Characterization of Fe(III) Adsorption onto Zeolite and Bentonite
Abstract
1. Introduction
2. Materials and Methods
- Kf (mg1−n.Ln.g−1) is adsorption capacity, n (1) is a constant related to the intensity of the adsorption; the isotherm represents sorption taking place on a heterogeneous surface with interaction between the adsorbed molecules [23];
- qm (mg.g−1) is maximum sorption capacity, aL (dm3.mg−1) is adsorption energy; the isotherm represents sorption taking place on a homogenous surface within the adsorbent [25],
- KR (dm3.g−1) and aR (dm3β.g−β) are constants, β (1) is exponent; the isotherm is used as compromise between the Langmuir and Freundlich systems [23].
3. Results
- Z-M20: 15.16% and 9.26%, in this only case the percentage of Fe in the surface layer is lower in the case of higher initial Fe concentration,
- Z-M50: 62.97% and 200.00%,
- B-BL: 14.00% and 37.20%, and
- B-BR: 7.80% and 8.10%.
4. Discussion
- Prior to the coating treatment, the zeolite or bentonite is treated by suspending in NaCl solution for a period of 24 h. The suspension is filtered and washed with deionized water. The resulting suspension is dried in oven at 100 °C [33]. Manganese oxide coated zeolite and bentonite are prepared utilizing a reductive procedure [34] modified to precipitate colloids of manganese oxides onto zeolite and bentonite surfaces. Manganese oxide is precipitated in aqueous solution by the reaction:2KMnO4 + 8HCl = 2MnO2 + 2KCl + 3Cl2 + 4H2ODried zeolites and bentonites samples are poured over a heated solution at 90 °C, containing potassium permanganate placed in a beaker, followed by dropwise addition of hydrochloric acid. After stirring for 1 h, the suspension is filtered, washed several times using distilled water, and dried in an oven at 100 °C [35]. MnO2 coating increases not only the Mn(II), but also Fe(II) and Fe(III) removal.
- Prior to modification, the zeolite or bentonite is washed with deionized water and dried at room temperature. The modified zeolite or bentonite are obtained by pouring a mixture of MnCl2 and NaOH over the washed zeolite in a heat resistant dish and then heating the mixture in a furnace at 150 °C for approximately 5 h. Afterwards, the modified adsorbent is heated at 500 °C for 3 h, cooled at room temperature, and washed several times with distilled water [36].
- The modified zeolite or bentonite is prepared by mixing with Fe(III) solution. The mixture is shaken for 20 h at 25 °C before pH is measured and NaOH solution is added to raise the pH. This procedure is repeated every 2 h for a total of three times to bring the final solution pH to 9. The mixture is allowed to settle, and the supernatant removed, followed by washing the adsorbent with de-ionized water [37].
- The natural zeolite or bentonite is first treated with NaCl solution under reflux for 3 h. The treated solution is filtered, washed with distilled water, and dried at 60 °C for 24 h. Next, it is treated with FeCl3 solution under reflux for 5 h. The mixture is filtered, washed with distilled water, and dried at 80 °C. The procedure mentioned before is applied in a similar manner using MnCl2 and a mixture of FeCl3 and MnCl2 solutions [38].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Edwards, M.; McNeill, L.S. Effect of phosphate inhibitors on lead release from pipes. J. AWWA 2002, 94, 79. [Google Scholar] [CrossRef]
- Qiu, W.; Li, W.; He, J.; Zhao, H.; Liu, X.; Yuan, Y. Variations regularity of microorganisms and corrosion of cast iron in water distribution system. J. Environ. Sci. (China) 2018, 74, 177. [Google Scholar] [CrossRef]
- Das, S.; Mishra, S. Insight into the isotherm modelling, kinetic and thermodynamic exploration of iron adsorption from aqueous media by activated carbon developed from Limonia acidissima shell. Mater. Chem. Phys. 2020, 245, 122751. [Google Scholar] [CrossRef]
