The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Outcomes
2.3. Governance and Ethics
2.4. Population
2.5. Sample Size
2.6. Clinical Parameters
- Plaque Index (PI),
- Probing Pocket Depth (PDD),
- Bleeding on Probing (BOP)
- Clinical Attachment Level (CAL)
2.7. Randomization
2.8. Study Treatment
- Rinsing with ozonated water (1:3) for two minutes;
- Decontamination of the oral cavity;
- Drying the periodontal sites;
- Irrigating the periodontal pockets with 150 mL ozonized water;
- Applying gaseous ozone as previously described.
2.9. Statistics
3. Results
Clinical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caton, J.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]
- Persson, G.R. What has ageing to do with periodontal health and disease? Int. Dent. J. 2006, 56, 240–249. [Google Scholar] [CrossRef]
- Lang, N.P.; Bartold, P.M. Periodontal health. J. Clin. Periodontol. 2018, 45, S230–S236. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, S.; Huizinga, J.D.; Loos, B.G. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J. Clin. Periodontol. 2008, 35, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Ocampo, G.L. Diabetes mellitus and periodontal disease. Periodontol. 2000 2007, 44, 127–153. [Google Scholar] [CrossRef]
- King, G.L. The role of inflammatory cytokines in diabetes and its complications. J. Periodontol. 2008, 79, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B.L.; Oates, T.W. The American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M.; Susin, C.; Hughes, F.J. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S171–S189. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J.; Loos, B.G.; van der Reijden, W.A.; van der Velden, U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 2002, 29, 1023–1028. [Google Scholar] [CrossRef]
