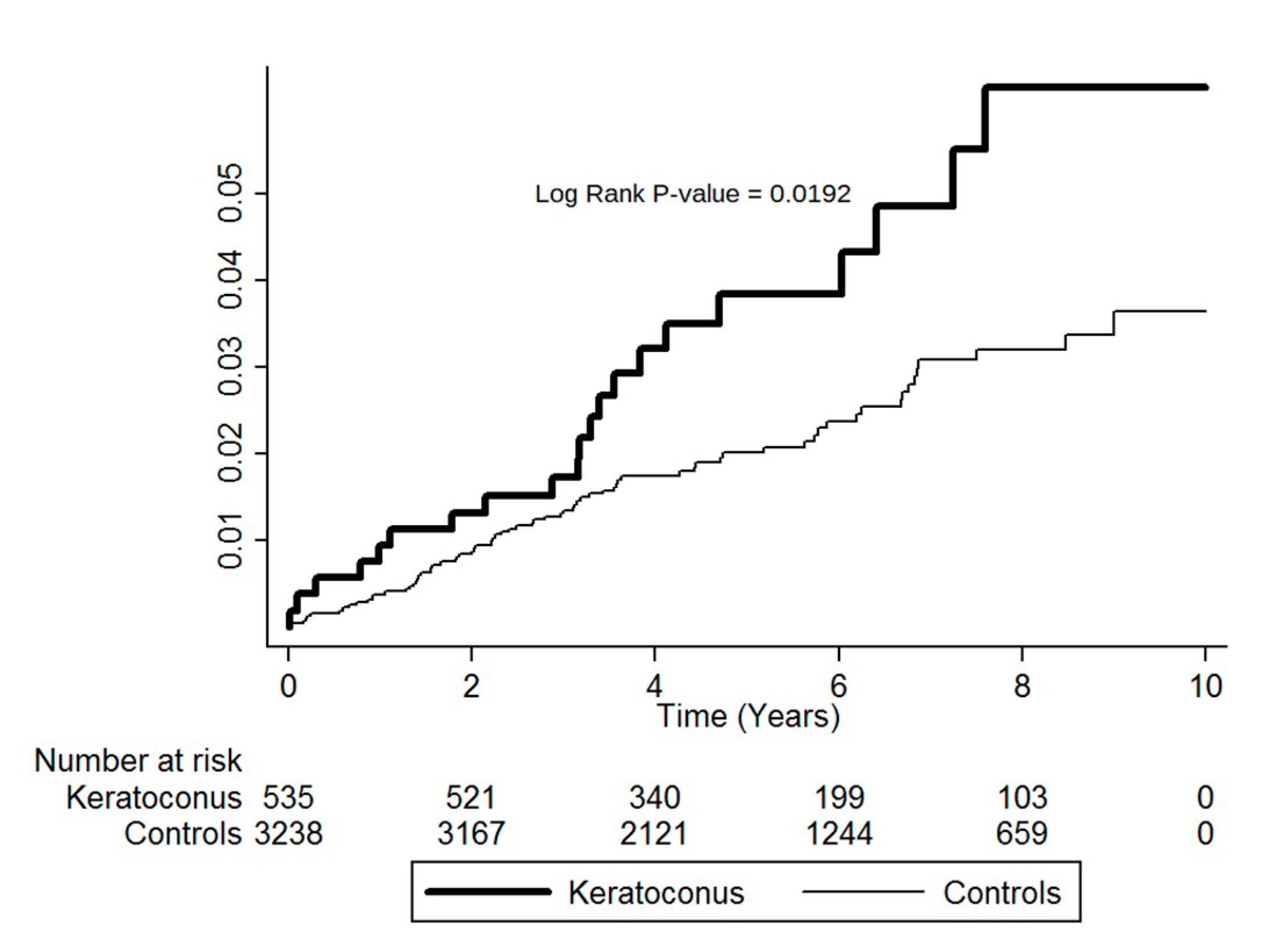
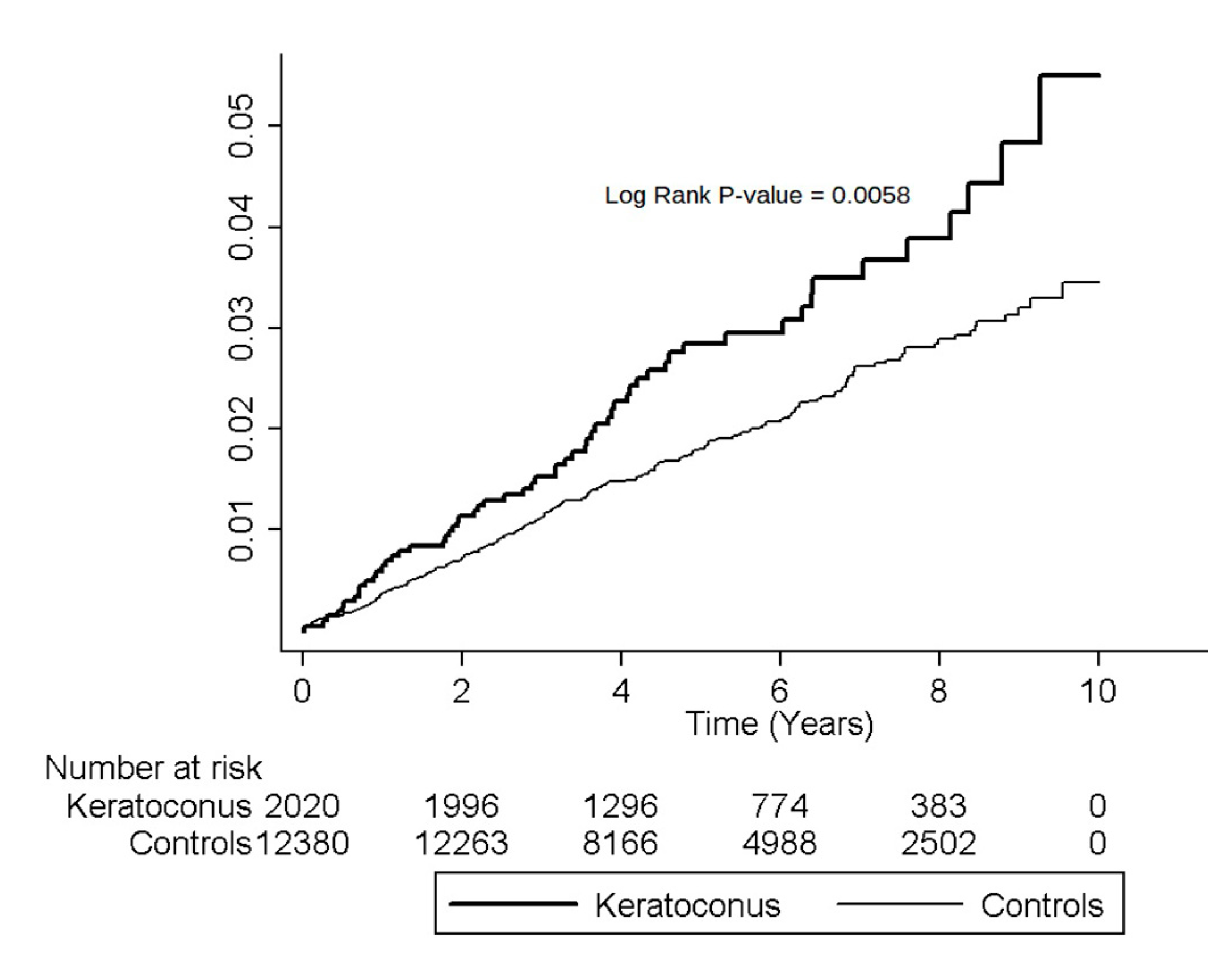
3.3. Cumulative Incidence Rates for MVP
3.4. Hazard Ratios for MVP
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Davidson, A.E.; Hayes, S.; Hardcastle, A.J.; Tuft, S.J. The pathogenesis of keratoconus. Eye 2014, 28, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas Tur, V.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar] [CrossRef] [PubMed]
- Zuppiroli, A.; Rinaldi, M.; Kramer-Fox, R.; Favilli, S.; Roman, M.J.; Devereux, R.B. Natural history of mitral valve prolapse. Am. J. Cardiol. 1995, 75, 1028–1032. [Google Scholar] [CrossRef]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Fuller, D.; Osypiuk, E.; Stantchev, P.; Hackman, B.; Manning, W.J.; Benjamin, E.J.; et al. Evolution of Mitral Valve Prolapse: Insights From the Framingham Heart Study. Circulation 2016, 133, 1688–1695. [Google Scholar] [CrossRef] [Green Version]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [Green Version]
- Devereux, R.B.; Kramer-Fox, R.; Shear, M.K.; Kligfield, P.; Pini, R.; Savage, D.D. Diagnosis and classification of severity of mitral valve prolapse: Methodologic, biologic, and prognostic considerations. Am. Heart J. 1987, 113, 1265–1280. [Google Scholar] [CrossRef]
- Luxereau, P.; Dorent, R.; De Gevigney, G.; Bruneval, P.; Chomette, G.; Delahaye, G. Aetiology of surgically treated mitral regurgitation. Eur. Heart J. 1991, 12, 2–4. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: The benign nature of echocardiographic features in the Framingham Heart Study. J. Am. College Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef] [Green Version]
- Bashey, R.I.; Bashey, H.M.; Jimenez, S.A. Characterization of pepsin-solubilized bovine heart-valve collagen. Biochem. J. 1978, 173, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.; Leier, C.V.; Baba, N.; Vasko, J.S.; Wooley, C.F.; Pinnell, S.R. Altered collagen composition in a prolapsing mitral valve with ruptured chordae tendineae. Am. J. Med. 1979, 67, 863–866. [Google Scholar] [CrossRef]