- Kang, Y.-G.; Vu, H.C.; Chang, Y.-Y.; Chang, Y.-S. Fe(III) adsorption on graphene oxide: A low-cost and simple modificationmethod for persulfate activation. Chem. Eng. J. 2020, 387, 124012. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture. Chem. Eng. J. 2019, 359, 363. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Zhang, Y.; Zhang, K.; Wang, J.; Jing, R.; Yang, X.; Hu, Z.; Lin, X.; Li, Y. Spectroscopic investigation of Cu2+, Pb2+ and Cd2+ adsorption behaviors by chitosan-coated argillaceous limestone: Competition and mechanisms. Environ. Pollut. 2019, 254, 112938. [Google Scholar] [CrossRef]
- Öztaş, N.A.; Karabakan, A.; Topal, Ö. Removal of Fe(III) ion from aqueous solution by adsorption on raw and treated clinoptilolite samples. Microporous Mesoporous Mater. 2008, 111, 200. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of Fe(III) from water by natural and acid activated clays: Studies on equilibrium isotherm, kinetics and thermodynamics of interactions. Adsorption 2006, 12, 185. [Google Scholar] [CrossRef]
- Ehssan, M.N. Utilization of bentonite as an adsprpent material in the removal of iron (III). Int. J. Eng. Sci. Technol. 2012, 4, 4480. [Google Scholar]
- Chan, Y.T.; Liu, Y.T.; Tzou, Y.M.; Kuan, W.H.; Chang, R.R.; Wang, M.K. Kinetics and equilibrium adsorption study of selenium oxyanions onto Al/Si and Fe/Si coprecipitates. Chemosphere 2018, 198, 59. [Google Scholar] [CrossRef]
- Ostovan, A.; Elhami, S. Evaluation of the sawdust modified with diethylenetriamine as an effective adsorbent for Fe (III) removal from water. J. Water Wastewater 2018, 29, 29. [Google Scholar]
- Kalak, T.; Dudczak-Hałabuda, J.; Tachibana, Y.; Cierpiszewsky, R. Effective use of elderberry (Sambucus nigra) pomace in biosorption processes of Fe(III) ions. Chemosphere 2020, 246, 125744. [Google Scholar] [CrossRef]
- Kyzioł-Komosińska, J.; Rosik-Dulewska, C.; Franus, M.; Antoszczyszyn-Szpicka, P.; Czupioł, J.; Krzyżewska, I. Sorption Capacities of Natural and Synthetic Zeolites for Cu(II) Ions. Pol. J. Environ. Stud. 2015, 24, 1111. [Google Scholar] [CrossRef]
- Le, T.D.; Tran, H.V.; Thu, L.D.; Quang, T.N.; Hang, N.T.M.; Khoi, N.G. Synthesis and application of chitosan/graphene oxide/magnetite nanostructured composite for Fe (III) removal from aqueous solution. Vietnam J. Sci. Technol. 2018, 56, 158. [Google Scholar] [CrossRef][Green Version]
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Xu, C.; Liu, P.; Lv, J.; Liu, Y.Y.; Wang, Q. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study. J. Hazard. Mater. 2020, 384, 121371. [Google Scholar] [CrossRef]
- Wu, K.; Liu, R.; Li, T.; Liu, H.; Peng, J.; Qu, J. Removal of arsenic(III) from aqueous solution using a low-cost by-product in Fe-removal plants—Fe-based backwashing sludge. Chem. Eng. J. 2013, 226, 393. [Google Scholar] [CrossRef]
- Medina-Ramirez, A.; Gamero-Melo, P.; Ruiz-Camacho, R.; Minchaca-Mojica, J.I.; Romero-Toledo, R.; Gamero-Vega, K.Y. Adsorption of Aqueous As (III) in Presence of Coexisting Ions by a Green Fe-Modified W Zeolite. Water 2019, 11, 281. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, Y.; Liu, H.; Jin, Z.; Chen, T. The kinetic and thermodynamic adsorption of Eu(III) on synthetic maghemite. J. Mol. Liq. 2016, 221, 171. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, W.; Tian, C.; Mo, S.; Liu, X.; Deng, H.; Lin, Z. Mussel-inspired functionalization of biological calcium carbonate for improving Eu(III) adsorption and the related mechanisms. Chem. Eng. J. 2018, 351, 816. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Z. Phys. Chem. 2017, 57. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Al-Muhtaseb, A.H.; Al-laqtah, N.A.; Walke, G.M.; Allen, S.J.; Ahmad, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362. [Google Scholar] [CrossRef] [PubMed]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Hüfner, S. Photoelectron Spectroscopy: Principles and Applications, 3rd ed.; Springer: Berlin, Germany, 2003; pp. 173–210, 411–500. [Google Scholar]
- Caridi, F.; Torrisi, L.; Cutroneo, M.; Barreca, F.; Gentile, C.; Serafino, T.; Castrizio, D. XPS and XRF depth patina profiles of ancient silver coins. Appl. Surf. Sci. 2013, 272, 82–87. [Google Scholar] [CrossRef]
- Comparison of Complimentary Techniques, University of Surrey, Surrey, Great Britain. Available online: http://www.surrey.ac.uk/sites/default/files/comparison-of-complementary-techniques.pdf (accessed on 18 March 2020).
