Proposal of New Health Risk Assessment Method for Deficient Essential Elements in Drinking Water—Case Study of the Slovak Republic
Abstract
1. Introduction
2. Materials and Methods
2.1. Area Description
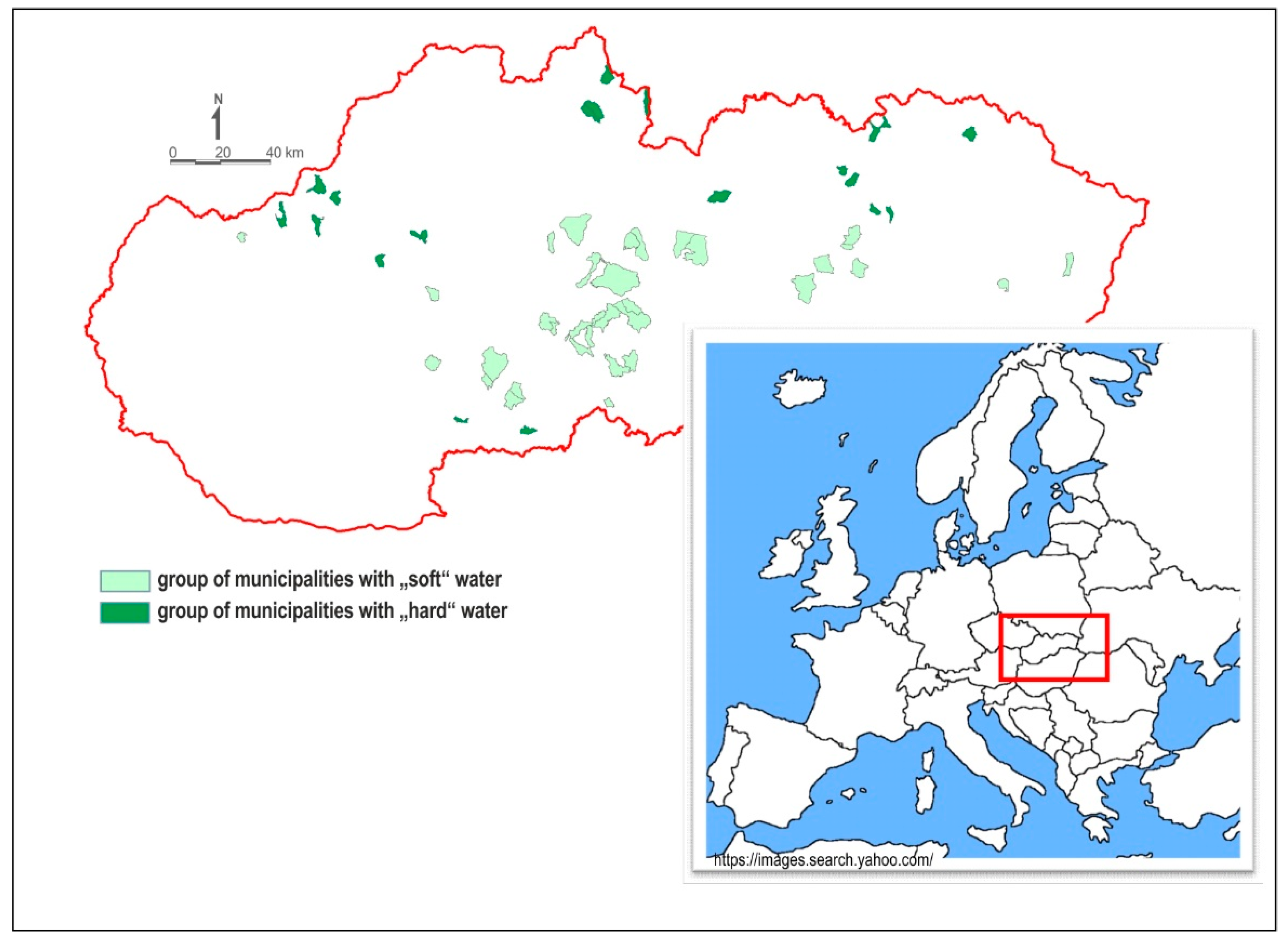
2.2. Selection of Municipalities
2.3. Characteristics of Ca, Mg Content and Water Hardness in Municipalities Supplied with Drinking Water of Different Hardness
2.4. Characteristics of the Health Status of the Population in Municipalities Supplied with Drinking Water of Different Hardness
2.5. Methodology of Hazard Quotient Calculation for Deficient Essential Elements
- Average Daily Required Dose (ADRD),
- Average Daily Accepted Dose (ADAD),
- Average Daily Missing Dose (ADMD).
