Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers?
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Active Aerobic Exercise Program
2.3. Body Composition and Function Measurements
2.4. Blood Collection and Biochemical Analysis
2.5. Analysis of Pro-Inflammatory and Anti-Inflammatory Markers
2.6. Calculation of the 10-Year Risk of Heart Disease
2.7. Statistical Analysis
3. Results
3.1. Body Composition
3.2. CVD Risk Factors
3.3. Physical Fitness
3.4. Pro-Inflammatory and Anti-Inflammatory Markers
3.5. Estimation of 10-Year Risk of Coronary Heart Disease
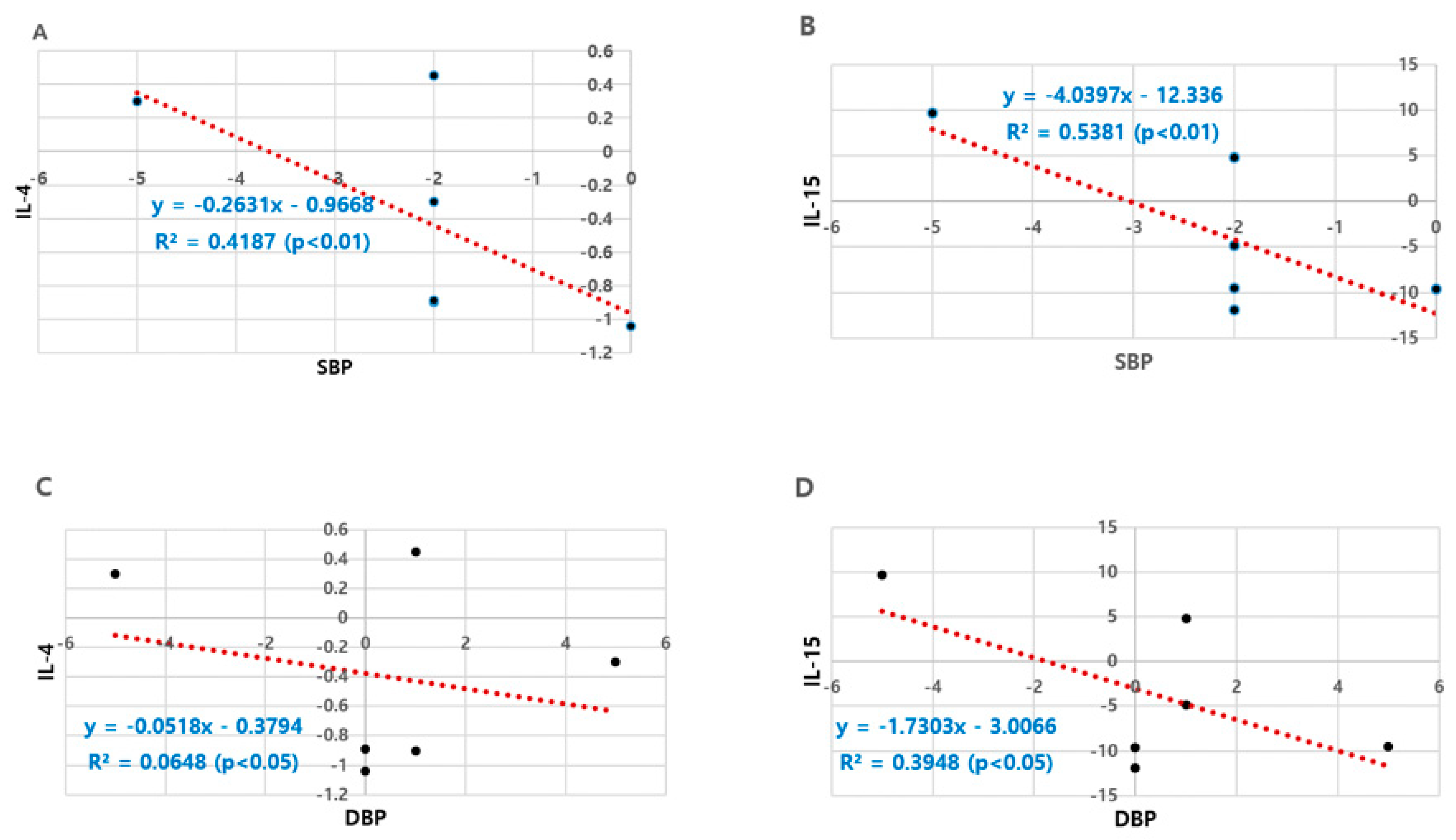
3.6. Correlations between Blood Pressure and Anti-Inflammation Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Rohani, H.A.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the global burden of disease study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. National high blood pressure education program coordinationg committee. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Stevens-Fabry, S. Prehypertension–prevalence, health risks, and management strategies. Nat. Rev. Cardiol. 2015, 12, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Park, H.S.; Han, J.H.; Lee, J.S.; Lee, J.; Ryu, O.H.; Lee, K.W.; Cho, K.H.; Yoon, D.; Bail, S.H.; et al. Prevalence of prehypertension and hypertension in a Korean population: Korean national health and nutrition survey 2001. J. Hypertens. 2006, 24, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Board of the E-mWGoERAE. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Speer, T.; Owala, F.O.; Holy, E.W.; Zewinger, S.; Frenzel, F.L.; Stahli, B.E.; Razavi, M.; Triem, S.; Cvija, H.; Rohrer, L.; et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart. J. 2014, 35, 3021–3032. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; de Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar]
- Riwanto, M.; Landmesser, U. High density lipoproteins and endothelial functions: Mechanistic insights and alterations in cardiovascular disease. J. Lipid Res. 2013, 54, 3227–3243. [Google Scholar] [CrossRef]
- Coetzee, G.A.; Strachan, A.F.; van der Westhuyzen, D.R.; Hoppe, H.C.; Jeenah, M.S.; de Beer, F.C. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem. 1986, 261, 9644–9651. [Google Scholar]
- Kopecky, C.; Genser, B.; Drechsler, C.; Krane, V.; Kaltenecker, C.C.; Hengstschläger, M.; März, W.; Wanner, C.; Säemann, M.D.M.; Weichhart, T. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin. J. Am. Soc. Nephrol. 2015, 10, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Keeney, J.T.; Swomley, A.M.; Forster, S.; Harris, J.L.; Sultana, R.; Butterfield, D.A. Apolipoprotein A-Ⅰ: Insights from redox proteomics for its role in neurodegeneration. Proteom. Clin. Appl. 2013, 7, 109–122. [Google Scholar] [CrossRef]
- Yazdani, R.; Marefati, H.; Shahesmaeili, A.; Nakhaei, S.; Bagheri, A.; Dastoorpoor, M. Effect of aerobic exercises on serum levels of apolipoprotein A1 and apolipoprotein B, and their ratio in patients with chronic obstructive pulmonary disease. Tanaffos 2018, 17, 82–89. [Google Scholar] [PubMed]