- Taylor, G.W.; Borgnakke, W.S. Periodontal disease: Associations with diabetes, glycemic control and complications. Oral. Dis. 2008, 14, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M., Jr.; Cullinan, M.P.; Atieh, M. An overview of systematic reviews on the effectiveness of periodontal treatment to improve glycaemic control. J. Periodontal Res. 2016, 51, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Sudhakara, P.; Gupta, A.; Bhardwaj, A.; Wilson, A. Oral Dysbiotic Communities and Their Implications in Systemic Diseases. Dent. J. (Basel) 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- De Miguel-Infante, A.; Martinez-Huedo, M.A.; Mora-Zamorano, E.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; de Burgos-Lunar, C.; Cardenas Valladolid, J.; Jiménez-García, R.; Lopez-de-Andrés, A. Periodontal disease in adults with diabetes, prevalence and risk factors. Results of an observational study. Int. J. Clin. Pract. 2018. [Google Scholar] [CrossRef]
- Chicharro-Luna, E.; Pomares-Gómez, F.J.; Ortega-Ávila, A.B.; Marchena-Rodríguez, A.; Blanquer-Gregori, J.F.J.; Navarro-Flores, E. Predictive model to identify the risk of losing protective sensibility of the foot in patients with diabetes mellitus. Int. Wound J. 2020, 17, 220–227. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Morales-Asencio, J.M.; Cervera-Marín, J.A.; Labajos-Manzanares, M.T.; Gijon-Nogueron, G. Development, validation and psychometric analysis of the diabetic foot self-care questionnaire of the University of Malaga, Spain (DFSQ-UMA). J. Tissue Viability 2015, 24, 24–34. [Google Scholar] [CrossRef]
