Coffee Consumption, Genetic Polymorphisms, and the Risk of Type 2 Diabetes Mellitus: A Pooled Analysis of Four Prospective Cohort Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ascertainment of Type 2 Diabetes
2.3. Assessments of Coffee and Other Factors
2.4. Genotyping and Single Nucleotide Polymorphisms (SNPs) Selection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
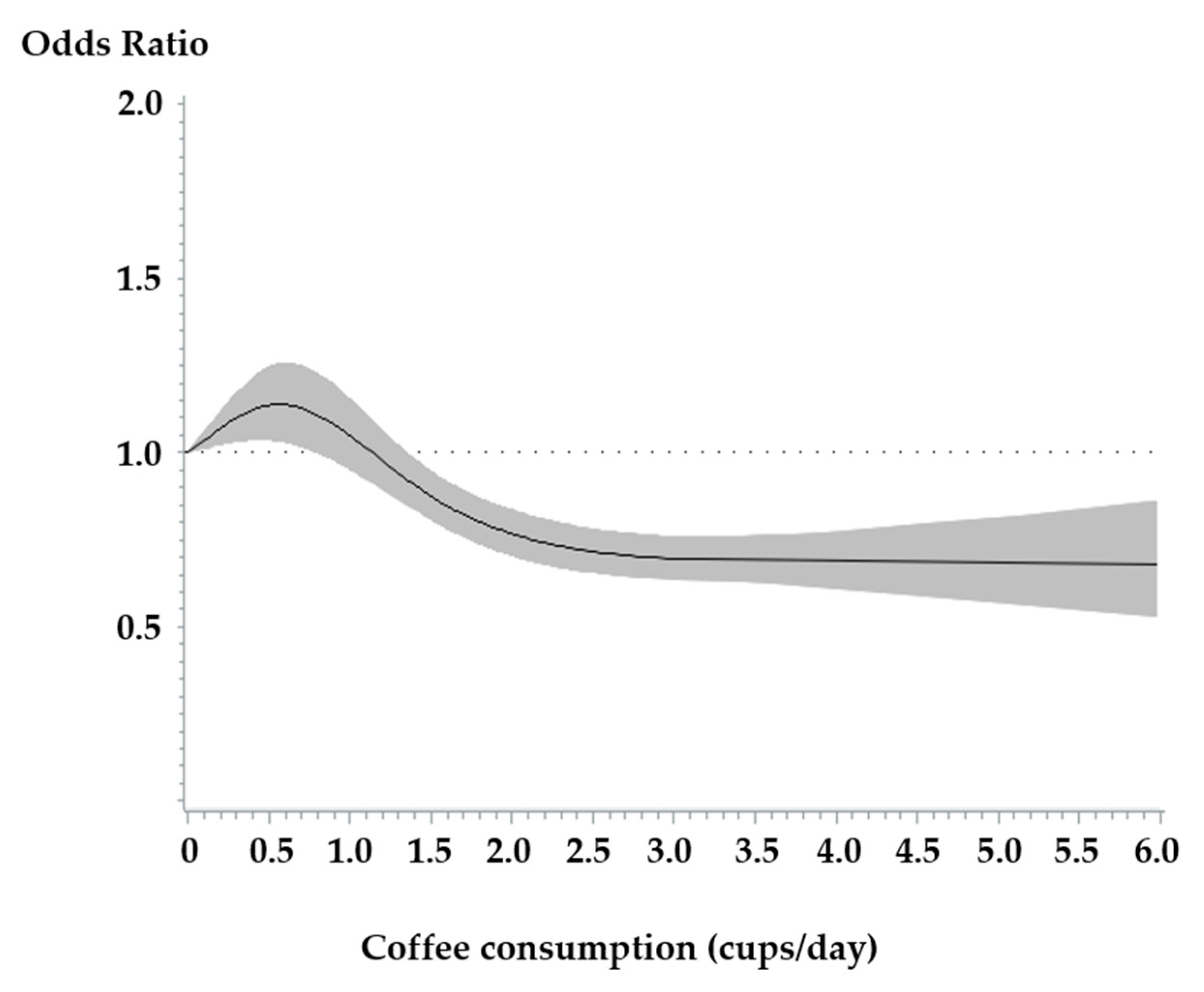
3.2. Association Between Coffee Consumption and the Risk of Type 2 Diabetes
3.3. Subgroup Analyses for the Association Between Coffee Consumption and Type 2 Diabetes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas Eighth Edition 2017, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- Noh, J. The Diabetes Epidemic in Korea. Endocrinol. Metab. 2016, 31, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare Korea Centers for Disease Control and Prevention. Korea Health Statistics 2018: Korea National Health and Nutrition Examination Survey (KNHANES VII-3); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2020.