- Malara, C.; Pierini, G.; Viola, A. Correlation, Analysis and Prediction of Adsorption Equilibria; Report EUR 13996 EN; European Commission: Luxembourg, 1992. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Huang, X.; Li, K.; Zhang, Y.; Qin, W.; Qiu, G.; Wang, J. Adsorption behavior and mechanism of Bi(III) ions on rutile−water interface in the presence of nonyl hydroxamic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 348. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner. Eng. 2009, 22, 336. [Google Scholar] [CrossRef]
- Richter, M.; Berndt, H.; Eckelt, R.; Schneider, M.; Fricke, R. Zeolite-mediated removal of NOx by NH3 from exhaust streams at low temperatures. Catal. Today 1999, 54, 531. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. Removal of Mn2+ from aqueous solution by manganese oxide coated zeolite. Miner. Eng. 2010, 23, 1131. [Google Scholar] [CrossRef]
- Camacho, L.M.; Parra, R.R.; Deng, S. Arsenic removal from groundwater by MnO2-modified natural clinoptilolite zeolite: Effects of pH and initial feed concentration. J. Hazard. Mater. 2011, 189, 286. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jean, J.-S.; Jiang, W.-T.; Chang, P.-H.; Chen, C.-J.; Liao, L. Removal of arsenic from water using Fe-exchanged natural zeolite. J. Hazard. Mater. 2011, 187, 318. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Cedillo, M.J.; Olguín, M.T.; Fall, C.H. Adsorption kinetic of arsenates as water pollutant on iron, manganese and iron–manganese-modified clinoptilolite-rich tuffs. J. Hazard. Mater. 2009, 163, 939. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421. [Google Scholar] [CrossRef]