2.5.1. Derivation of MRC Based on Standard Values
2.5.2. Derivation of MRC Based on Real Data
2.5.3. Calculation of Hazard Quotient—HQd
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- US EPA. Risk Assessment Guidance for Superfund (RAGS), Volume I: Human Health Evaluation Manual (HHEM), Part A-Baseline Risk Assessment, Interim Final; (EPA/540/1-89/002); United States Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 1989.
- US EPA. Exposure Factors Handbook I., II, III; (EPA/600/P-95/002Fa); United States Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment: Washington, DC, USA, 1997.
- US EPA. Risk Assessment Guidance for Superfund (RAGS), Volume I: Human Health Evaluation Manual (HHEM), Part E-Supplemental Guidance for Dermal Risk Assessment, Final; United States Environmental Protection Agency, Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2004.
- US EPA. Guidelines for Carcinogen Risk Assessment; (EPA/630/P-03/001F); United States Environmental Protection Agency, Risk Assessment Forum: Washington, DC, USA, 2005.
- US EPA. Child-Specific Exposure Factors Handbook; (EPA/600/R-06/096F); United States Environmental Protection Agency, National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2008.
- US EPA. Risk Assessment Guidance for Superfund (RAGS), Volume I: Human Health Evaluation Manual (HHEM), Part F-Supplemental Guidance for Inhalation Risk Assessment, Final; (EPA-540-R-070-002); United States Environmental Protection Agency, Office of Superfund Remediation and Technology Innovation: Washington, DC, USA, 2009.
- Commission Regulation (EC) No 1488/94 of 28 June 1994 Laying down the Principles for the Assessment of Risk to Man and the Environment of Existing Substances in Accordance with Council Regulation (EEC) No 793/93 (Text with EEA Relevance). Official Journal No L 161, 29/06/1994:0003-0011. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31994R1488:EN:HTML (accessed on 13 March 2020).
- Commission Directive 93/67/EEC Lying down the Principles for Assessment of Risks to Man and the Environment of Substances Notified in Accordance with Council Directive 67/548/EEC. Official Journal L 227, 0009-0018. Available online: https://op.europa.eu/en/publication-detail/-/publication/1b5257d9-fe8c-4071-9c79-d0b9a8079b8b (accessed on 13 March 2020).
- Methodical Instruction of the Ministry of the Environment of the Slovak Republic No. 623/98-2 on the Risk Assessment and Management Procedure. Bulletin of the Ministry of the Environment of the Slovak Republic, VI, 6, 51–71. Available online: https://www.enviroportal.sk/uploads/files/EZ/arsmernicafinal.pdf (accessed on 20 January 2020). (In Slovak).
- Methodical Instruction of the Ministry of the Environment of the Czech Republic No. 12/2005 for Risk Analysis of Contaminated Areas + 7 annexes, Bulletin of the Ministry of the Environment-Issue 9/9/2005. Available online: https://www.mzp.cz/C1257458002F0DC7/cz/metodiky_ekologicke_zateze/$FILE/Met%20pokyn%2013.pdf (accessed on 20 January 2020). (In Czech).