- Neuenschwander, M.; Ballon, A.; Weber, K.S.; Norat, T.; Aune, D.; Schwingshackl, L.; Schlesinger, S. Role of diet in type 2 diabetes incidence: Umbrella review of meta-analyses of prospective observational studies. BMJ (Clin. Res. Ed.) 2019, 366, l2368. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Unwin, D.; Finucane, F. Low-Carbohydrate Diets in the Management of Obesity and Type 2 Diabetes: A Review from Clinicians Using the Approach in Practice. Int. J. Environ. Res. Public Health 2020, 17, 2557. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Tekos, F.; Mesnage, R.; Kouretas, D.; Tsatsakis, A. Chronic Inflammation in the Context of Everyday Life: Dietary Changes as Mitigating Factors. Int. J. Environ. Res. Public Health 2020, 17, 4135. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Han, W.; Wang, Y.; Zhang, Y.; Wu, S.; Zhang, H.; Jiang, L.; Wang, R.; Zhang, P.; Yu, Y.; et al. Identification of Risk Factors Affecting Impaired Fasting Glucose and Diabetes in Adult Patients from Northeast China. Int. J. Environ. Res. Public Health 2015, 12, 12662. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare Korea Centers for Disease Control and Prevention. Korea Health Statistics 2016: Korea National Health and Nutrition Examination Survey (KNHANES VII-1); Korea Centers for Disease Control and Prevention: Chengju, Korea, 2018.
- van Dam, R.; Hu, F. Coffee consumption and risk of type 2 diabetes. A systematic review. JAMA J. Am. Med. Assoc. 2005, 294, 97–104. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Chen, M.; van Dam, R.M.; Hu, F.B. Caffeinated and Decaffeinated Coffee Consumption and Risk of Type 2 Diabetes: A Systematic Review and a Dose-Response Meta-analysis. Diabetes Care 2014, 37, 569–586. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, D.; Jiang, W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: A meta-analysis of prospective studies. Eur. J. Nutr. 2014, 53, 25–38. [Google Scholar] [CrossRef]
- Carlström, M.; Larsson, S.C. Coffee consumption and reduced risk of developing type 2 diabetes: A systematic review with meta-analysis. Nutr. Rev. 2018, 76, 395–417. [Google Scholar] [CrossRef]
- van Dam, R.M. Coffee and type 2 diabetes: From beans to beta-cells. Nutr. Metab. Cardiovasc. Dis. NMCD 2006, 16, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Sarnsamak, K.; Hauge-Evans, A.C. Coffee, type 2 diabetes and pancreatic islet function–A mini-review. J. Funct. Foods 2018, 45, 409–416. [Google Scholar] [CrossRef]
- Bidel, S.; Hu, G.; Tuomilehto, J. Coffee consumption and type 2 diabetes—An extensive review. Cent. Eur. J. Med. 2008, 3, 9. [Google Scholar] [CrossRef]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segrè, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; Hu, F.B. Gene-Environment Interactions in the Development of Type 2 Diabetes: Recent Progress and Continuing Challenges. Annu. Rev. Nutr. 2012, 32, 245–259. [Google Scholar] [CrossRef]
- Dietrich, S.; Jacobs, S.; Zheng, J.-S.; Meidtner, K.; Schwingshackl, L.; Schulze, M.B. Gene-lifestyle interaction on risk of type 2 diabetes: A systematic review. Obes. Rev. 2019, 20, 1557–1571. [Google Scholar] [CrossRef]
- Diabetes Genetics Replication and Meta-analysis Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes Consortium; South Asian Type 2 Diabetes Consortium; Mexican American Type 2 Diabetes Consortium; Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in muylti-Ethnic Samples Consortium. Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat. Genet. 2014, 46, 234–244. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; Ko, G.E.S.G. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Ahn, Y.O.; Paik, H.Y.; Ahn, Y.; Tokudome, Y.; Hamajima, N.; Inoue, M.; Tajima, K. Development of a food frequency questionnaire in Koreans. Asia Pac. J. Clin. Nutr. 2003, 12, 243–250. [Google Scholar] [PubMed]