- Agrawal, A.; Hammond, D.J.; Singh, S.K. Atherosclerosis-related functions of C-reactive protein. Cardiovasc. Hematol. Disord. Drug Targets 2010, 10, 235–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- DeSouza, C.A.; Dengel, D.R.; Macko, R.F.; Cox, K.; Seals, D.R. Elevated levels of circulation cell adhesion molecule in uncomplicated essential hypertension. Am. J. Hypertens. 1997, 10, 1335–1341. [Google Scholar] [CrossRef]
- de Faria, A.P.; Ritter, A.M.; Sabbatini, A.R.; Corrêa, N.B.; Brunelli, V.; Modolo, R.; Moreno, H. Deregulation of soluble adhesion molecules in resistant hypertension and its role in cardiovascular remodeling. Circ. J. 2016, 80, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, S.; Panes, J.; Russell, J.M.; Anderson, D.C.; Muzykantov, V.R.; Miyasaka, M.; Granger, D.N. Effects of chronic arterial hypertension on constitutive and induced intercellular adhesion molecule-1 expression in vivo. Hypertension 1997, 29, 683–689. [Google Scholar] [CrossRef]
- Ridker, P.M.; Buring, J.E.; Cook, N.R.; Rifai, N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14719 initially healthy American women. Circulation 2003, 107, 391–397. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Roitman-Johnson, B.; Buring, J.E.; Grodstein, F. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998, 351, 88–92. [Google Scholar] [CrossRef]
- Faselis, C.; Doumas, M.; Kokkinos, J.P.; Panagiotakos, D.; Kheirbek, R.; Sheriff, H.M.; Hare, K.; Papademetriou, V.; Fletcher, R.; Kokkinos, P. Exercise capacity and progression from prehypertension to hypertension. Hypertension 2012, 60, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Faselis, C.; Doumas, M.; Pittaras, A.; Narayan, P.; Myers, J.; Tsimploulis, A.; Kokkinos, P. Exercise capacity and all-cause mortality in male veterans with hypertension aged ≥70 years. Hypertension 2014, 64, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, P.; Manolis, A.; Pittaras, A.; Doumas, M.; Giannelou, A.; Panagiotakos, D.B.; Faselis, C.; Narayan, P.; Singh, S.; Myers, J. Exercise capacity and mortality in hypertensive men with and without additional risk factors. Hypertension 2009, 53, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Galeano, H.; Garatachea, N.; Lucia, A. Exercise as a polypill for chronic diseases. Prog. Mol. Biol. Transl. Sci. 2015, 135, 497–526. [Google Scholar]
- Huang, G.; Shi, X.; Gibson, C.A.; Huang, S.C.; Coudret, N.A.; Ehlman, M.C. Controlled aerobic exercise training reduces resting blood pressure in sedentary older adults. Blood Press. 2013, 22, 386–394. [Google Scholar] [CrossRef]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am. J. Hypertens. 2013, 26, 1093–1102. [Google Scholar] [CrossRef]
- Barlow, C.E.; LaMonte, M.J.; Fitzgerald, S.J.; Kampert, J.B.; Perrin, J.L.; Blair, S.N. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am. J. Epidemiol. 2006, 163, 142–150. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef]
- Jung, M.H.; Ihm, S.H.; Lee, D.H.; Chung, W.B.; Jung, H.O.; Youn, H.J. Prehypertension is associated with early complications of atherosclerosis but not with exercise capacity. Int. J. Cardiol. 2017, 227, 387–392. [Google Scholar] [CrossRef]