- Syahmani, M.; Leny, M.; Iriani, R.; Sanjaya, R.E. Potency of Chitin as an Adsorbent in Black Water Treatment Process at Peatland Environment. Adv. Soc. Sci. Educ. Humanit. Res. 2018, 147, 316. [Google Scholar]
- Jooste, J.H.; De Bruyn, J.A. Desorption of absorbed iron in bean root and leaf tissues. J. S. Afr. Bot. 1979, 45, 249. [Google Scholar]
- Zhang, S.; Liu, C.; Yuan, Y.; Fan, M.; Zhang, D.; Wang, D.; Xu, Y. Selective, highly efficient extraction of Cr(III), Pb(II) and Fe(III) from complex water environment with a tea residue derived porous gel adsorbent. Bioresour. Technol. 2020, 311, 123520. [Google Scholar] [CrossRef]
- Harrison, K.; Prince, R.H.; Lambert, R.M. Structure and properties of ultrathin iron films on RU1010: The formation of metastable surface phases of γ-Fe. Surf. Sci. 1988, 201, 393. [Google Scholar] [CrossRef]
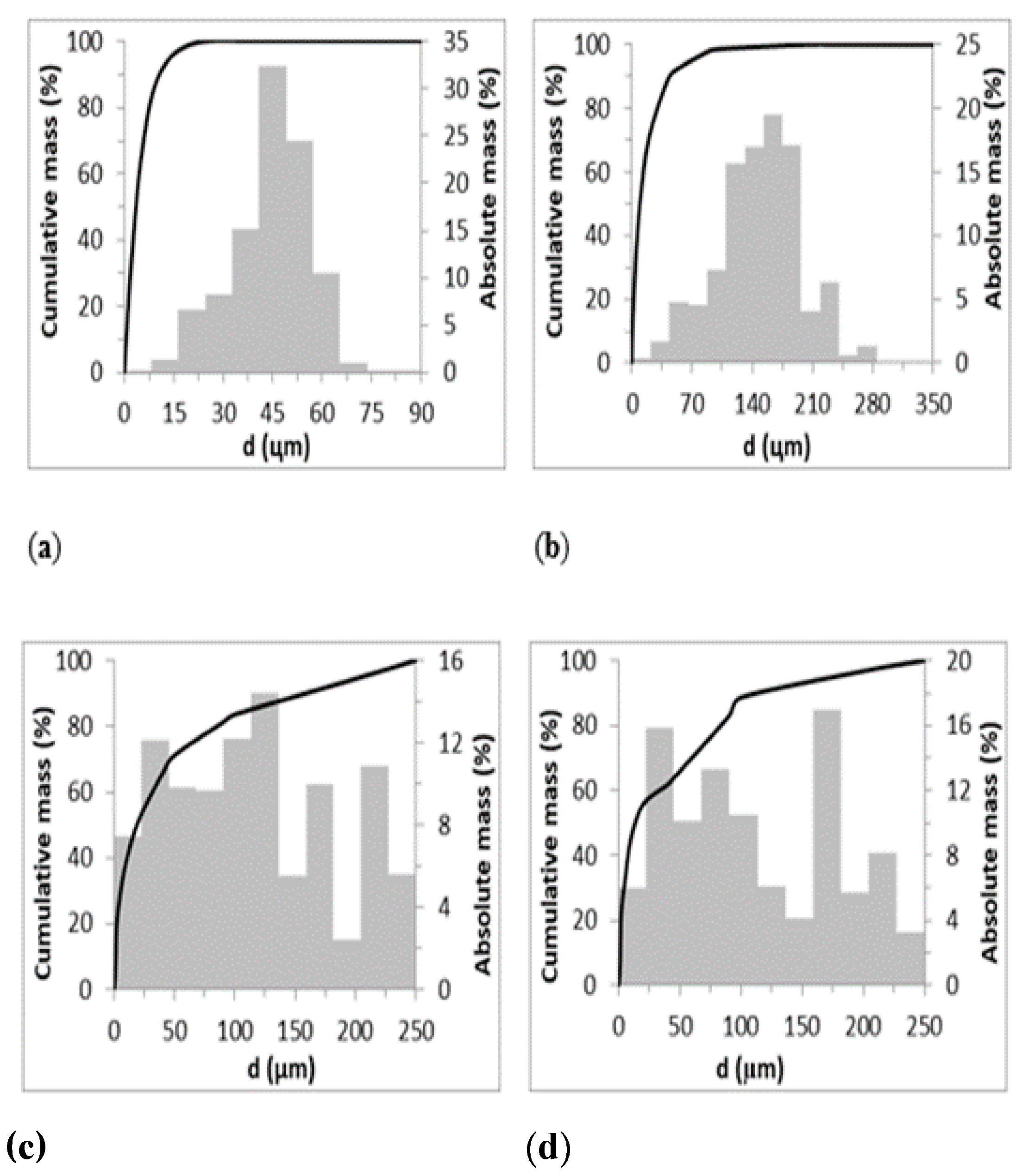

| Parameter | Z-M20 | Z-M50 | B-BL | B-BR |
|---|---|---|---|---|
| Particle size (µm) | 0-90 | 0–350 | 0–250 | 0–250 |
| d32 (µm) | 19.553 | 50.862 | 199 | 180 |
| d50 (µm) | 3.493 | 9.549 | 19 | 13 |
| Surface area (m2.g−1) | 25.8394 | 26.3208 | 21.8874 | 20.1231 |
| Compound | Z-M20 | Z-M50 | B-BL | B-BR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Low | High | Raw | Low | High | Raw | Low | High | Raw | Low | High | |
| SiO2 (%) | 51.54 | 51.31 | 51.73 | 54.33 | 53.38 | 55.82 | 41.27 | 40.63 | 40.94 | 44.78 | 41.29 | 40.87 |
| Al2O3 (%) | 8.66 | 8.11 | 7.92 | 7.35 | 7.13 | 6.76 | 10.54 | 11.37 | 11.32 | 11.66 | 11.87 | 11.71 |
| CaO (%) | 1.79 | 0.26 | 0.45 | 1.26 | 0.15 | 0.44 | 1.97 | 1.51 | 1.02 | 2.53 | 1.69 | 1.38 |