- Korea Health Promotion Institute. Low-Risk Alcohol Drinking Guideline; Korea Health Promotion Institute: Seoul, Korea, 2013. [Google Scholar]
- Korea Centers for Disease Control and Prevention. Korean Genome and Epidemiology Study User Guideline; Korea Centers for Diesease Control and Prevention: Cheongju, Korea, 2017. [Google Scholar]
- Kim, Y.J.; Go, M.J.; Hu, C.; Hong, C.B.; Kim, Y.K.; Lee, J.Y.; Hwang, J.-Y.; Oh, J.H.; Kim, D.-J.; Kim, N.H.; et al. Large-scale genome-wide association studies in east Asians identify new genetic loci influencing metabolic traits. Nat. Genet. 2011, 43, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Moon, S.; Hwang, M.Y.; Kim, D.J.; Oh, J.H.; Kim, Y.J.; Han, B.G.; Lee, J.Y.; Kim, B.J. Gene-based copy number variation study reveals a microdeletion at 12q24 that influences height in the Korean population. Genomics 2013, 101, 134–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, Y.S.; Go, M.J.; Kim, Y.J.; Heo, J.Y.; Oh, J.H.; Ban, H.-J.; Yoon, D.; Lee, M.H.; Kim, D.-J.; Park, M.; et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat. Genet. 2009, 41, 527–534. [Google Scholar] [CrossRef]
- Moon, S.; Kim, Y.J.; Han, S.; Hwang, M.Y.; Shin, D.M.; Park, M.Y.; Lu, Y.; Yoon, K.; Jang, H.-M.; Kim, Y.K.; et al. The Korea Biobank Array: Design and Identification of Coding Variants Associated with Blood Biochemical Traits. Sci. Rep. 2019, 9, 1382. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Thompson, S.G.; Higgins, J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002, 21, 1559–1573. [Google Scholar] [CrossRef]
- Orsini, N.; Li, R.; Wolk, A.; Khudyakov, P.; Spiegelman, D. Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am. J. Epidemiol. 2012, 175, 66–73. [Google Scholar] [CrossRef]
- Yamaji, T.; Mizoue, T.; Tabata, S.; Ogawa, S.; Yamaguchi, K.; Shimizu, E.; Mineshita, M.; Kono, S. Coffee consumption and glucose tolerance status in middle-aged Japanese men. Diabetologia 2004, 47, 2145–2151. [Google Scholar] [CrossRef]
- Odegaard, A.O.; Pereira, M.A.; Koh, W.P.; Arakawa, K.; Lee, H.P.; Yu, M.C. Coffee, tea, and incident type 2 diabetes: The Singapore Chinese Health Study. Am. J. Clin. Nutr. 2008, 88, 979–985. [Google Scholar] [CrossRef]
- The InterAct Consortium. Investigation of gene–diet interactions in the incretin system and risk of type 2 diabetes: The EPIC-InterAct study. Diabetologia 2016, 59, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, K.; Ahn, Y.; Yang, M.; Lee, J.E. Habitual coffee intake, genetic polymorphisms, and type 2 diabetes. Eur. J. Endocrinol. 2015, 172, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Imamura, F.; Ye, Z.; Schulze, M.B.; Zheng, J.; Ardanaz, E.; Arriola, L.; Boeing, H.; Dow, C.; Fagherazzi, G.; et al. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: Systematic review and findings from European Prospective Investigation into Cancer (EPIC)-InterAct. Am. J. Clin. Nutr. 2017, 106, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Sham, P.C.; Purcell, S.M. Statistical power and significance testing in large-scale genetic studies. Nat. Rev. Genet. 2014, 15, 335–346. [Google Scholar] [CrossRef]
- Dimas, A.S.; Lagou, V.; Barker, A.; Knowles, J.W.; Mägi, R.; Hivert, M.-F.; Benazzo, A.; Rybin, D.; Jackson, A.U.; Stringham, H.M.; et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. Diabetes 2014, 63, 2158–2171. [Google Scholar] [CrossRef]
- Wei, F.-Y.; Suzuki, T.; Watanabe, S.; Kimura, S.; Kaitsuka, T.; Fujimura, A.; Matsui, H.; Atta, M.; Michiue, H.; Fontecave, M.; et al. Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Investig. 2011, 121, 3598–3608. [Google Scholar] [CrossRef]