- Müller, J.; Meyer, J.; Elmenhorst, J.; Oberhoffer, R. Body weight and not exercise capacity determines central systolic blood pressure, a surrogate for arterial stiffness, in children and adolescents. J. Clin. Hypertens. 2015, 18, 762–765. [Google Scholar] [CrossRef]
- Green, D.J.; Eijsvogels, T.; Bouts, Y.M.; Maiorana, A.J.; Naylor, L.H.; Scholten, R.R.; Spaanderman, M.E.A.; Pugh, C.J.A.; Sprung, V.S.; Schreuder, T.; et al. Exercise training and artery function in humans: Nonresponse and its relationship to cardiovascular risk factors. J. Appl. Physiol. 2014, 117, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sponder, M.; Kopecky, C.; Campean, I.A.; Emich, M.; Fritzer-Szekeres, M.; Litschauer, B.; Graf, S.; Säemann, M.D.; Strametz-Juranek, J. Sports and HDL-quality reflected by serum amyloid A and surfactant protein B. Int. J. Med. Sci. 2017, 14, 1040–1048. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niku, K.J.; Oksala, E.; David, E.; Laaksonen, J.; Savita, L.; Nakao, C.; Hänninen, O.; Sen, C.K.; Atalay, M. Heat shock protein 60 response to exercise in diabetes effects of a-lipoic acid supplementation. J. Diabetes Its Complicat. 2006, 20, 257–261. [Google Scholar]
- Schmitt, E.; Parcellier, A.; Gurbuxani, S.; Cande, C.; Hammann, A.; Morales, M.C.; Hunt, C.R.; Dix, D.J.; Kroemer, R.T.; Giodanetto, F.; et al. Chemosensitization by a non-apoptogenic heat shock protein 70-binding apoptosis-inducing factor mutant. Cancer Res. 2003, 63, 8233–8240. [Google Scholar]
- Rajdev, S.; Hara, K.; Kokubo, Y.; Mestril, R.; Dillmann, W.; Weinstein, P.R.; Sharp, F.R. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann. Neurol. 2000, 47, 782–791. [Google Scholar] [CrossRef]
- Moran, M.; Delgado, J.; Gonzalez, B.; Manso, R.; Megias, A. Responses of rat myocardial antioxidant defences and heat shock protein HSP72 induced by 12 and 24-week treadmill training. Acta Physiol. Scand. 2004, 180, 157–166. [Google Scholar] [CrossRef]
- Durrant, J.R.; Seals, D.R.; Connell, M.L.; Russell, M.J.; Lawson, B.R.; Folian, B.J.; Donato, A.J.; Lesniewski, L.A. Voluntary wheel running restores endothelial function in conduit arteries of old mice: Direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J. Physiol. 2009, 587, 3271–3285. [Google Scholar] [CrossRef]
- Trott, D.W.; Gunduz, F.; Laughlin, M.H.; Woodman, C.R. Exercise training reverses age-related decrements in endothelium-dependent dilation in skeletal muscle feed arteries. J. Appl. Physiol. 2009, 106, 1925–1934. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales; Human Kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Rikli, R.; Jones, C. Senior Fitness Test Manual; Human Kinetics: Champaign, IL, USA, 2013. [Google Scholar]