| K2O (%) | 1.36 | 0.42 | 0.29 | 1.45 | 0.18 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Fe2O3 (%) | 0.83 | 0.99 | 2.27 | 2.27 | 0.08 | 1.47 | 2.92 | 3.26 | 5.22 | 2.17 | 2.48 | 4.81 |
| FeO (%) | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Isotherm | Parameter | Z-M20 | Z-M50 | B-BL | B-BR |
|---|---|---|---|---|---|
| Freundlich | Kf, mg1−n.dm3n.g−1 | 3.07 | 1.29 | 1.17 | 3.05 |
| n | 5.85 | 2.87 | 3.21 | 4.44 | |
| R2 | 0.78 | 0.57 | 0.52 | 0.83 | |
| Langmuir | qm, mg.g−1 | 10.19 | 9.72 | 11.64 | 16.86 |
| aL, dm3.mg−1 | 0.05 | 1.18 | 0.03 | 0.01 | |
| R2 | 0.92 | 0.91 | 0.91 | 0.95 | |
| Redlich-Peterson | KR, dm3.g−1 | 0.31 | 0.32 | 0.08 | 2.50 |
| bR, dm3β.g−β | 0.05 | 0.02 | 0.01 | 0.32 | |
| β | 0.94 | 1.04 | 1.04 | 0.91 | |
| R2 | 0.92 | 0.82 | 0.87 | 0.99 |
| Method | Z-M20 | Z-M50 | B-BL | B-BR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Low | High | Raw | Low | High | Raw | Low | High | Raw | Low | High | |
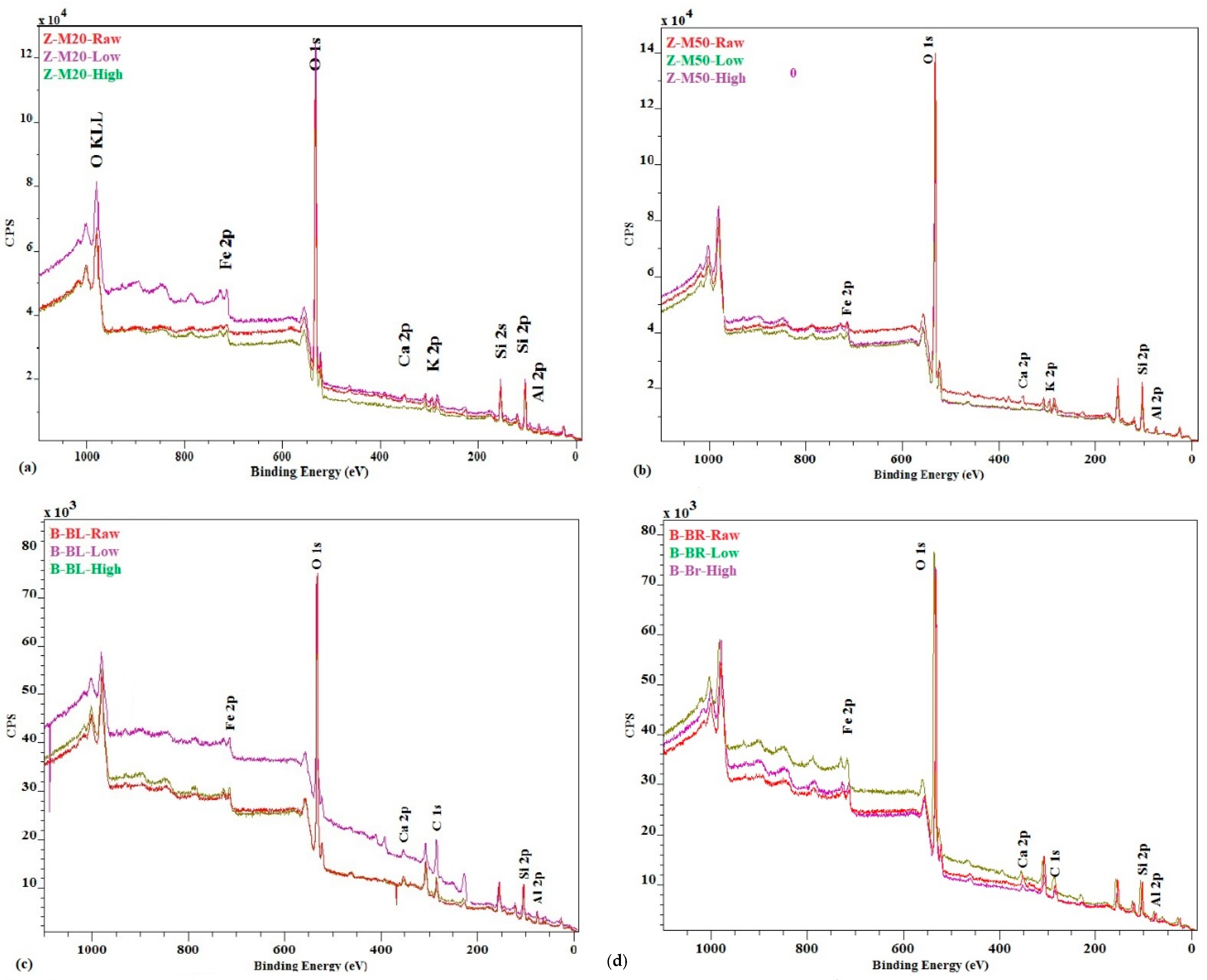
| XPS | 7.02 ± 0.31 | 8.08 ± 0.31 | 7.67 ± 0.25 | 1.85 ± 0.05 | 3.02 ± 0.10 | 5.55 ± 0.21 | 15.00 ± 0.63 | 17.10± 0.65 | 20.58 ± 0.91 | 20.38 ± 0.97 | 21.97 ± 0.90 | 22.03 ± 0.94 |
| XRF | 7.11 ± 0.29 | 8.27 ± 0.29 | 17.19 ± 0.47 | 1.89 ± 0.06 | 3.06 ± 0.09 | 11.61 ± 0.52 | 15.90 ± 0.63 | 18.07 ± 0.74 | 34.31 ± 1.06 | 21.11 ± 0.96 | 23.47 ± 0.99 | 37.22 ± 1.09 |
| Adsorbed | 1.16 | 10.08 | 1.17 | 9.72 | 2.17 | 18.41 | 2.36 | 16.10 | ||||
| XPS + ads | 8.18 | 17.10 | 3.02 | 11.57 | 17.17 | 33.41 | 22.74 | 36.48 | ||||
| XRF + ads | 8.27 | 17.19 | 3.06 | 11.61 | 18.07 | 34.31 | 23.47 | 37.21 | ||||
| Ratio, Method | Z-M20 | Z-M50 | B-BL | B-BR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Low | High | Raw | Low | High | Raw | Low | High | Raw | Low | High | |