- Krentz, N.A.J.; Gloyn, A.L. Insights into pancreatic islet cell dysfunction from type 2 diabetes mellitus genetics. Nat. Rev. Endocrinol. 2020, 16, 202–212. [Google Scholar] [CrossRef]
- Wei, F.Y.; Nagashima, K.; Ohshima, T.; Saheki, Y.; Lu, Y.F.; Matsushita, M.; Yamada, Y.; Mikoshiba, K.; Seino, Y.; Matsui, H.; et al. Cdk5-dependent regulation of glucose-stimulated insulin secretion. Nat. Med. 2005, 11, 1104–1108. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, H.; Shi, X.; Warren, C.R.; Lotta, L.A.; Friesen, M.; Meissner, T.B.; Langenberg, C.; Wabitsch, M.; Wareham, N.; et al. Functional Screening of Candidate Causal Genes for Insulin Resistance in Human Preadipocytes and Adipocytes. Circ. Res. 2020, 126, 330–346. [Google Scholar] [CrossRef]
- Mandal, S.S. Gene Regulation, Epigenetics and Hormone Signaling; John Wiley & Sons, Incorporated: Berlin, Germany, 2017. [Google Scholar]
- Müssig, K.; Staiger, H.; Machicao, F.; Kirchhoff, K.; Guthoff, M.; Schäfer, S.A.; Kantartzis, K.; Silbernagel, G.; Stefan, N.; Holst, J.J.; et al. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes 2009, 58, 1715–1720. [Google Scholar] [CrossRef]
- Kasuga, M. KCNQ1, a susceptibility gene for type 2 diabetes. J. Diabetes Investig. 2011, 2, 413–414. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.-J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic Acid and Hydroxynitrobenzaldehyde: New Inhibitors of Hepatic Glucose 6-Phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Buscemi, S.; Batsis, J.A.; Arcoleo, G.; Verga, S. Coffee and endothelial function: A battle between caffeine and antioxidants? Eur. J. Clin. Nutr. 2010, 64, 1242–1243. [Google Scholar] [CrossRef][Green Version]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Laskaris, A.; Stefanadis, C. The evaluation of inflammatory and oxidative stress biomarkers on coffee-diabetes association: Results from the 10-year follow-up of the ATTICA Study (2002–2012). Eur. J. Clin. Nutr. 2015, 69, 1220–1225. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Trepanowski, J.F.; Farney, T.M. Influence of acute coffee consumption on postprandial oxidative stress. Nutr. Metab. Insights 2013, 6, 35–42. [Google Scholar] [CrossRef]
- Natella, F.; Scaccini, C. Role of coffee in modulation of diabetes risk. Nutr. Rev. 2012, 70, 207–217. [Google Scholar] [CrossRef]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee Consumption Is Associated With Higher Plasma Adiponectin Concentrations in Women With or Without Type 2 Diabetes. A Prospect. Cohort Study 2008, 31, 504–507. [Google Scholar] [CrossRef]
- Imatoh, T.; Tanihara, S.; Miyazaki, M.; Momose, Y.; Uryu, Y.; Une, H. Coffee consumption but not green tea consumption is associated with adiponectin levels in Japanese males. Eur. J. Nutr. 2011, 50, 279–284. [Google Scholar] [CrossRef]
- Salvini, S.; Hunter, D.J.; Sampson, L.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Willett, W.C. Food-based validation of a dietary questionnaire: The effects of week-to-week variation in food consumption. Int. J. Epidemiol. 1989, 18, 858–867. [Google Scholar] [CrossRef]
- Tsubono, Y.; Kobayashi, M.; Sasaki, S.; Tsugane, S. Validity and Reproducibility of a Self-administered Food Frequency Questionnaire Used in the Baseline Survey of the JPHC Study Cohort I. J. Epidemiol. 2003, 13, 125–133. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Axen, K.V.; Schnoll, R.; Boozer, C.N. Coffee, tea and diabetes: The role of weight loss and caffeine. Int. J. Obes. 2005, 29, 1121–1129. [Google Scholar] [CrossRef]
- Pereira, M.A.; Parker, E.D.; Folsom, A.R. Coffee consumption and risk of type 2 diabetes mellitus: An 11-year prospective study of 28 812 postmenopausal women. Arch. Intern. Med. 2006, 166, 1311–1316. [Google Scholar] [CrossRef]