- Kalkan Akcay, E.; Akcay, M.; Uysal, B.S.; Kosekahya, P.; Aslan, A.N.; Caglayan, M.; Koseoglu, C.; Yulek, F.; Cagil, N. Impaired corneal biomechanical properties and the prevalence of keratoconus in mitral valve prolapse. J. Ophthalmol. 2014, 2014, 402193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devereux, R.B.; Perloff, J.K.; Reichek, N.; Josephson, M.E. Mitral valve prolapse. Circulation 1976, 54, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif, K.W.; Casey, T.A.; Coltart, J. Prevalence of mitral valve prolapse in keratoconus patients. J. R. Soci. Med. 1992, 85, 446–448. [Google Scholar]
- Rabbanikhah, Z.; Javadi, M.A.; Rostami, P.; Aghdaie, A.; Yaseri, M.; Yahyapour, F.; Katibeh, M. Association between acute corneal hydrops in patients with keratoconus and mitral valve prolapse. Cornea 2011, 30, 154–157. [Google Scholar] [CrossRef]
- Beardsley, T.L.; Foulks, G.N. An association of keratoconus and mitral valve prolapse. Ophthalmology 1982, 89, 35–37. [Google Scholar] [CrossRef]
- Street, D.A.; Vinokur, E.T.; Waring, G.O., 3rd; Pollak, S.J.; Clements, S.D.; Perkins, J.V. Lack of association between keratoconus, mitral valve prolapse, and joint hypermobility. Ophthalmology 1991, 98, 170–176. [Google Scholar] [CrossRef]
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zoller, B.; Sundquist, K.; Smith, J.G. Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef]
- Subki, A.H.; Bakhaidar, M.G.; Bakhaider, M.A.; Alkhowaiter, A.A.; Al-Harbi, R.S.; Almalki, M.A.; Alzahrani, K.A.; Fakeeh, M.M.; Subki, S.H.; Alhejily, W.A. Trends in mitral valve prolapse: A tertiary care center experience in Jeddah, Saudi Arabia. Int. J. Gen. Med. 2019, 12, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Belliveau, M.J.; Liao, W.N.; Brownstein, S.; Manusow, J.S.; Jordan, D.R.; Gilberg, S.; Mintsioulis, G. Myxomatous corneal degeneration: A clinicopathological study of six cases and a review of the literature. Surv. Ophthalmol. 