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Fuge, R.; Johnson, C.C. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Arredondo, M.; González, M.; Latorre, M. Copper. In Trace Elements and Minerals in Health and Longevity. Healthy Ageing and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 8, pp. 35–62. [Google Scholar] [CrossRef]
- Catling, L.A.; Abubakar, I.; Lake, I.R.; Swift, L.; Hunter, P.R. A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J. Water Health 2008, 6, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Rapant, S.; Cvečková, V.; Fajčíková, K.; Sedláková, D.; Stehlíková, B. Impact of calcium and magnesium in groundwater and drinking water on the health of inhabitants of the Slovak Republic. Int. J. Environ. Res. Public Health 2017, 14, 278. [Google Scholar] [CrossRef]
- Rosborg, I.; Kožíšek, F. Drinking Water Mineral and Balance. Importance, Health Significance, Safety Precautions, 2nd ed.; Springer: Cham, Switzerland, 2020; p. 175. [Google Scholar] [CrossRef]
- Tam, M.; Gómez, S.; Gonzáles-Gross, M.; Marcos, A. Possible roles of magnesium on the immune system. Eur. J. Clin. Nutr. 2003, 57, 1193–1197. [Google Scholar] [CrossRef]
- Schwarz, E.C.; Qu, B.; Hoth, M. Calcium, cancer and killing: The role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim. Biophys. Acta 2013, 1833, 1603–1611. [Google Scholar] [CrossRef]
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018, 2018, 9041694. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, E. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Rapant, S.; Cvečková, V.; Dietzová, Z.; Fajčíková, K.; Hiller, E.; Finkelman, R.B.; Škultétyová, S. The potential impact of geological environment on health status of residents of the Slovak Republic. Environ. Geochem. Health 2014, 36, 543–561. [Google Scholar] [CrossRef]
- Fajčíková, K.; Stehlíková, B.; Cvečková, V.; Rapant, S. Application of artificial neural network in medical geochemistry. Environ. Geochem. Health 2017, 39, 1513–1529. [Google Scholar] [CrossRef] [PubMed]
- Kondratyuk, V.A. On the health significance of microelements in low-mineral water. Gig. Sanit. 1989, 68, 81–82. (In Russian) [Google Scholar]
- WHO. Guidelines on Health Aspects of Water Desalination; ETS/80.4; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Geological Map of the Slovak Republic, 1:50 000. Available online: http://www.geology.sk (accessed on 20 February 2020).
- Rapant, S.; Vrana, K.; Bodiš, D. Geochemical Atlas of Slovakia-Part I. Groundwater; Geological Survey of Slovak Republic: Bratislava, Slovakia, 1996; p. 127. (In Slovak) [Google Scholar]
- Life–Water and Health. Available online: http://fns.uniba.sk/lifewaterhealth/ (accessed on 23 November 2019).
- Rapant, S.; Letkovičová, M.; Cvečková, V.; Fajčíková, K.; Galbavý, J.; Letkovič, M. Environmental and Health Indicators of the Slovak Republic; State Geological Institute of Dionyz Stur: Bratislava, Slovakia, 2010; p. 245. (In Slovak) [Google Scholar]
- Geohealth. Available online: http://www.geology.sk/geohealth (accessed on 15 April 2020).
- Decree of the Ministry of Health of the Slovak Republic No. 247/2017 Coll. Slovak Standard Values for Drinking Water. Available online: http://www.zakonypreludi.sk/zz/2017-247 (accessed on 28 April 2020). (In Slovak).
- Statistical Office of the Slovak Republic. Available online: https://slovak.statistics.sk (accessed on 27 April 2020).
- International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10)-WHO. Available online: https://icd.who.int/browse10/2016/en#/IX (accessed on 28 April 2020).
- Bonita, R.; Beaglehole, R.; Kjellstrom, T. Basic Epidemiology, 2nd ed.; World Health Organization: Geneva, Switzerland, 2006; pp. 15–38. Available online: https://apps.who.int/iris/bitstream/handle/10665/43541/9241547073_eng.pdf?sequence=1&isAllowed=y (accessed on 22 February 2020).