- Quintero, A.J.; Chaparro, A.; Quirynen, M.; Ramirez, V.; Prieto, D.; Morales, H.; Prada, P.; Hernández, M.; Sanz, A. Effect of two periodontal treatment modalities in patients with uncontrolled type 2 diabetes mellitus: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1098–1106. [Google Scholar] [CrossRef]
- Christgau, M.; Palitzsch, K.D.; Schmalz, G.; Kreiner, U.; Frenzel, S. Healing response to non-surgical periodontal therapy in patients with diabetes mellitus: Clinical, microbiological, and immunologic results. J. Clin. Periodontol. 1998, 25, 112–124. [Google Scholar] [CrossRef]
- Ioannidou, E.; Kao, D.; Chang, N.; Burleson, J.; Dongari-Bagtzoglou, A. Elevated serum interleukin-6 (IL-6) in solid-organ transplant recipients is positively associated with tissue destruction and IL-6 gene expression in the periodontium. J. Periodontol. 2006, 77, 1871–1878. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Nishimura, F.; Nakagawa, M.; Sugimoto, H.; Shikata, K.; Makino, H.; Fukuda, T.; Tsuji, T.; Iwamoto, M.; Murayama, Y. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J. Periodontol. 2001, 72, 774–778. [Google Scholar] [CrossRef]
- Menabde, G.T.; Natroshvili, N.D.; Natroshvili, T.D. Ozonotherapy for the treatment of periodontitis. Georgian Med. News 2006, 134, 43–46. [Google Scholar]
- Andreula, C.F.; Simonetti, L.; De Santis, F.; Agati, R.; Ricci, R.; Leonardi, M. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. AJNR Am. J. Neuroradiol. 2003, 24, 996–1000. [Google Scholar] [PubMed]