2012, 57, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Bykhovskaya, Y.; Li, X.; Epifantseva, I.; Haritunians, T.; Siscovick, D.; Aldave, A.; Szczotka-Flynn, L.; Iyengar, S.K.; Taylor, K.D.; Rotter, J.I.; et al. Variation in the lysyl oxidase (LOX) gene is associated with keratoconus in family-based and case-control studies. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4152–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudakova, L.; Liskova, P.; Trojek, T.; Palos, M.; Kalasova, S.; Jirsova, K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Dudakova, L.; Jirsova, K. The impairment of lysyl oxidase in keratoconus and in keratoconus-associated disorders. J. Neural Transm. 2013, 120, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Laiquzzaman, M.; Bhojwani, R.; Mantry, S.; Cunliffe, I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3026–3031. [Google Scholar] [CrossRef] [Green Version]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Touboul, D.; Roberts, C.; Kerautret, J.; Garra, C.; Maurice-Tison, S.; Saubusse, E.; Colin, J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J. Cataract Refract. Surg. 2008, 34, 616–622. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Hickey, A.J. Lifetime risk for patients with mitral valve prolapse of developing severe valve regurgitation requiring surgery. Circulation 1988, 78, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A., Jr.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef]
- Devereux, R.B.; Brown, W.T.; Kramer-Fox, R.; Sachs, I. Inheritance of mitral valve prolapse: Effect of age and sex on gene expression. Ann. Intern. Med. 1982, 97, 826–832. [Google Scholar] [CrossRef]
- Nanna, M.; Stergiopoulos, K. Pregnancy complicated by valvular heart disease: An update. J. Am. Heart Assoc. 2014, 3, e000712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.M. Cardiac myxoma in pregnancy: A comprehensive review. Revista brasileira de cirurgia cardiovascular. Orgao Of. Soc. Bras. Cir. Cardiovasc. 