- van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Coffee, caffeine, and risk of type 2 diabetes: A prospective cohort study in younger and middle-aged U.S. women. Diabetes Care 2006, 29, 398–403. [Google Scholar] [CrossRef]
- Hjellvik, V.; Tverdal, A.; Strøm, H. Boiled coffee intake and subsequent risk for type 2 diabetes. Epidemiology 2011, 22, 418–421. [Google Scholar] [CrossRef]
- Tuomilehto, J.; Hu, G.; Bidel, S.; Lindström, J.; Jousilahti, P. Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA 2004, 291, 1213–1219. [Google Scholar] [CrossRef]
| Coffee Consumption (cups/Day) | |||||
|---|---|---|---|---|---|
| Study | 0 to <0.5 | 0.5 to <1 | 1 to <3 | ≥3 | p Value 2 |
| HEXA | |||||
| Total population no. | 15,480 | 5310 | 22,598 | 10,734 | |
| Age at baseline (years of age) | 54.51 ± 7.82 | 52.73 ± 7.86 | 52.89 ± 7.87 | 51.12 ± 7.70 | <0.001 |
| Sex | <0.001 | ||||
| Men | 3980 (25.71) | 1692 (31.86) | 6883 (30.46) | 4981 (46.40) | |
| Women | 11,500 (74.29) | 3618 (68.14) | 15,715 (69.54) | 5753 (53.60) | |
| BMI (kg/m2) | <0.001 | ||||
| <18.5 | 354 (2.29) | 88 (1.66) | 344 (1.52) | 147 (1.37) | |
| 18.5–<23 | 6848 (44.27) | 2082 (39.23) | 8689 (38.46) | 3839 (35.79) | |
| 23–<25 | 4266 (27.58) | 1546 (29.13) | 6433 (28.47) | 3087 (28.78) | |
| ≥25 | 4001 (25.86) | 1591 (29.98) | 7126 (31.54) | 3654 (34.06) | |
| Cigarette smoking (pack-years) 1 | 2.79 ± 8.57 | 4.13 ± 10.41 | 4.47 ± 10.84 | 9.33 ± 15.35 | <0.001 |
| Alcohol drinking (g/day) 1 | 4.98 ± 26.92 | 6.00 ± 16.80 | 6.03 ± 15.20 | 9.19 ± 21.81 | <0.001 |
| Regular exercise 1 | <0.001 | ||||
| no | 6572 (42.61) | 2151 (40.59) | 10,069 (44.72) | 5279 (49.30) | |
| yes | 8852 (57.39) | 3148 (59.41) | 12,447 (55.28) | 5428 (50.70) | |
| Education level 1 | <0.001 | ||||
| Elementary school or less | 2778 (18.20) | 643 (12.22) | 2996 (13.42) | 1026 (9.65) | |
| Middle school | 2828 (18.53) | 754 (14.33) | 3462 (15.51) | 1443 (13.57) | |
| High school or above | 9655 (63.27) | 3863 (73.44) | 15,866 (71.07) | 8163 (76.78) | |
| Total energy intake (kcal/day) | 1649.55 ± 493.61 | 1697.68 ± 494.80 | 1780.62 ± 490.32 | 1881.3 ± 541.84 | <0.001 |
| Green tea consumption (cups/day) | <0.001 | ||||
| 0–<1 | 6057 (39.13) | 1583 (29.81) | 8864 (39.22) | 4727 (44.04) | |
| 1–<2 | 7653 (49.44) | 3134 (59.02) | 9211 (40.76) | 3919 (36.51) | |
| ≥2 | 1770 (11.44) | 593 (11.17) | 4523 (20.02) | 2088 (19.45) | |
| CAVAS | |||||
| Total population no. | 3375 | 889 | 3991 | 1739 | |
| Age at baseline (years of age) | 61.84 ± 9.07 | 59.79 ± 9.25 | 60.50 ± 9.09 | 58.50 ± 9.27 | <0.001 |
| Sex | <0.001 | ||||
| Men | 1038 (30.76) | 354 (39.82) | 1451 (36.36) | 937 (53.88) | |
| Women | 2337 (69.24) | 535 (60.18) | 2540 (63.64) | 802 (46.12) | |
| BMI (kg/m2) | <0.001 | ||||
| <18.5 | 70 (2.07) | 17 (1.91) | 64 (1.60) | 30 (1.73) | |
| 18.5-<23 | 1238 (36.68) | 278 (31.27) | 1198 (30.02) | 549 (31.57) | |
| 23-<25 | 865 (25.63) | 218 (24.52) | 1061 (26.58) | 445 (25.59) | |
| ≥25 | 1202 (35.61) | 376 (42.29) | 1668 (41.79) | 715 (41.12) | |
| Cigarette smoking (pack-years) 1 | 4.75 ± 13.26 | 6.99 ± 15.64 | 7.15 ± 15.96 | 14.17 ± 21.63 | <0.001 |
| Alcohol drinking (g/day) 1 | 7.01 ± 22.73 | 10.69 ± 25.78 | 8.40 ± 22.47 | 10.26 ± 23.08 | <0.001 |
| Regular exercise 1 | 0.141 | ||||
| no | 2303 (68.34) | 580 (65.24) | 2756 (69.11) | 1174 (67.55) | |
| yes | 1067 (31.66) | 309 (34.76) | 1232 (30.89) | 564 (32.45) | |
| Education level 1 | <0.001 | ||||
| Elementary school or less | 2085 (61.98) | 433 (48.82) | 2165 (54.30) | 754 (43.43) | |
| Middle school | 532 (15.81) | 150 (16.91) | 713 (17.88) | 345 (19.87) | |
| High school or above | 747 (22.21) | 304 (34.27) | 1109 (27.82) | 637 (36.69) | |