- Clavo, B.; Pérez, J.L.; López, L.; Suárez, G.; Lloret, M.; Rodríguez, V.; Macías, D.; Santana, M.; Morera, J.; Fiuza, D.; et al. Effect of ozone therapy on muscle oxygenation. J. Altern. Complement. Med. 2003, 9, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. Is it true that ozone is always toxic? The end of a dogma. Toxicol. Appl. Pharmacol. 2006, 16, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Menéndez, S.; Wong, R. Decrease of blood cholesterol and stimulation of antioxidative response in cardiopathy patients treated with endovenous ozone therapy. Free Radical. Biol. Med. 1995, 19, 115–119. [Google Scholar] [CrossRef]
- Folinsbee, L.J. Effects of ozone exposure on lung function in man. Rev. Environ. Health 1981, 3, 211–240. [Google Scholar]
- Holt, G.R. Declaration of Helsinki—The World’s Document of Conscience and Responsibility. South. Med. J. 2014, 107, 407. [Google Scholar] [CrossRef]
- Bocci, V. Biological and clinical effects of ozone: Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 1999, 56, 270–279. [Google Scholar]
- Di Paolo, N.; Bocci, V.; Gaggioti, E. Ozone therapy editorial review. Int. J. Artif. Organs 2004, 27, 168–175. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Pérez-Ros, P.; FM, M.-A.; Julían-Rochina, I.; Cauli, O. Neuro-psychiatric alterations in patients with diabetic foot syndrome. CNS Neurol. Disord. Drug Targets 2019, 18, 598–608. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Cauli, O. Quality of life in individuals with diabetic foot syndrome. Endocr. Metab. Immune Disord.-Drug Targets 2020. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.A.; Miller, D.R.; Wehler, C.J.; Rich, S.E.; Krall-Kaye, E.A.; McCoy, L.C.; Christiansen, C.L.; Rothendler, J.A.; Garcia, R.I. Does periodontal care improve glycemic control? The Department of Veterans Affairs Dental Diabetes Study. J. Clin. Periodontol. 