| Fe:Ca, XPS | 0.52 | 2.06 | 1.95 | 0.21 | 0.70 | 1.23 | 0.84 | 1.27 | 1.95 | 1.48 | 1.26 | 2.43 |
| Fe:Ca, XRF | 0.56 | 4.45 | 5.39 | 0.21 | 2.81 | 3.69 | 0.88 | 1.49 | 3.47 | 1.50 | 2.17 | 5.08 |
| ΔFe:Ca, XPS, % | 298.64 | 276.31 | 237.75 | 490.02 | 51.04 | 86.74 | −14.80 | 64.29 | ||||
| ΔFe:Ca, XRF, % | 701.42 | 871.05 | 1238.92 | 1659.31 | 70.14 | 294.72 | 45.15 | 239.62 | ||||
| Ratio, Method | Z-M20 | Z-M50 | B-BL | B-BR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Low | High | Raw | Low | High | Raw | Low | High | Raw | Low | High | |
| Fe:Se, XPS | 3.21 | 0.79 | 2.22 | 1.01 | 0.42 | 0.75 | 3.31 | 3.62 | 2.42 | 3.89 | 2.28 | 2.12 |
| Fe:Se, XRF | 3.57 | 3.97 | 8.76 | 0.24 | 0.44 | 1.53 | 2.88 | 4.35 | 5.02 | 1.79 | 2.55 | 4.18 |
| ΔFe:Se, XPS, % | −75.42 | −30.84 | −58.00 | −25.51 | 9.41 | −26.97 | −41.34 | −45.48 | ||||
| ΔFe:Se, XRF, % | 11.17 | 145.27 | 82.63 | 538.68 | 51.17 | 74.46 | 42.69 | 133.9 | ||||
| Ratio, Method | Z-M20 | Z-M50 | ||||
|---|---|---|---|---|---|---|
| Raw | Low | High | Raw | Low | High | |
| Fe:K, XPS | 0.57 | 1.07 | 1.12 | 0.16 | 0.51 | 1.56 |
| Fe:K, XRF | 2.38 | 7.25 | 0.16 | 0.16 | 2.04 | 4.64 |
| ΔFe:K, XPS, % | 87.19 | 94.95 | 227.88 | 898.60 | ||
| ΔFe:K, XRF, % | 278.50 | 1055.91 | 1196.55 | 2853.99 | ||
| Adsorbent | qm mg.g−1 | Temperature °C | Initial pH | Source |
|---|---|---|---|---|
| Zeolite—M20 | 10.19 | 25 | * | this study |
| Zeolite—M50 | 9.73 | 25 | * | this study |
| Bentonite—BL | 11.64 | 25 | * | this study |
| Bentonite—BR | 16.86 | 25 | * | this study |
| Zeolite | 98.00 | room | 3.0 | [8] |
| Bentonite | 28.90 | 30 | 3.0 | [9] |