| Total energy intake (kcal/day) | 1541.67 ± 474.21 | 1634.86 ± 492.65 | 1679.10 ± 469.95 | 1846.12 ± 545.15 | <0.001 |
| Green tea consumption (cups/day) | <0.001 | ||||
| 0–<1 | 1351 (40.03) | 346 (38.92) | 1826 (45.75) | 825 (47.44) | |
| 1–<2 | 1676 (49.66) | 478 (53.77) | 1479 (37.06) | 592 (34.04) | |
| ≥2 | 348 (10.31) | 65 (7.31) | 686 (17.19) | 322 (18.52) | |
| KARE | |||||
| Total population no. | 2079 | 546 | 2280 | 793 | |
| Age at baseline (years of age) | 53.41 ± 8.70 | 50.71 ± 8.16 | 50.20 ± 8.14 | 48.60 ± 7.48 | <0.001 |
| Sex | <0.001 | ||||
| Men | 804 (38.67) | 280 (51.28) | 1027 (45.04) | 551 (69.48) | |
| Women | 1275 (61.33) | 266 (48.72) | 1253 (54.96) | 242 (30.52) | |
| BMI (kg/m2) | <0.001 | ||||
| <18.5 | 42 (2.02) | 3 (0.55) | 25 (1.10) | 14 (1.77) | |
| 18.5–<23 | 696 (33.48) | 148 (27.11) | 659 (28.90) | 214 (26.99) | |
| 23–<25 | 524 (25.20) | 159 (29.12) | 622 (27.28) | 209 (26.36) | |
| ≥25 | 817 (39.30) | 236 (43.22) | 974 (42.72) | 356 (44.89) | |
| Cigarette smoking (pack-years) 1 | 5.61 ± 12.29 | 8.19 ± 13.33 | 8.37 ± 14.72 | 17.35 ± 19.62 | <0.001 |
| Alcohol drinking (g/day) 1 | 6.86 ± 17.69 | 9.64 ± 19.92 | 9.35 ± 21.12 | 13.22 ± 25.44 | <0.001 |
| Exercise (MET-h/wk) 1 | 1596.83 ± 970.33 | 1574.97 ± 985.81 | 1507.31 ± 911.05 | 1441.54 ± 961.17 | <0.001 |
| Education level 1 | <0.001 | ||||
| Elementary school or less | 1315 (63.68) | 274 (50.46) | 1108 (48.85) | 305 (38.56) | |
| Middle school | 538 (26.05) | 182 (33.52) | 810 (35.71) | 329 (41.59) | |
| High school or above | 212 (10.27) | 87 (16.02) | 350 (15.43) | 157 (19.85) | |
| Total energy intake (kcal/day) | 1861.91 ± 614.64 | 1933.38 ± 609.98 | 1971.82 ± 589.86 | 2115.02 ± 647.64 | <0.001 |
| Green tea consumption (cups/day) | <0.001 | ||||
| 0–<1 | 795 (38.24) | 170 (31.14) | 857 (37.59) | 361 (45.52) | |
| 1–<2 | 1063 (51.13) | 330 (60.44) | 982 (43.07) | 281 (35.44) | |
| ≥2 | 221 (7.89) | 46 (8.42) | 441 (19.34) | 151 (19.05) | |
| TWIN | |||||
| Total population no. | 478 | 252 | 590 | 393 | |
| Age at baseline (years of age) | 43.21 ± 14.66 | 43.99 ± 13.78 | 42.58 ± 11.60 | 41.73 ± 10.12 | 0.121 |
| Sex | <0.001 | ||||
| Men | 161 (33.68) | 86 (34.13) | 209 (35.42) | 194 (49.36) | |
| Women | 317 (66.32) | 166 (65.87) | 381 (64.58) | 199 (50.64) | |
| BMI (kg/m2) | 0.012 | ||||
| <18.5 | 18 (3.77) | 11 (4.37) | 6 (1.02) | 10 (2.54) | |
| 18.5–<23 | 226 (47.38) | 104 (41.27) | 265 (44.92) | 154 (39.19) | |
| 23–<25 | 110 (23.06) | 57 (22.62) | 145 (24.58) | 93 (23.66) | |
| ≥25 | 123 (25.79) | 80 (31.75) | 174 (29.49) | 136 (34.61) | |
| Cigarette smoking (pack-years) 1 | 2.54 ± 7.75 | 3.22 ± 8.24 | 4.37 ± 9.57 | 9.55 ± 16.96 | <0.001 |
| Alcohol drinking (g/day) 1 | 7.09 ± 16.94 | 7.36 ± 14.26 | 8.94 ± 18.36 | 16.36 ± 53.90 | <0.001 |
| Regular exercise 1 | 0.176 | ||||
| no | 291 (62.58) | 161 (65.18) | 384 (66.67) | 270 (70.13) | |
| yes | 174 (37.42) | 86 (34.82) | 192 (33.33) | 115 (29.87) | |
| Education level 1 | 0.051 | ||||
| Elementary school or less | 75 (15.82) | 30 (11.95) | 64 (10.87) | 38 (9.69) | |
| Middle school | 28 (5.91) | 24 (9.56) | 38 (6.45) | 28 (7.14) | |
| High school or above | 371 (78.27) | 197 (78.49) | 487 (82.68) | 326 (83.16) | |
| Total energy intake (kcal/day) | 1815.86 ± 707.23 | 1869.54 ± 647.56 | 1904.06 ± 632.84 | 2069.78 ± 697.32 | <0.001 |
| Green tea consumption (cups/day) | <0.001 | ||||
| 0–<1 | 142 (29.71) | 49 (19.44) | 147 (24.92) | 100 (25.45) | |
| 1–<2 | 210 (43.93) | 125 (49.60) | 214 (36.27) | 132 (33.59) | |
| ≥2 | 126 (26.36) | 88 (30.96) | 229 (38.81) | 161 (40.96) | |
| Coffee Consumption (cups/Day) | p for Trend | p for Heterogeneity 1 | |||||
|---|---|---|---|---|---|---|---|
| Study | Median Follow-Up Period (Years) | 0 to <0.5 | 0.5 to <1 | 1 to <3 | ≥3 | ||