- Mattews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985, 28, 412–429. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’ Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart diseases using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Park, Y.; Blair, A.; Ballard-Barbash, R.; Mouw, T.; Hollenbeck, A.R.; Schatzkin, A. Physical activity recommendations and decreased risk of mortality. Arch. Intern. Med. 2007, 167, 2453–2460. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dikareva, A.; Bacon, S.L.; Daskalopoulou, S.S. The impact of physical activity on mortality in patients with high blood pressure: A systematic review. J. Hypertens. 2012, 30, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.; de Boer, J.F.; Annema, W.; Tietge, U.J. The inverse relation of HDL anti-oxidative functionality with serum amyloid a is lost in metabolic syndrome subjects. Obesity 2013, 21, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Drechsler, C.; Kleber, M.E.; Dressel, A.; Riffel, J.; Triem, S.; Lehmann, M.; Kopecky, C.; Säemann, M.D.; Lepper, P.M.; et al. Serum amyloid A: High-density lipoproteins interaction and cardiovascular risk. Eur. Heart J. 2015, 36, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Florentin, M.; Liberopoulos, E.N.; Wierzbicki, A.S.; Mikhailidis, D.P. Multiple actions of high-density lipoprotein. Curr. Opin. Cardiol. 2008, 23, 370–378. [Google Scholar] [CrossRef]
- Podrez, E.A. Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin. Exp. Pharm. Physiol. 2010, 37, 719–725. [Google Scholar] [CrossRef]
- Navab, M.; Hama, S.Y.; Anantharamaiah, G.M.; Hassan, K.; Hough, G.P.; Watson, A.D.; Reddy, S.T.; Sevanian, A.; Fonarow, G.C.; Fogelman, A.M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: Steps 2 and 3. J. Lipid Res. 2000, 41, 1495–1508. [Google Scholar]
- Ansell, B.J.; Navab, M.; Hama, S.N.; Kamranpour, N.; Fonarow, G.; Hough, G.; Rahmani, S.; Mottahedeh, R.; Dave, R.; Reddy, S.T.; et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003, 108, 2751–2756. [Google Scholar] [CrossRef]
- Zheng, L.; Nukuna, B.; Brennan, M.L.; Sun, M.; Goormastic, M.; Settle, M.; Schmitt, D.; Fu, X.; Thoson, L.; Fox, P.L.; et al. Apolipoprotein A-I is a selective target for myeloperoxidase catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Investig. 2004, 114, 529–541. [Google Scholar] [CrossRef]
- Shlomai, G.; Grassi, G.; Grossman, E.; Mancia, G. Assessment of target organ damage inthe evaluation and follow-up of hypertensive patients: Where do we stand? J. Clin. Hypertens. 2013, 15, 742–747. [Google Scholar]
- Totsikas, C.; Röhm, J.; Kantartzis, K.; Claus, T.; Kilian, R.; Jürgen, M.; Fritz, S.; Jochen, H.; Andreas, N.; Andreas, F.; et al. Cardiorespiratory fitness determines the reduction in blood pressure and insulin resistance during lifestyle intervention. J. Hypertens. 2011, 29, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Hopman, M.T.; Padilla, J.; Laughlin, M.H.; Thijssen, D.H. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol. Rev. 2017, 97, 495–528. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Quyyumi, A.A.; Wu, H.; Csako, G.; Rott, D.; Zalles-Ganley, A.; Ogunmakinwa, J.; Halcox, J.; Epstein, S.E. Increased serum levels of heat shock protein 70 are associated with low risk of coronary artery disease. Arter. Thromb. Vasc. Biol. 2003, 23, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ahn, N.Y.; Hong, C.B.; Kim, K.J. The effect of acute and prolonged endurance swim exercise on antioxidant and mitochondrial enzymes in rat skeletal muscle. Exerc. Sci. 2011, 20, 359–366. [Google Scholar]