- Jenicek, M. Epidemiology. The Logic of Modern Medicine; Epimed: Montreal, QC, Canada, 1995; p. 322. [Google Scholar]
- Last, J.M. A Dictionary of Epidemiology, 4th ed.; Oxford University Press: New York, NY, USA, 2001; p. 219. [Google Scholar]
- Klinda, J.; Lieskovská, Z. State of the Environment Report of the Slovak Republic; Ministry of Environment of the Slovak Republic: Bratislava, Slovakia, 2010; p. 192.
- US EPA. Exposure Factors Handbook; (EPA/600/8-89/43); Office of Health and Environmental Assessment: Washington, DC, USA, 1989.
- NHIC. Health Statistics Year Book of the Slovak Republic 2013; National Health Information Center: Bratislava, Slovakia, 2013; p. 241. [Google Scholar]
- Jiang, L.; He, P.; Chen, J.; Liu, Y.; Liu, D.; Qin, G.; Tan, N. Magnesium levels in drinking water and coronary heart disease mortality risk: A meta-analysis. Nutrients 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Bragazzi, N.L.; Nucci, D.; Villarini, M.; Moretti, M. Cardiovascular diseases and hard drinking waters: Implications from a systematic review with meta-analysis of case-control studies. J. Water Health 2017, 15, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Nutrient Recommendations: Dietary Reference Intakes (DRIs). 2018. Available online: https://ods.od.nih.gov/Health_Information/Dietary_Reference_Intakes.aspx (accessed on 4 March 2020).
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural mineral waters: Chemical characteristics and health effects. Clin. Cases Miner. Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Halpern, G.M.; Van de Water, J.; Delabroise, A.M.; Keen, C.L.; Gershwin, M.E. Comparative uptake of calcium from milk and a calcium-rich mineral water in lactose intolerant adults: Implications for treatment of osteoporosis. Am. J. Prev. Med. 1991, 7, 379–383. [Google Scholar] [CrossRef]
- Van Dokkum, W.; de la Gueronniere, V.; Schaafsma, G.; Bouley, C.; Luten, J.; Latgé, C. Bioavailability of calcium of fresh cheeses, enteral food and mineral water: A study with stable calcium isotopes in young adult women. Br. J. Nutr. 1996, 75, 893–903. [Google Scholar] [CrossRef]
- Wynckel, A.; Hanrotel, C.; Wuillai, C.H.A.; Charnard, J. Intestinal calcium absorption from mineral water. Miner. Electrolyte Metab. 1997, 23, 88–92. [Google Scholar]
- Bacciottini, L.; Tanini, A.; Falchetti, A.; Masi, L.; Franceschelli, F.; Pampaloni, B.; Guanluca, G.; Brandi, M.L. Calcium bioavailability from a calcium-rich mineral water, with some observations on method. J. Clin. Gastroenterol. 2001, 38, 761–766. [Google Scholar] [CrossRef]
- Brandolini, M.; Guéguen, L.; Boirie, Y.; Rousset, P.; Bertière, M.C.; Beaufrère, B. Higher calcium urinary loss induced by a calcium sulphate-rich mineral water intake than by milk in young women. Br. J. Nutr. 2005, 93, 225–231. [Google Scholar] [CrossRef]
- Greupner, T.; Schneider, I.; Hahn, A. Calcium bioavailability from mineral waters with different mineralization in comparison to milk and a supplement. J. Am. Coll. Nutr. 2017, 36, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Altura, B.M.; Li, W.; Zhang, A.; Zheng, T.; Shah, N.C. Sudden cardiac death in infants, children and young adults: Possible roles of dietary magnesium intake and generation of platelet-activating factor in coronary arteries. J. Heart Health 2016, 2, 1–5. [Google Scholar] [CrossRef]
- Costello, R.B.; Elin, R.J.; Rosanoff, A.; Wallace, T.C.; Guerrero-Romero, F.; Hruby, A.; Lutsey, P.L.; Nielsen, F.H.; Rodriguez-Moran, M.; Song, Y.; et al. Perspective: The case for an evidence-based reference interval for serum magnesium: The time has come. Adv. Nutr. 2016, 7, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Food and Supplement Intake Data. What We Eat in America, NHANES 2011–2012. 2018. Available online: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1112/Table_37_SUP_GEN_11.pdf (accessed on 27 April 2020).