2007, 34, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lira Junior, R.; Santos, C.M.M.; Oliveira, B.H.; Fischer, R.G.; Santos, A.P.P. Effects on HbA1c in diabetic patients of adjunctive use of systemic antibiotics in nonsurgical periodontal treatment: A systematic review. J. Dent. 2017, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol. 2000 2013, 62, 218–231. [Google Scholar] [CrossRef]
- Grellmann, A.P.; Sfreddo, C.S.; Maier, J.; Lenzi, T.L.; Zanatta, F.B. Systemic antimicrobials adjuvant to periodontal therapy in diabetic subjects: A meta-analysis. J. Clin. Periodontol. 2016, 43, 250–260. [Google Scholar] [CrossRef]
- Bocci, V. Does ozone therapy normalize the cellular redox balance? Implications for the therapy of human immunodeficiency virus infection and several other diseases. Med. Hypothesis 1996, 46, 150–154. [Google Scholar] [CrossRef]
- Bocci, V. The Antioxidant System and the Defence System against Ozone; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Pryor, W.A.; Wu, M. Ozonation of methyl oleate in hexane, in a thin film, in SDS micelles, and in distearoylphosphatidylcholine liposomes: Yields and properti of the Criegee ozonide. Chem. Res. Toxicol. 1992, 5, 505e511. [Google Scholar] [CrossRef]
- Skurska, A.; Pietruska, M.D.; Paniczko-Drężek, A.; Dolińska, E.; Zelazowska-Rutkowska, B.; Zak, J.; Pietruski, J.; Milewski, R.; Wysocka, J. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv. Med. Sci. 2010, 55, 297–307. [Google Scholar] [CrossRef]