- Ookawara, T.; Haga, S.; Ha, S.; Oh-ishi, S.; Toshinai, K.; Kizaki, T.; Ji, L.L.; Suzuki, K.; Ohno, H. Effects of endurance training on three superoxide dismutase isoenzymes in human plasma. Free Radic. Res. 2003, 37, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.L.; Donato, A.J.; Larocca, T.J.; Eskurza, I.; Silver, A.E.; Seals, D.R. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 2011, 10, 1032–1037. [Google Scholar] [CrossRef]
- Fehrenbach, E.; Passek, F.; Niess, A.M.; Pohla, H.; Weistock, C.; Dickhuth, H.H.; Northoff, H. HSP expression in human leukocytes is modulated by endurance exercise. Med. Sci. Sports Exerc. 2000, 32, 592–600. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Ott, P.; Nielsen, H.B.; Steensberg, A.; Keller, C.; Peter, K.; Secher, N.H.; Pedersen, B.K. Exercise induces hepatosplanchnic release of heat shock protein 72 in humans. J. Physiol. 2002, 544, 957–962. [Google Scholar] [CrossRef]
- Walsh, R.C.; Koukoulas, I.; Garnham, A.; Moseley, P.L.; Hargreaves, M.; Febbraio, M.A. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 2001, 6, 386–393. [Google Scholar] [CrossRef]
- Kohut, M.L.; McCann, D.A.; Russell, D.W.; Konopka, D.N.; Cunnick, J.E.; Franke, W.D.; Castillo, M.C.; Reighard, A.E.; Vanderah, E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of beta-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006, 20, 201–209. [Google Scholar] [CrossRef]
- Larsen, A.I.; Aukrust, P.; Aarsland, T.; Dickstein, K. Effect of aerobic exercise training on plasma levels of tumor necrosis factor alpha in patients with heart failure. Am. J. Cardiol. 2001, 88, 805–808. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Hsu, F.C.; Brinkley, T.J.; Church, T.; Goodpaster, B.H.; Kritchevsky, S.B.; Pahor, M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008, 56, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Kemmler, W.; von Stengel, S.; Engelke, K.; Kalender, W.A. Exercise decreases the risk of metabolic syndrome in elderly females. Med. Sci. Sports Exerc. 2009, 41, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.; Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxidative Med. Cell. Longev. 2015, 2016, 1–32. [Google Scholar] [CrossRef]
- Conraads, V.M.; Beckers, P.; Bosmans, J.; de Clerck, L.S.; Stevens, W.J.; Vrints, C.J.; Brutsaert, D.L. Combined endurance/resistance training reduces plasma TNF-alpha receptor levels in patients with chronic heart failure and coronary artery disease. Eur. Heart J. 2002, 23, 1854–1860. [Google Scholar] [CrossRef]
- Stewart, L.K.; Flynn, M.G.; Campbell, W.W.; Craig, B.A.; Robinson, J.P.; Timmerman, K.L.; McFarlin, B.K.; Coen, P.M.; Talbert, E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med. Sci. Sports Exerc. 2007, 39, 1714–1719. [Google Scholar] [CrossRef]