- Workinger, J.L.; Doyle, R.P.; Bortz, J. Challenges in the diagnosis of magnesium status. Nutrients 2018, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar]
- Barbagallo, M.; Dominguez, L.J. Magnesium Role in Health and Longevity. In Trace Elements and Minerals in Health and Longevity. Healthy Ageing and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 8, pp. 235–264. [Google Scholar] [CrossRef]
- Rapant, S.; Cvečková, V.; Fajčíková, K.; Hajdúk, I.; Hiller, E.; Stehlíková, B. Hard water, more elastic arteries: A case study from Krupina district, Slovakia. Int. J. Environ. Res. Public Health 2019, 16, 1521. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chiu, H.F.; Chiu, J.F.; Tsai, S.S.; Cheng, M.F. Calcium and magnesium in drinking water and risk of death from colon cancer. Jpn. J. Cancer Res. 1997, 88, 928–933. [Google Scholar] [CrossRef]
- Chiu, H.F.; Chang, C.C.; Yang, C.Y. Magnesium and calcium in drinking water and risk of death from ovarian cancer. Magnes. Res. 2004, 17, 28–34. [Google Scholar]
- Castiglioni, S.; Maier, J.A.M. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, S92–S100. [Google Scholar] [CrossRef]
- Kozisek, F. Regulatory Aspects of Arsenic in Drinking Water. In Best Practice Guide on the Control of Arsenic in Drinking Water; Bhattacharya, P., Polya, D.A., Jovanonic, D., Eds.; IWA Publishing: London, UK, 2017; pp. 39–48. [Google Scholar]
- Council of the European Union. Proposal for a Directive of the European Parliament and of the Council on the Quality of Water Intended for Human Consumption (recast). Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:8c5065b2-074f-11e8-b8f5-01aa75ed71a1.0016.02/DOC_1&format=PDF (accessed on 2 May 2020).
- Kozisek, F. Regulations for calcium, magnesium or hardness in drinking water in the European Union member states. Reg. Toxicol. Pharmacol. 2020, 112, 104589. [Google Scholar] [CrossRef]
- Haring, B.S.A.; Van Delft, W. Changes in the mineral composition of food as a result of cooking in “hard” and “soft” waters. Arch. Environ. Health 1981, 36, 33–35. [Google Scholar] [CrossRef] [PubMed]
| Group of Municipalities | Ca | Mg | (Ca + Mg) | |
|---|---|---|---|---|
| mg·L−1 | mg·L−1 | mmol·L−1 | ||
| “soft” water | Average | 21.2 | 5.66 | 0.77 |
| Minimum | 5.40 | 2.00 | 0.26 | |
| Maximum | 48.9 | 16.8 | 1.69 | |
| Median | 20.7 | 4.45 | 0.75 | |
| “hard” water | Average | 70.1 | 26.4 | 2.84 |
| Minimum | 38.5 | 12.6 | 1.58 | |
| Maximum | 96.8 | 38.0 | 3.84 | |
| Median | 73.7 | 27.6 | 2.75 |
| Group of Municipalities | LE 1 | ReC 2 | ReI 3 | ReJ 4 | ReK 5 |
|---|---|---|---|---|---|
| “soft” water | 69.40 | 282.53 | 852.42 | 100.92 | 82.65 |
| “hard” water | 74.68 | 171.11 | 455.11 | 45.37 | 34.27 |
| Slovak Republic | 72.60 | 212.79 | 531.05 | 58.08 | 45.83 |
| Parameter | Value | Unit | Source |
|---|---|---|---|
| BW—body weight | 70 | kg | [2] |
| AT—averaging exposure time | 365 | day | [38] |