2015, 30, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.M.; Yan, S.L. Mitral Valve Prolapse in Pregnancy. Braz. J. Cardiovasc. Surg. 2016, 31, 158–162. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Keratoconus (N = 4488) | Controls (N = 26,928) | p-Value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age | 27.38 ± 13.08 | 27.50 ± 12.88 | 0.5513 |
| Age group | |||
| 12–19 years | 1250 (27.85) | 7450 (27.67) | 0.9892 |
| 20–29 years | 1830 (40.78) | 11,024 (40.94) | |
| 30–39 years | 873 (19.45) | 5216 (19.37) | |
| ≥40 years | 535 (11.92) | 3238 (12.02) | |
| Gender | |||
| Male | 2468 (54.99) | 14,548 (54.03) | 0.2294 |
| Female | 2020 (45.01) | 12,380 (45.97) | |
| Baseline comorbidity | |||
| Hypertension | 145 (3.23) | 871 (3.23) | 0.9896 |
| Hyperlipidemia | 66 (1.47) | 403 (1.50) | 0.8942 |
| Congestive heart failure | 7 (0.16) | 30 (0.10) | 0.4203 |
| Characteristics | Keratoconus | Controls | IRR (95% CI) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | MVP | PYs | Rate a | N | MVP | PYs | Rate a | |||
| All | 4488 | 88 | 24,658 | 35.69 | 26,928 | 433 | 149,769 | 28.91 | 1.23 (0.98–1.55) | 0.0717 |
| Age | ||||||||||
| 12–19 years | 1250 | 17 | 6884 | 24.69 | 7450 | 115 | 42,431 | 27.10 | 0.91 (0.55–1.52) | 0.7202 |
| 20–29 years | 1830 | 36 | 10,353 | 34.77 | 11,024 | 174 | 62,462 | 27.86 | 1.25 (0.87–1.79) | 0.2259 |
| 30–39 years | 873 | 14 | 4555 | 30.74 | 5216 | 71 | 27,204 | 26.10 | 1.18 (0.66–2.09) | 0.5760 |
| ≥40 years | 535 | 21 | 2866 | 73.27 | 3238 | 73 | 17,672 | 41.31 | 1.77 (1.09–2.88) | 0.0206 |
| Gender | ||||||||||
| Male | 2468 | 29 | 13,790 | 21.03 | 14,548 | 185 | 81,775 | 22.62 | 0.93 (0.63–1.38) | 0.7146 |
| Female | 2020 | 59 | 10,868 | 54.29 | 12,380 | 248 | 67,994 | 36.47 | 1.49 (1.12–1.98) | 0.0060 |
| Comorbidity | ||||||||||
| Hypertension | 145 | 2 | 782 | 25.58 | 871 | 27 | 4708 | 57.35 | 0.45 (0.11–1.88) | 0.2705 |
| Hyperlipidemia | 66 | 1 | 310 | 32.26 | 403 | 5 | 2006 | 24.93 | 1.29 (0.15–11.07) | 0.8146 |
| Congestive heart failure | 7 | 0 | 26 | 0 | 30 | 2 | 155 | 129.03 | - | - |