- Murray, C.J.; Lauer, J.A.; Hutubessy, R.C.; Niessen, L.; Tomijima, N.; Rodgers, A.; Lawes, C.M.M.; Evans, D.B. Effectiveness and costs of intervention to lower systolic blood pressure and cholesterol: A global and regional analysis on reduction of cardiovascular-disease risk. Lancet 2003, 361, 717–725. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. The distribution of 10-Year risk for coronary heart disease among US adults: Findings from the national health and nutrition examination survey III. J. Am. Coll. Cardiol. 2004, 43, 1791–1796. [Google Scholar] [CrossRef]
- Brindle, P.M.; Beswick, A.D.; Fahey, T.; Ebrahim, S.B. The accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: A systematic review. Heart 2006, 92, 1752–1759. [Google Scholar] [CrossRef]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Faramarzi, M.; Nasiri, S. The effects of 10-week different exercise interventions on Framingham risk score and metabolic syndrome severity scores in overweight women with type 2 diabetes. J. Shahrekord Univ. Med. Sci. 2019, 20, 1–8. [Google Scholar] [CrossRef]
- Tully, M.A.; Cupples, M.E.; Chan, W.S.; McGlade, K.; Young, I.S. Brisk walking, fitness, and cardiovascular risk: A randomized controlled trial in primary care. Prev. Med. 2005, 41, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Balducci, S.; Zanuso, S.; Cardelli, P.; Salvi, L.; Bazuro, A.; Pugliese, L.; Maccora, C.; Lacobini, C.; Conti, F.G.; Nicolucci, A.; et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes; the Italian Diabetes and Exercise Study (IDES). PLoS ONE 2012, 7, e49297. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisloff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.; Brown, A.W.; Brown, M.M.B.; Alcorn, A.; Noles, C.; Winwood, L.; Resuehr, H.; George, B.; Jeansonne, M.M.; Allison, D.B. High intensity interval–vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: A randomized controlled trial. PLoS ONE 2015, 10, e0138853. [Google Scholar] [CrossRef]
| Variable | NBP (n = 18) | HNBP (n = 12) |
|---|---|---|
| Age (yr) | 69.22 | 72.00 |
| 4.14 | 3.81 | |
| Height (cm) | 154.11 | 152.17 |
| 4.35 | 5.31 | |
| Body weight (kg) | 61.20 | 58.43 |
| 9.36 | 7.30 | |
| BMI (kg/m2) | 25.23 | 25.27 |
| 3.87 | 2.53 | |
| %fat | 34.06 | 37.08 |
| 6.03 | 4.09 | |
| WHR | 0.90 | 0.90 |
| 0.07 | 0.09 |
| Variable | NBP (n = 18) | HNBP (n = 12) | Source | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Body composition | |||||||
| Body weight (kg) | 61.20 | 61.37 | 58.43 | 58.82 | Time | 0.015 | 0.902 |
| 9.36 | 9.36 | 7.30 | 6.49 | Groups | 1.391 | 0.001 | |
| T × G | 0.002 | 0.961 | |||||
| BMI (kg/m2) | 25.23 | 25.27 | 25.27 | 25.37 | Time | 0.005 | 0.946 |
| 3.87 | 3.81 | 2.53 | 2.16 | Groups | 0.007 | 0.932 | |
| T × G | 0.001 | 0.973 | |||||
| %fat | 34.06 | 33.56 | 37.08 | 38.97 *,# | Time | 0.272 | 0.606 |
| 6.03 | 6.06 | 4.09 | 3.83 | Groups | 8.160 | 0.008 | |
| T × G | 0.807 | 0.377 | |||||
| WHR | 0.90 | 0.87 # | 0.90 | 0.87 # | Time | 13.440 | 0.001 |
| 0.07 | 0.05 | 0.09 | 0.05 | Groups | 0.001 | 1.000 | |
| T × G | 0.001 | 1.000 | |||||
| Cardiovascular disease risk factors | |||||||