| H2SO4 activated bentonite | 30.00 | 30 | 3.0 | [9] |
| Egyptian Bentonite | 52.63 | 20 | 4.0 | [10] |
| Egyptian Bentonite | 56.18 | 40 | 4.0 | [10] |
| Egyptian Bentonite | 58.48 | 50 | 4.0 | [10] |
| Egyptian Bentonite | 63.69 | 60 | 4.0 | [10] |
| Sawdust modified with diethylenetriamine | 200.00 | room | 3.0 | [12] |
| Elderberry pomace | 33.25 | 23 | 3.4 | [13] |
| Chitosan/Fe3O4/graphene oxide nanocomposite | 6.50 | room | 2.5 | [15] |
| Y zeolite | 31.45 | 25 | 6.5 | [16] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakalár, T.; Kaňuchová, M.; Girová, A.; Pavolová, H.; Hromada, R.; Hajduová, Z. Characterization of Fe(III) Adsorption onto Zeolite and Bentonite. Int. J. Environ. Res. Public Health 2020, 17, 5718. https://doi.org/10.3390/ijerph17165718
Bakalár T, Kaňuchová M, Girová A, Pavolová H, Hromada R, Hajduová Z. Characterization of Fe(III) Adsorption onto Zeolite and Bentonite. International Journal of Environmental Research and Public Health. 2020; 17(16):5718. https://doi.org/10.3390/ijerph17165718
Chicago/Turabian StyleBakalár, Tomáš, Mária Kaňuchová, Anna Girová, Henrieta Pavolová, Rudolf Hromada, and Zuzana Hajduová. 2020. "Characterization of Fe(III) Adsorption onto Zeolite and Bentonite" International Journal of Environmental Research and Public Health 17, no. 16: 5718. https://doi.org/10.3390/ijerph17165718
APA StyleBakalár, T., Kaňuchová, M., Girová, A., Pavolová, H., Hromada, R., & Hajduová, Z. (2020). Characterization of Fe(III) Adsorption onto Zeolite and Bentonite. International Journal of Environmental Research and Public Health, 17(16), 5718. https://doi.org/10.3390/ijerph17165718