- Uraz, A.; Karaduman, B.; Isler, S.C.; Gönen, S.; Çetiner, D. Ozone application as adjunctive therapy in chronic periodontitis: Clinical, microbiological and biochemical aspects. J. Dent. Sci. 2019, 14, 27–37. [Google Scholar] [CrossRef]
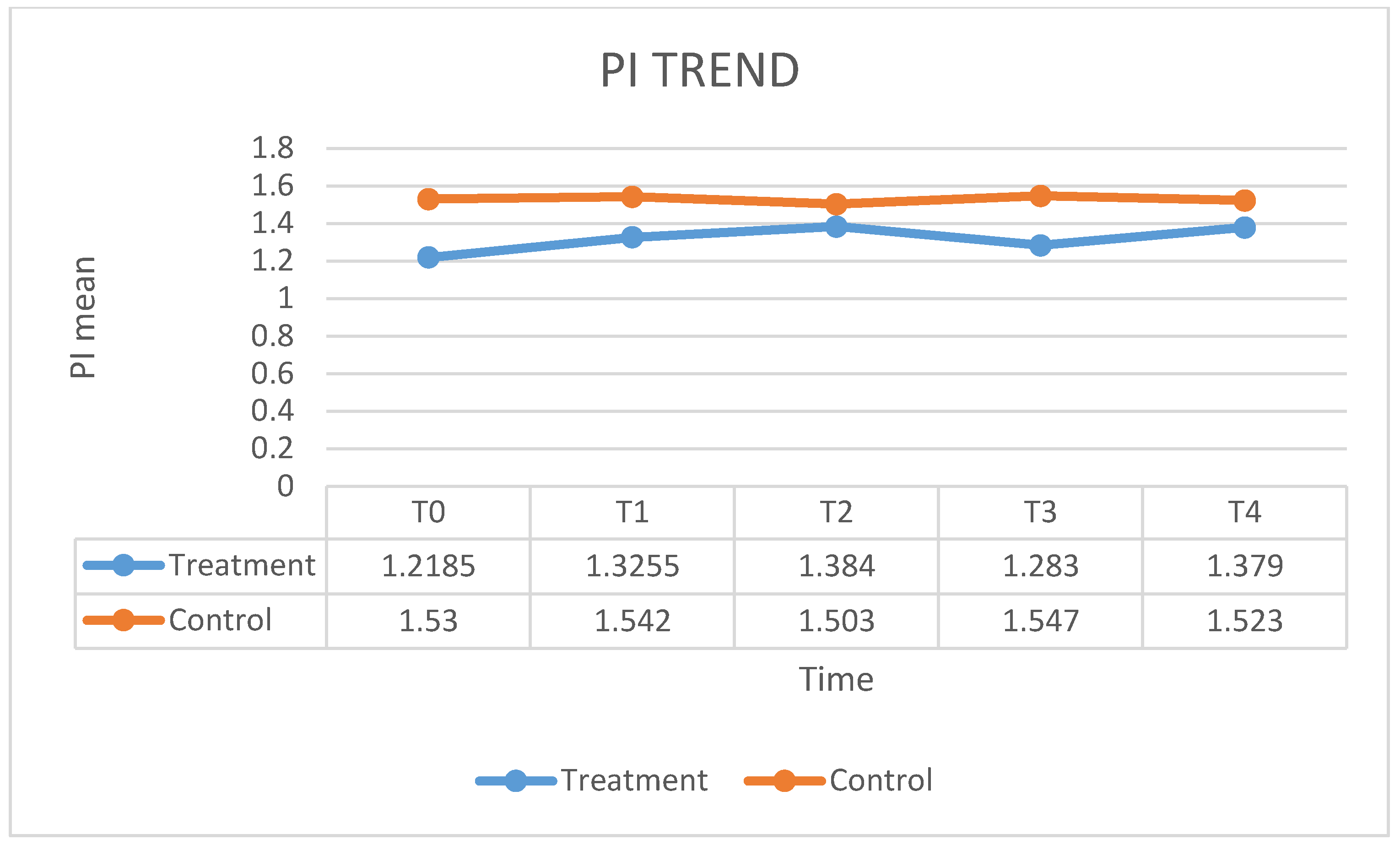

| PI T0 | PI T1 | PI T2 | PI T3 | PI T4 | |
| Mean | 77.35 | 22.2 | 14.05 | 9.2 | 6.8 |
| SD | 18.582 | 8.224 | 6.476 | 3.874 | 5.908 |
| BoP T0 | BoP T1 | BoP T2 | BoP T3 | BoP T4 | |
| Mean | 79.35 | 24 | 13.8 | 8.55 | 6.2 |
| SD | 23.058 | 12.388 | 7.112 | 3.677 | 4.047 |
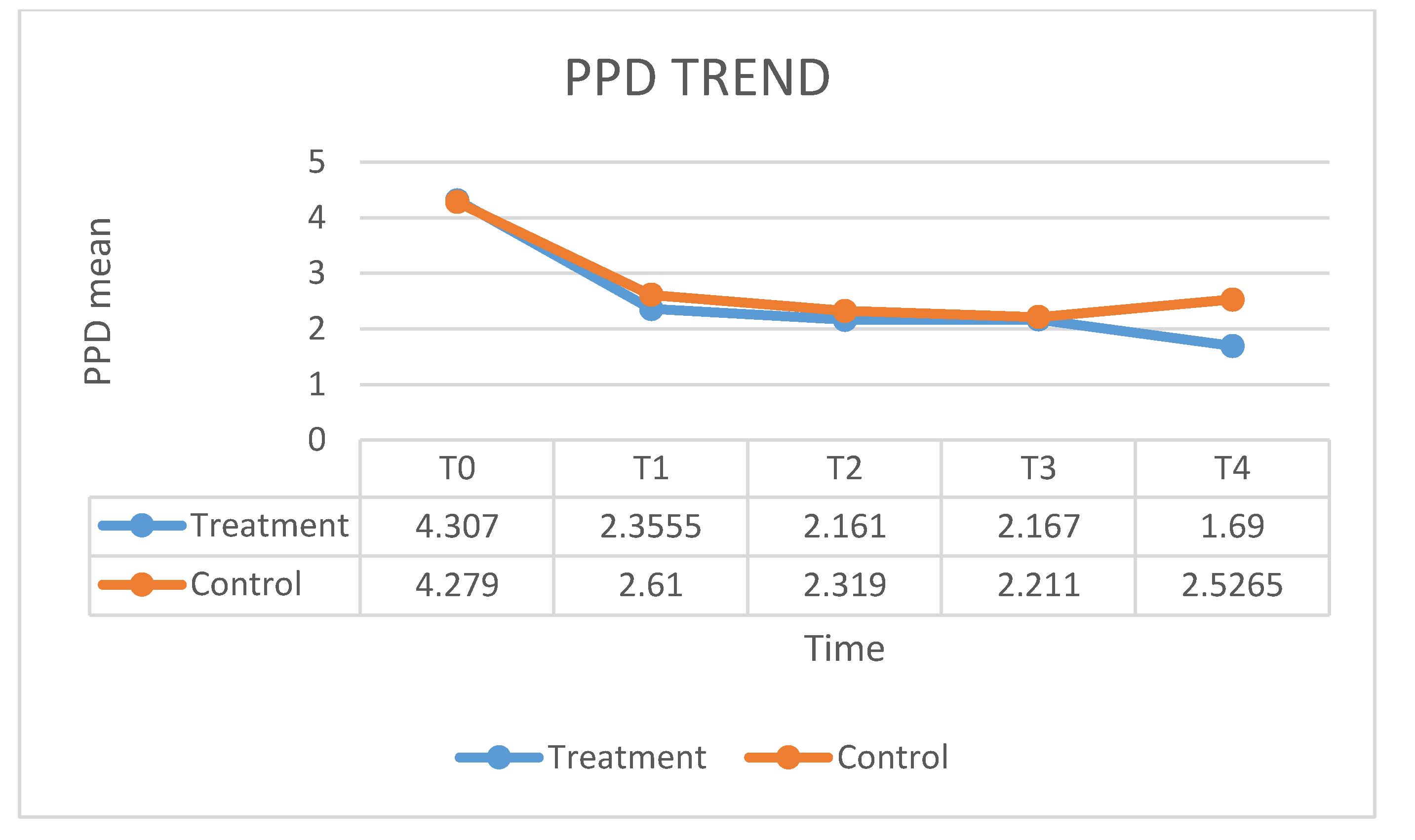
| PPD T0 | PPD T1 | PPD T2 | PPD T3 | PPD T4 | |
| Mean | 4.279 | 2.61 | 2.319 | 2.211 | 2.526 |
| SD | 0.945 | 0.775 | 0.726 | 0.683 | 0.831 |
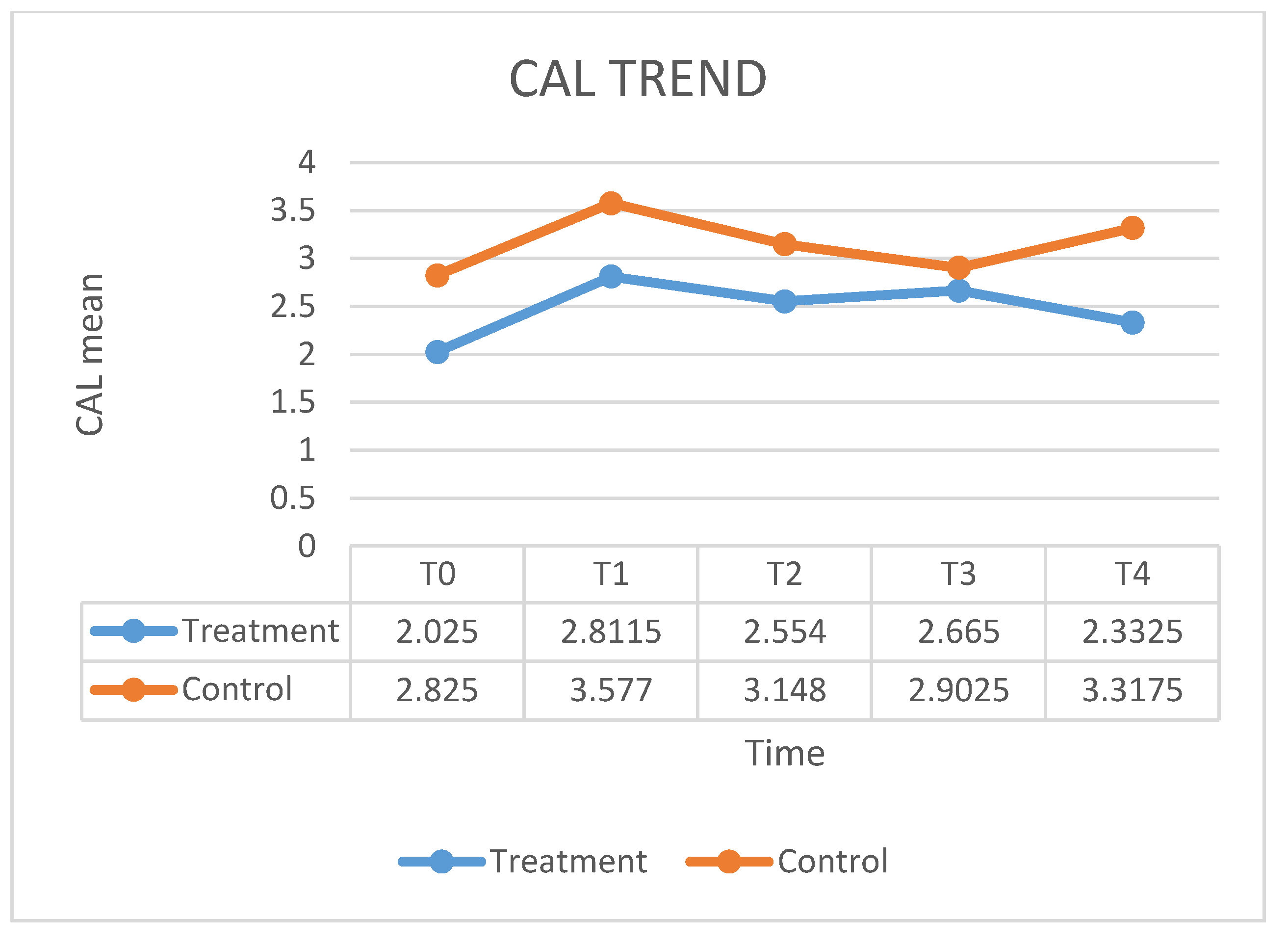
| CAL T0 | CAL T1 | CAL T2 | CAL T3 | CAL T4 | |
| Mean | 2.825 | 3.577 | 3.148 | 2.902 | 3.317 |
| SD | 0.899 | 0.915 | 0.987 | 0.849 | 1.147 |
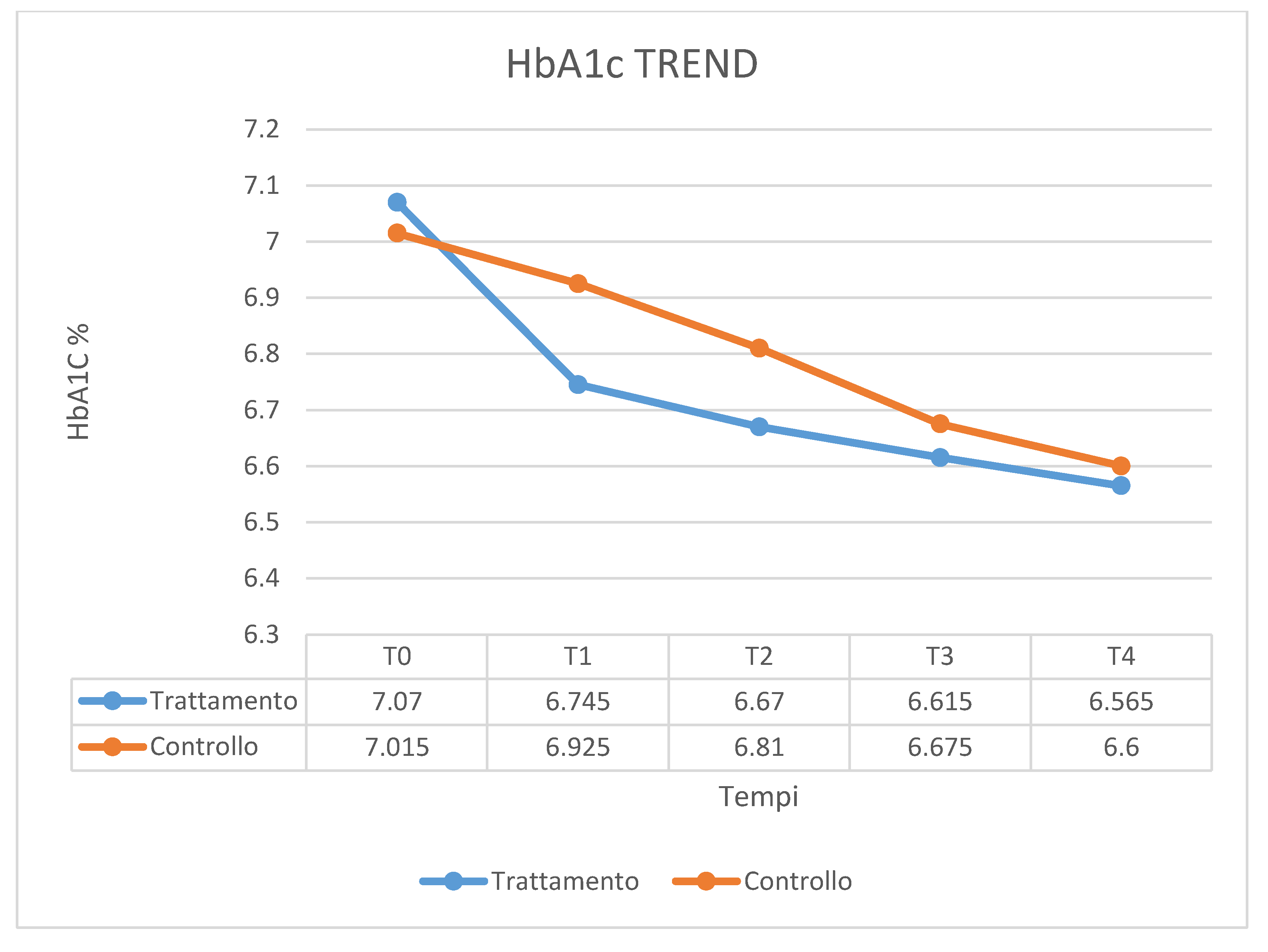
| HbA1c T0 | HbA1c T1 | HbA1c T2 | HbA1c T3 | HbA1c T4 | |
| Mean | 7.015 | 6.925 | 6.81 | 6.675 | 6.6 |
| SD | 0.767 | 0.841 | 0.881 | 0.829 | 0.773 |
| Indices | U of Mann–Whitney | W of Wilcoxon | Z | Exact Sig. [2 × (1 − Taliled Sig.)] |
|---|---|---|---|---|
| PI T0 | 185,500 | 395,500 | −0.397 | 0.698 b |
| PI T1 | 166,000 | 376,000 | −0.922 | 0.369 b |
| PI T2 | 161,000 | 371,000 | −1.060 | 0.301 b |
| PI T3 | 190,500 | 400,500 | −0.258 | 0.799 b |
| PI T4 | 165,500 | 375,500 | −0.949 | 0.355 b |
| BoP T0 | 175,000 | 385,000 | −0.691 | 0.512 b |
| BoP T1 | 192,500 | 402,500 | −0.203 | 0.841 b |
| BoP T2 | 190,500 | 400,500 | −0.258 | 0.799 b |
| BoP T3 | 195,000 | 405,000 | −0.136 | 0.904 b |
| BoP T4 | 185,500 | 395,500 | −0.394 | 0.698 b |