| SBP (mmHg) | 118.44 | 118.89 | 135.67 *** | 133.50 ***,### | Time | 2.033 | 0.165 |
| 10.65 | 7.19 | 3.94 | 4.66 | Groups | 33.285 | 0.001 | |
| T × G | 4.673 | 0.039 | |||||
| DBP (mmHg) | 77.44 | 78.89 # | 83.00 * | 83.33 | Time | 2.621 | 0.117 |
| 7.97 | 7.19 | 4.22 | 3.89 | Groups | 4.583 | 0.041 | |
| T × G | 1.024 | 0.320 | |||||
| TC (mg/dL) | 178.56 | 193.11 | 182.00 | 189.33 | Time | 2.052 | 0.163 |
| 48.80 | 40.36 | 47.17 | 48.69 | Groups | 0.001 | 0.991 | |
| T × G | 0.223 | 0.640 | |||||
| HDL-C (mg/dL) | 54.67 | 55.44 | 51.17 | 57.67 ## | Time | 7.681 | 0.010 |
| 6.77 | 9.14 | 7.32 | 7.25 | Groups | 0.062 | 0.806 | |
| T × G | 4.749 | 0.038 | |||||
| LDL-C (mg/dL) | 111.44 | 126.11 | 105.00 | 116.83 | Time | 4.880 | 0.036 |
| 41.40 | 36.49 | 34.60 | 41.47 | Groups | 0.359 | 0.554 | |
| T × G | 0.056 | 0.815 | |||||
| TG (mg/dL) | 97.67 | 104.78 | 157.33 *** | 124.67 ## | Time | 2.224 | 0.147 |
| 37.59 | 43.48 | 39.04 | 37.04 | Groups | 10.922 | 0.003 | |
| T × G | 5.389 | 0.028 | |||||
| Insulin | 7.56 | 8.14 | 6.30 | 9.00 # | Time | 5.692 | 0.024 |
| 3.79 | 3.21 | 2.45 | 2.29 | Groups | 0.046 | 0.832 | |
| T × G | 2.345 | 0.137 | |||||
| Glucose | 91.78 | 95.89 | 110.00 ** | 123.67 **,## | Time | 25.080 | 0.001 |
| 10.97 | 18.45 | 23.53 | 31.83 | Groups | 8.963 | 0.006 | |
| T × G | 7.246 | 0.012 | |||||
| HOMA IR | 1.68 | 1.95 | 1.64 | 2.77 * | Time | 9.791 | 0.004 |
| 0.79 | 0.91 | 0.54 | 1.26 | Groups | 2.403 | 0.132 | |
| T × G | 3.714 | 0.064 | |||||
| Variable | NBP (n = 18) | HNBP (n = 12) | Source | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Chair sit and reach (cm) | 21.88 | 21.26 | 17.45 | 22.50 # | Time | 5.531 | 0.027 |
| 3.11 | 6.80 | 3.76 | 6.59 | Groups | 1.603 | 0.217 | |
| T × G | 4.190 | 0.051 | |||||
| Back scratch (Right, cm) | −7.02 | −4.00 ## | −9.88 | −7.25 | Time | 2.548 | 0.125 |
| 6.63 | 3.53 | 7.86 | 4.63 | Groups | 3.000 | 0.097 | |
| T × G | 0.288 | 0.567 | |||||
| 30 s chair stand (time) | 16.56 | 25.33 ### | 14.17 | 25.83 ### | Time | 107.785 | 0.001 |
| 2.75 | 5.86 | 1.53 | 4.57 | Groups | 1171.705 | 0.001 | |
| T × G | 2.152 | 0.154 | |||||
| Arm curl (Right, time) | 25.38 | 31.00 ## | 22.33 * | 29.50 ## | Time | 36.774 | 0.001 |
| 3.72 | 4.50 | 3.85 | 2.61 | Groups | 3.653 | 0.067 | |
| T × G | 1.166 | 0.290 | |||||
| 8 foot up-and-go (sec) | 14.67 | 14.90 | 15.83 | 15.98 ** | Time | 0.190 | 0.666 |
| 4.32 | 1.79 | 1.82 | 1.91 | Groups | 3.675 | 0.065 | |
| T × G | 0.008 | 0.929 | |||||
| PEI (Harvard step test) | 99.09 | 114.57 | 94.24 | 103.76 # | Time | 3.813 | 0.065 |
| 15.13 | 14.15 | 7.09 | 8.75 | Groups | 10.090 | 0.005 | |
| T × G | 0.130 | 0.722 | |||||
| Variable | NBP (n = 18) | HNBP (n = 12) | Source | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| HDL function markers | |||||||
| SAA(ng/mL) | 17.33 | 17.03 | 17.98 | 14.05 *,## | Time | 3.909 | 0.058 |
| 3.26 | 4.20 | 3.18 | 0.69 | Groups | 4.048 | 0.054 | |
| T × G | 2.872 | 0.101 | |||||
| SAA/HDL | 0.32 | 0.32 | 0.37 | 0.25 *,### | Time | 11.459 | 0.002 |
| 0.05 | 0.08 | 0.09 | 0.04 | Groups | 0.205 | 0.654 | |
| T × G | 9.881 | 0.004 | |||||