| HEXA | 4.25 | ||||||
| Case/Total no. | 810/15,480 | 299/5310 | 1199/22,598 | 561/10,734 | |||
| Age-sex adjusted OR (CIs) | 1.00 (reference) | 1.12 (0.97–1.28) | 1.05 (0.96–1.15) | 1.03 (0.92–1.15) | 0.94 | ||
| MV adjusted OR (CIs) | 1.00 (reference) | 1.05 (0.92–1.21) | 0.97 (0.88–1.06) | 0.90 (0.80–1.01) | 0.04 | ||
| CAVAS | 2.08 | ||||||
| Case/Total no. | 92/3375 | 28/889 | 121/3991 | 43/1739 | |||
| Age-sex adjusted OR (CIs) | 1.00 (reference) | 1.17 (0.76–1.80) | 1.12 (0.85–1.48) | 0.90 (0.62–1.31) | 0.62 | ||
| MV adjusted OR (CIs) | 1.00 (reference) | 1.11 (0.72–1.71) | 1.05 (0.79–1.39) | 0.84 (0.57–1.24) | 0.38 | ||
| KARE | 11.67 | ||||||
| Case/Total no. | 515/1564 | 126/420 | 556/1724 | 192/601 | |||
| Age-sex adjusted OR (CIs) | 1.00 (reference) | 0.95 (0.76–1.19) | 1.06 (0.92–1.22) | 1.02 (0.84–1.25) | 0.71 | ||
| MV adjusted OR (CIs) | 1.00 (reference) | 0.90 (0.72–1.14) | 0.99 (0.85–1.15) | 0.88 (0.71–1.09) | 0.29 | ||
| TWIN | 3.17 | ||||||
| Case/Total no. | 18/478 | 10/252 | 17/590 | 13/393 | |||
| Age-sex adjusted OR (CIs) | 1.00 (reference) | 1.04 (0.44–1.46) | 0.86 (0.43–1.68) | 1.08 (0.48–1.40) | 0.90 | ||
| MV adjusted OR (CIs) | 1.00 (reference) | 1.00 (0.42–1.39) | 0.75 (0.38–1.48) | 0.82 (0.33–1.06) | 0.65 | ||
| Pooled | |||||||
| MV adjusted OR (CIs) | 1.00 (reference) | 1.02 (0.91–1.14) | 0.97 (0.90–1.05) | 0.89 (0.80–0.98) | 0.01 | 0.99 | |
| Coffee Consumption (cups/Day) | p for Trend | p for Interaction | p for Heterogeneity 1 | |||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | 0 to <0.5 | 0.5 to <1 | 1 to <3 | ≥3 | ||||
| Age at baseline (years) | 0.24 | |||||||
| <50 | Case/Total no. | 355/5811 | 131/2522 | 559/10,335 | 311/5986 | |||
| Pooled OR (CIs) | 1.00 (reference) | 0.84 (0.68–1.05) | 0.87 (0.75–1.01 | 0.79 (0.67–0.95) | 0.01 | 0.49 | ||
| ≥50 | Case/Total no. | 1080/15,601 | 332/4475 | 1334/19,124 | 498/7673 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.10 (0.96–1.26) | 1.01 (0.93–1.11) | 0.91 (0.81–1.03) | 0.11 | 0.63 | ||
| Sex | 0.51 | |||||||
| Men | Case/Total no. | 527/5983 | 216/2412 | 850/9570 | 503/6663 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.03 (0.86–1.23) | 1.01 (0.89–1.14) | 0.86 (0.75–0.99) | 0.02 | 0.97 | ||
| Women | Case/Total no. | 908/15,429 | 247/4585 | 1043/19,889 | 306/6996 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.02 (0.88–1.19) | 0.95 (0.86–1.04) | 0.93 (0.80–1.07) | 0.15 | 0.86 | ||
| BMI (kg/m2) | 0.12 | |||||||
| <25 | Case/Total no. | 766/15,257 | 211/4711 | 846/19,511 | 335/8791 | |||
| Pooled OR (CIs) | 1.00 (reference) | 0.96 (0.82–1.14) | 0.90 (0.81–1.00) | 0.80 (0.69–0.92) | <0.01 | 0.34 | ||
| ≥25 | Case/Total no. | 669/6143 | 252/2283 | 1046/9942 | 473/4861 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.09 (0.93–1.28) | 1.04 (0.93–1.16) | 0.96 (0.83–1.10) | 0.27 | 0.45 | ||
| Smoking status | 0.31 | |||||||
| Never | Case/Total no. | 1011/17,420 | 290/5233 | 1197/21,918 | 341/7769 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.06 (0.96–1.17) | 1.01 (0.94–1.07) | 0.93 (0.84–1.02) | 0.18 | 0.47 | ||
| Ever | Case/Total no. | 411/3894 | 169/1741 | 689/7427 | 466/5852 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.06 (0.92–1.21) | 0.99 (0.91–1.08) | 0.89 (0.81–0.98) | 0.01 | 0.81 | ||
| Alcohol drinking | 0.73 | |||||||
| Non-drinker | Case/Total no. | 889/14,070 | 200/3687 | 944/15,277 | 323/5993 | |||
| Pooled OR (CIs) | 1.00 (reference) | 0.92 (0.78–1.09) | 1.00 (0.90–1.11) | 0.87 (0.75–1.00) | 0.11 | 0.87 | ||
| Current drinker | Case/Total no. | 540/7251 | 260/3282 | 941/14,070 | 483/7617 | |||
| Pooled OR (CIs) | 1.00 (reference) | 1.13 (0.96–1.33)) | 0.95 (0.85- 1.07) | 0.90 (0.78- 1.04) | 0.04 | 0.70 | ||