| PPD T0 | 183,000 | 393,000 | −0.460 | 0.659 b |
| PPD T1 | 158,500 | 368,500 | −1.124 | 0.265 b |
| PPD T2 | 172,500 | 382,500 | −0.744 | 0.461 b |
| PPD T3 | 196,500 | 406,500 | −0.095 | 0.925 b |
| PPD T4 | 85,000 | 295,000 | −3.113 | 0.001 b |
| CAL T0 | 102,500 | 312,500 | −2.643 | 0.007 b |
| CAL T1 | 120,500 | 330,500 | −2.151 | 0.030 b |
| CAL T2 | 130,000 | 340,000 | −1.894 | 0.060 b |
| CAL T3 | 174,500 | 384,500 | −0.690 | 0.495 b |
| CAL T4 | 100,000 | 310,000 | −2.705 | 0.006 b |
| HbA1c T0 | 200,000 | 410,000 | 0.000 | 1.000 b |
| HbA1c T1 | 178,000 | 388,000 | −0.599 | 0.565 b |
| HbA1c T2 | 172,500 | 382,500 | −0.746 | 0.461 b |
| HbA1c T3 | 188,000 | 398,000 | −0.326 | 0.758 b |
| HbA1c T4 | 199,000 | 409,000 | −0.027 | 0.989 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. https://doi.org/10.3390/ijerph17155467
Rapone B, Ferrara E, Corsalini M, Converti I, Grassi FR, Santacroce L, Topi S, Gnoni A, Scacco S, Scarano A, et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(15):5467. https://doi.org/10.3390/ijerph17155467
Chicago/Turabian StyleRapone, Biagio, Elisabetta Ferrara, Massimo Corsalini, Ilaria Converti, Felice Roberto Grassi, Luigi Santacroce, Skender Topi, Antonio Gnoni, Salvatore Scacco, Antonio Scarano, and et al. 2020. "The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 15: 5467. https://doi.org/10.3390/ijerph17155467
APA StyleRapone, B., Ferrara, E., Corsalini, M., Converti, I., Grassi, F. R., Santacroce, L., Topi, S., Gnoni, A., Scacco, S., Scarano, A., & Delvecchio, M. (2020). The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 17(15), 5467. https://doi.org/10.3390/ijerph17155467