| APOA1 (ng/mL) | 2182.20 | 3841.46 # | 2311.20 | 2769.78 | Time | 0.116 | 0.739 |
| 1818.67 | 1971.41 | 1450.49 | 1297.58 | Groups | 0.141 | 0.713 | |
| T × G | 6.376 | 0.024 | |||||
| APOA1/HDL-C | 27.33 | 51.67 | 84.24 ** | 55.19 # | Time | 0.023 | 0.881 |
| 32.85 | 39.70 | 32.88 | 62.86 | Groups | 6.845 | 0.014 | |
| T × G | 2.921 | 0.098 | |||||
| Antioxidant and cell repair markers | |||||||
| SOD2 (pg/mL) | 50,126.30 | 56,463.16 ## | 51,464.40 | 49,984.00 | Time | 2.863 | 0.102 |
| 13,904.92 | 13,360.68 | 12,462.32 | 10,517.19 | Groups | 0.317 | 0.578 | |
| T × G | 7.418 | 0.011 | |||||
| HSP70 (ng/mL) | 0.59 | 0.79 # | 0.49 | 0.73 ### | Time | 12.721 | 0.001 |
| 0.25 | 0.47 | 0.09 | 0.08 | Groups | 0.707 | 0.408 | |
| T × G | 0.129 | 0.722 | |||||
| Pro-inflammation markers | |||||||
| TNF-α (pg/mL) | 9.59 | 8.71 | 8.19 | 7.71 * | Time | 2.334 | 0.138 |
| 2.75 | 0.96 | 1.65 | 1.13 | Groups | 5.370 | 0.028 | |
| T × G | 0.204 | 0.655 | |||||
| CRP (mg/dL) | 0.630 | 0.820 | 0.537 | 0.652 | Time | 1.754 | 0.196 |
| 0.511 | 0.667 | 0.281 | 0.596 | Groups | 0.593 | 0.448 | |
| T × G | 0.106 | 0.748 | |||||
| IL-6 (pg/mL) | 14.68 | 11.39 | 9.85 | 9.54 * | Time | 2.427 | 0.130 |
| 10.51 | 2.88 | 0.85 | 1.04 | Groups | 2.994 | 0.095 | |
| T × G | 1.659 | 0.208 | |||||
| Anti-inflammation markers | |||||||
| IL-15 (pg/mL) | 22.39 | 17.59 | 20.93 | 17.34 | Time | 4.110 | 0.052 |
| 11.31 | 8.75 | 10.09 | 6.82 | Groups | 0.087 | 0.770 | |
| T × G | 0.086 | 0.772 | |||||
| IL-4 (pg/mL) | 14.41 | 13.88 | 12.83 * | 12.43 *,# | Time | 1.535 | 0.226 |
| 2.03 | 2.40 | 0.45 | 0.61 | Groups | 7.826 | 0.009 | |
| T × G | 0.032 | 0.860 | |||||
| Variable | NBP (n = 18) | HNBP (n = 12) | Source | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| FRS Your Heart-10 Risk (%) | 7.98 | 7.47 | 17.63 *** | 16.17 ***,# | Time | 4.361 | 0.046 |
| 2.82 | 2.29 | 6.39 | 5.40 | Groups | 37.561 | 0.001 | |
| T × G | 1.018 | 0.322 | |||||
| Your Heart/Vascular Age (yr) | 65.22 | 63.56 | 82.00 *** | 81.67 *** | Time | 0.479 | 0.494 |
| 9.60 | 9.05 | 6.12 | 6.89 | Groups | 40.191 | 0.001 | |
| T × G | 0.213 | 0.648 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, N.; Kim, K. Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers? Int. J. Environ. Res. Public Health 2020, 17, 5910. https://doi.org/10.3390/ijerph17165910
Ahn N, Kim K. Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers? International Journal of Environmental Research and Public Health. 2020; 17(16):5910. https://doi.org/10.3390/ijerph17165910
Chicago/Turabian StyleAhn, Nayoung, and Kijin Kim. 2020. "Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers?" International Journal of Environmental Research and Public Health 17, no. 16: 5910. https://doi.org/10.3390/ijerph17165910
APA StyleAhn, N., & Kim, K. (2020). Can Active Aerobic Exercise Reduce the Risk of Cardiovascular Disease in Prehypertensive Elderly Women by Improving HDL Cholesterol and Inflammatory Markers? International Journal of Environmental Research and Public Health, 17(16), 5910. https://doi.org/10.3390/ijerph17165910