| Subgroup | Coffee Consumption (cups/Day) | p for Trend | p for Interaction | p for Heterogeneity 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP (Risk/Other) | Chr | Locus | 0 to <0.5 | 0.5 to <1 | 1 to <3 | ≥3 | ||||
| rs7756992(G/A) | 6 | CDKAL1 | 0.56 | |||||||
| GG+AG | Case/Total no. | 424/2610 | 112/758 | 448/2957 | 165/1228 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.92 (0.72–1.17) | 0.93 (0.79–1.09) | 0.83 (0.66–1.04) | 0.11 | 0.96 | ||||
| AA | Case/Total no. | 111/757 | 21/192 | 117/806 | 38/289 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.79 (0.45–1.39) | 1.03 (0.75–1.42) | 0.99 (0.61–1.59) | 0.87 | 0.70 | ||||
| rs10811661(T/C) | 9 | CDKN2A/B | 0.97 | |||||||
| TT+CT | Case/Total no. | 437/2697 | 113/776 | 452/3020 | 170/1228 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.97 (0.75–1.23) | 0.93 (0.79–1.09) | 0.85 (0.68–1.06) | 0.15 | 0.99 | ||||
| CC | Case/Total no. | 97/670 | 20/174 | 113/743 | 34/290 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.75 (0.44–1.31) | 1.03 (0.74–1.43) | 0.86 (0.52–1.41) | 0.69 | 0.78 | ||||
| rs5215(C/T) | 11 | KCNJ11 | 0.73 | |||||||
| CC+CT | Case/Total no. | 347/2183 | 77/605 | 371/2425 | 131/997 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.83 (0.62–1.11) | 0.98 (0.83–1.17) | 0.87 (0.68–1.12) | 0.34 | 0.61 | ||||
| TT | Case/Total no. | 187/1179 | 55/343 | 194/1335 | 72/519 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 1.08 (0.74–1.56) | 0.90 (0.71–1.15) | 0.80 (0.56–1.13) | 0.22 | 0.42 | ||||
| rs163184(G/T) | 11 | KCNQ1 | 0.62 | |||||||
| GG+TG | Case/Total no. | 381/2203 | 85/633 | 399/2428 | 133/1000 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.81 (0.62–1.07) | 0.99 (0.83–1.17) | 0.81 (0.63–1.04) | 0.15 | 0.34 | ||||
| TT | Case/Total no. | 154/1164 | 48/314 | 166/1336 | 71/517 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 1.10 (0.74–1.63) | 0.90 (0.69–1.17) | 0.94 (0.65–1.35) | 0.78 | 0.20 | ||||
| rs3786897(A/G) * | 19 | PEPD | 0.68 | |||||||
| AA+AG | Case/Total no. | 403/2180 | 98/561 | 440/2453 | 149/909 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.88 (0.68–1.14) | 0.95 (0.80–1.12) | 0.82 (0.65–1.04) | 0.81 | 0.07 | ||||
| GG | Case/Total no. | 87/543 | 19/123 | 87/538 | 34/200 | |||||
| Pooled OR (CIs) | 1.00 (reference) | 0.94 (0.52–1.70) | 1.15 (0.79–1.65) | 1.31 (0.78–2.18) | 0.27 | 0.93 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.N.; Cho, H.J.; Youn, J.; Jin, T.; Kang, M.; Sung, J.; Lee, J.E. Coffee Consumption, Genetic Polymorphisms, and the Risk of Type 2 Diabetes Mellitus: A Pooled Analysis of Four Prospective Cohort Studies. Int. J. Environ. Res. Public Health 2020, 17, 5379. https://doi.org/10.3390/ijerph17155379
Kim AN, Cho HJ, Youn J, Jin T, Kang M, Sung J, Lee JE. Coffee Consumption, Genetic Polymorphisms, and the Risk of Type 2 Diabetes Mellitus: A Pooled Analysis of Four Prospective Cohort Studies. International Journal of Environmental Research and Public Health. 2020; 17(15):5379. https://doi.org/10.3390/ijerph17155379
Chicago/Turabian StyleKim, An Na, Hyun Jeong Cho, Jiyoung Youn, Taiyue Jin, Moonil Kang, Joohon Sung, and Jung Eun Lee. 2020. "Coffee Consumption, Genetic Polymorphisms, and the Risk of Type 2 Diabetes Mellitus: A Pooled Analysis of Four Prospective Cohort Studies" International Journal of Environmental Research and Public Health 17, no. 15: 5379. https://doi.org/10.3390/ijerph17155379
APA StyleKim, A. N., Cho, H. J., Youn, J., Jin, T., Kang, M., Sung, J., & Lee, J. E. (2020). Coffee Consumption, Genetic Polymorphisms, and the Risk of Type 2 Diabetes Mellitus: A Pooled Analysis of Four Prospective Cohort Studies. International Journal of Environmental Research and Public Health, 17(15), 5379. https://doi.org/10.3390/ijerph17155379