| CW—content of chemical element in water | site specific | mg·L−1 | |
| IR—daily water intake | 2 | L·day−1 | [38] |
| EF—exposure frequency | 365 | day·year−1 | [1] |
| ED—duration of exposure | 1 | year | [38] |
| Calcium | ||||||
|---|---|---|---|---|---|---|
| Mean “soft” water | CaReC 1 | CaReI | CaReJ | CaReK | Ca | |
| LV 2 | MRC 3 | |||||
| 28.15 | 34.03 | 36.84 | 38.23 | 31.21 | 62.42 | |
| Magnesium | ||||||
| Mean “soft” water | MgReC | MgReI | MgReJ | MgReK | Mg | |
| LV | MRC | |||||
| 7.52 | 9.09 | 9.83 | 10.21 | 8.33 | 16.66 | |
| Water Hardness | ||||||
| Mean “soft” water | (Ca + Mg)ReC | (Ca + Mg)ReI | (Ca + Mg)ReJ | (Ca + Mg)ReK | (Ca + Mg) | |
| LV | MRC | |||||
| 1.02 | 1.24 | 1.34 | 1.39 | 1.13 | 2.26 | |
| Risk Level | HQd | Risk of Chronic Diseases |
|---|---|---|
| 1 | ≤0.1 | Without risk |
| 2 | >0.1 | Low |
| 3 | >1.0 | Medium |
| 4 | >4.0 | High |
| ADADMg 1 | ADADCa | ADAD(Ca + Mg) | HQdMg 2 | HQdCa | HQd(Ca + Mg) | |
|---|---|---|---|---|---|---|
| “soft” water group | ||||||
| Average | 0.162 | 0.606 | 0.022 | 2.94 | 2.94 | 2.95 |
| Median | 0.127 | 0.591 | 0.021 | 3.75 | 3.01 | 3.01 |
| Maximum | 0.480 | 1.397 | 0.048 | 8.33 | 11.6 | 8.69 |
| Minimum | 0.057 | 0.154 | 0.007 | 0.99 | 1.28 | 1.34 |
| “hard” water group | ||||||
| Average | 0.754 | 2.003 | 0.081 | 0.63 | 0.89 | 0.80 |
| Median | 0.789 | 2.106 | 0.079 | 0.60 | 0.85 | 0.82 |
| Maximum | 1.086 | 2.766 | 0.110 | 1.32 | 1.62 | 1.43 |
| Minimum | 0.360 | 1.100 | 0.045 | 0.44 | 0.64 | 0.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapant, S.; Cvečková, V.; Hiller, E.; Jurkovičová, D.; Kožíšek, F.; Stehlíková, B. Proposal of New Health Risk Assessment Method for Deficient Essential Elements in Drinking Water—Case Study of the Slovak Republic. Int. J. Environ. Res. Public Health 2020, 17, 5915. https://doi.org/10.3390/ijerph17165915
Rapant S, Cvečková V, Hiller E, Jurkovičová D, Kožíšek F, Stehlíková B. Proposal of New Health Risk Assessment Method for Deficient Essential Elements in Drinking Water—Case Study of the Slovak Republic. International Journal of Environmental Research and Public Health. 2020; 17(16):5915. https://doi.org/10.3390/ijerph17165915
Chicago/Turabian StyleRapant, Stanislav, Veronika Cvečková, Edgar Hiller, Dana Jurkovičová, František Kožíšek, and Beáta Stehlíková. 2020. "Proposal of New Health Risk Assessment Method for Deficient Essential Elements in Drinking Water—Case Study of the Slovak Republic" International Journal of Environmental Research and Public Health 17, no. 16: 5915. https://doi.org/10.3390/ijerph17165915
APA StyleRapant, S., Cvečková, V., Hiller, E., Jurkovičová, D., Kožíšek, F., & Stehlíková, B. (2020). Proposal of New Health Risk Assessment Method for Deficient Essential Elements in Drinking Water—Case Study of the Slovak Republic. International Journal of Environmental Research and Public Health, 17(16), 5915. https://doi.org/10.3390/ijerph17165915