| Cohort | Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|
| Keratoconus | ||
| Yes | 1.77 * (1.09–2.88) | 1.77 * (1.09–2.88) |
| No | 1.00 | 1.00 |
| Gender | ||
| Male | 1.00 | 1.00 |
| Female | 1.88 * (1.20–2.96) | 1.89 * (1.20–2.97) |
| Comorbidity | ||
| Hypertension | ||
| Yes | 1.15 (0.72–1.83) | 1.24 (0.76–2.03) |
| No | 1.00 | 1.00 |
| Hyperlipidemia | ||
| Yes | 0.64 (0.26–1.58) | 0.58 (0.23–1.47) |
| No | 1.00 | 1.00 |
| Congestive heart failure | ||
| Yes | 1.34 (0.19–9.58) | 1.26 (0.17–9.15) |
| No | 1.00 | 1.00 |
| Cohort | Crude Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|
| Keratoconus | ||
| Yes | 1.49 * (1.12–1.98) | 1.48 * (1.11–1.97) |
| No | 1.00 | 1.00 |
| Age | ||
| 12–19 years | 1.00 | 1.00 |
| 20–29 years | 1.33 (0.96–1.83) | 1.32 (0.96–1.81) |
| 30–39 years | 1.37 (0.96–1.94) | 1.35 (0.95–1.93) |
| ≥40 years | 1.98 * (1.39–2.82) | 1.85 * (1.26–2.71) |
| Comorbidity | ||
| Hypertension | ||
| Yes | 1.79 * (1.14–2.82) | 1.40 (0.82–2.30) |
| No | 1.00 | 1.00 |
| Hyperlipidemia | ||
| Yes | 0.91 (0.34–2.45) | 0.55 (0.19–1.54) |
| No | 1.00 | 1.00 |
| Congestive heart failure | ||
| Yes | 5.73 * (1.43–23.02) | 3.63 (0.88–15.00) |
| No | 1.00 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-S.; Tai, M.-C.; Weng, S.-F.; Wang, J.-J.; Tseng, S.-H.; Jan, R.-L. Risk of Mitral Valve Prolapse in Patients with Keratoconus in Taiwan: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 6049. https://doi.org/10.3390/ijerph17176049
Chang Y-S, Tai M-C, Weng S-F, Wang J-J, Tseng S-H, Jan R-L. Risk of Mitral Valve Prolapse in Patients with Keratoconus in Taiwan: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(17):6049. https://doi.org/10.3390/ijerph17176049
Chicago/Turabian StyleChang, Yuh-Shin, Ming-Cheng Tai, Shih-Feng Weng, Jhi-Joung Wang, Sung-Huei Tseng, and Ren-Long Jan. 2020. "Risk of Mitral Valve Prolapse in Patients with Keratoconus in Taiwan: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 17, no. 17: 6049. https://doi.org/10.3390/ijerph17176049






