Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California
Abstract
1. Introduction
Coccidioides spp. and Coccidioidomycosis
- (i)
- To assess whether Coccidioides DNA can be detected in soils in study areas near military bases in California were coccidioidomycosis cases were reported in previous years;
- (ii)
- To investigate whether the presence of Coccidioides is supported in soils that are characterized by specific pH, soil texture, soil ionic content, and possibly other parameters (identified parameters could be linked to large-scale datasets, or maps to indicate the distribution of supportive Coccidioides habitats);
- (iii)
- To investigate whether wind-suspendable dust at sites where surface soils do test positive for the pathogen’s DNA, and to investigate whether dust suspended by travel on unpaved roads in endemic areas of Coccidioides are potential or significant pathways for exposure.
2. Material and Methods
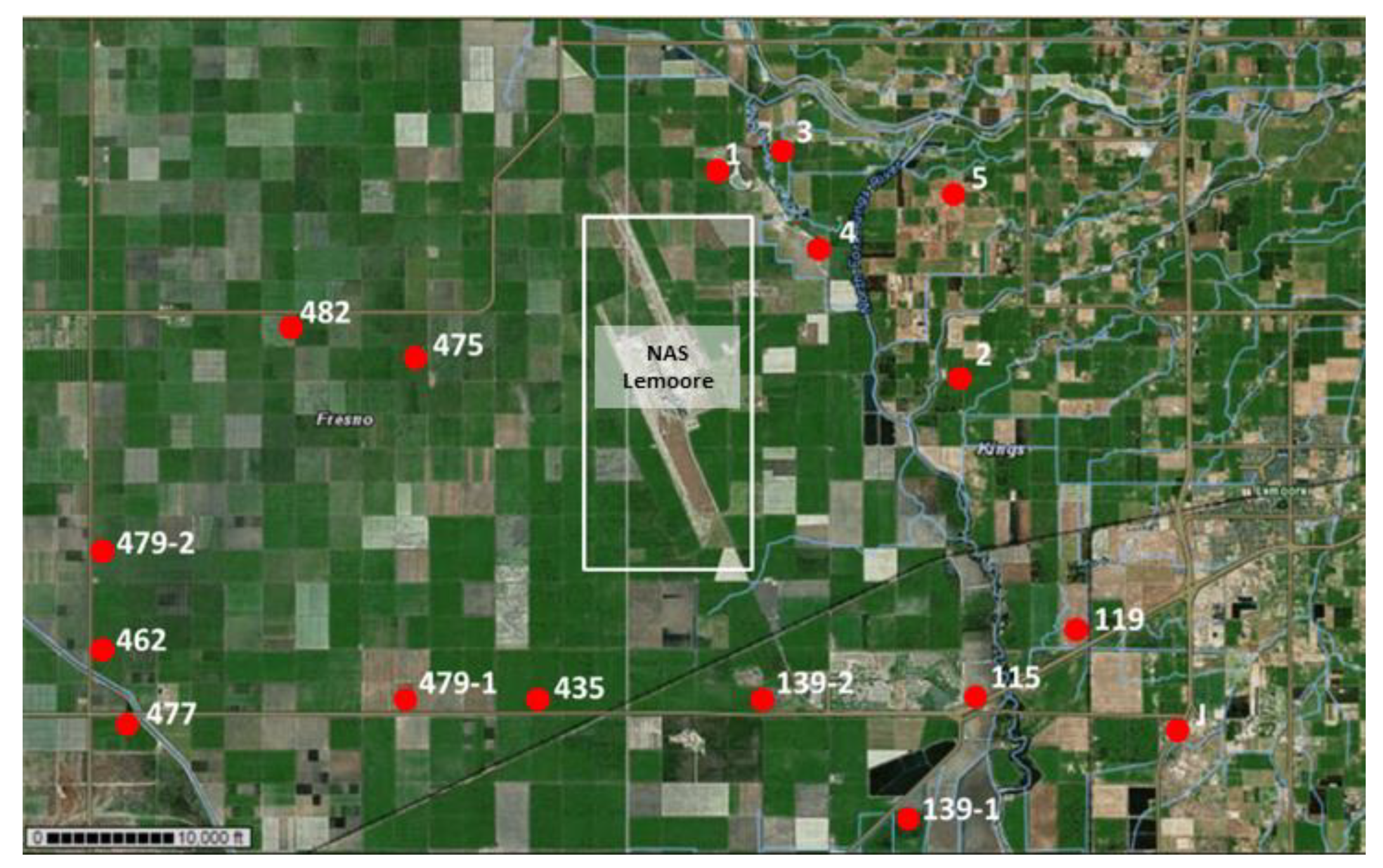
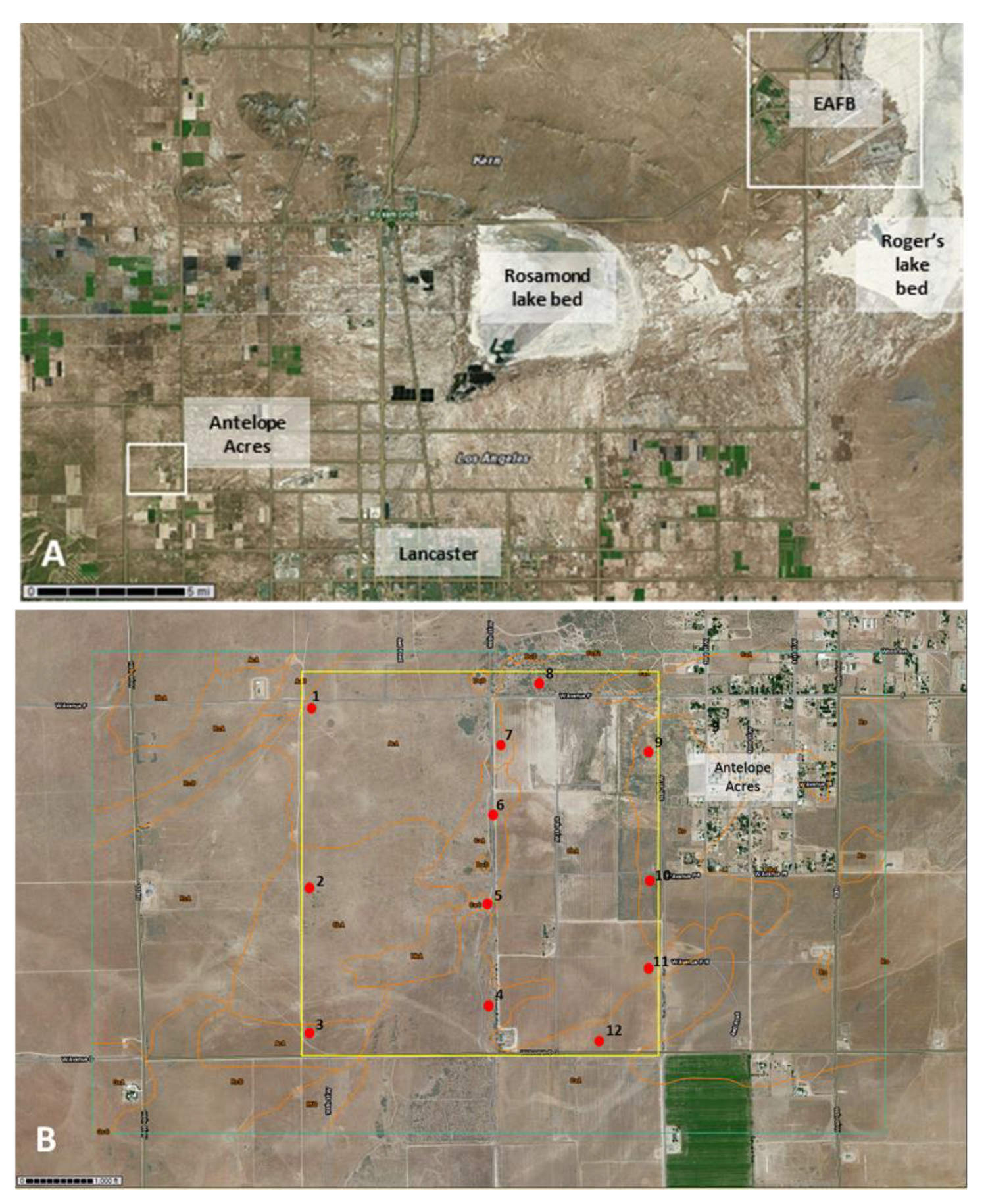
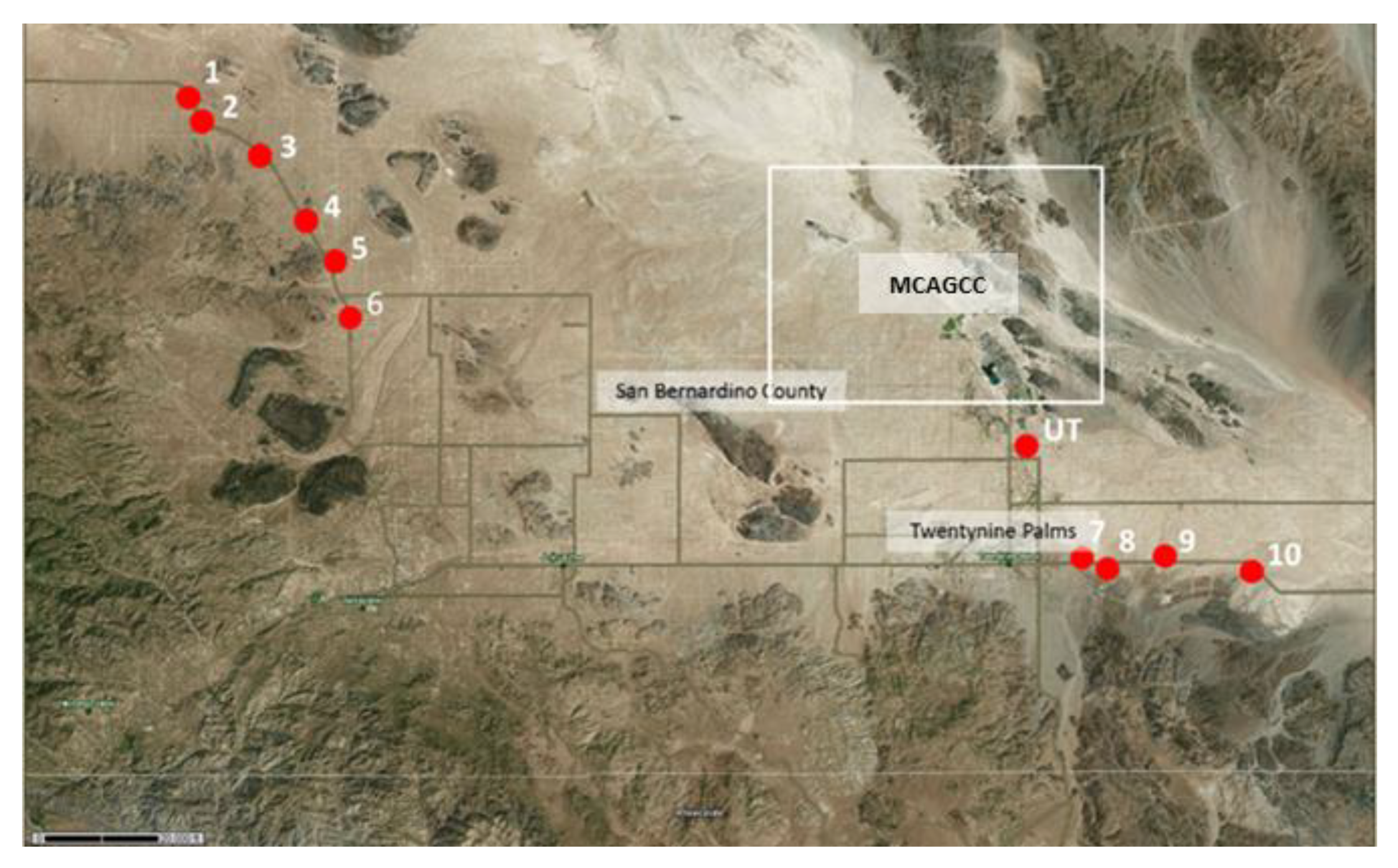
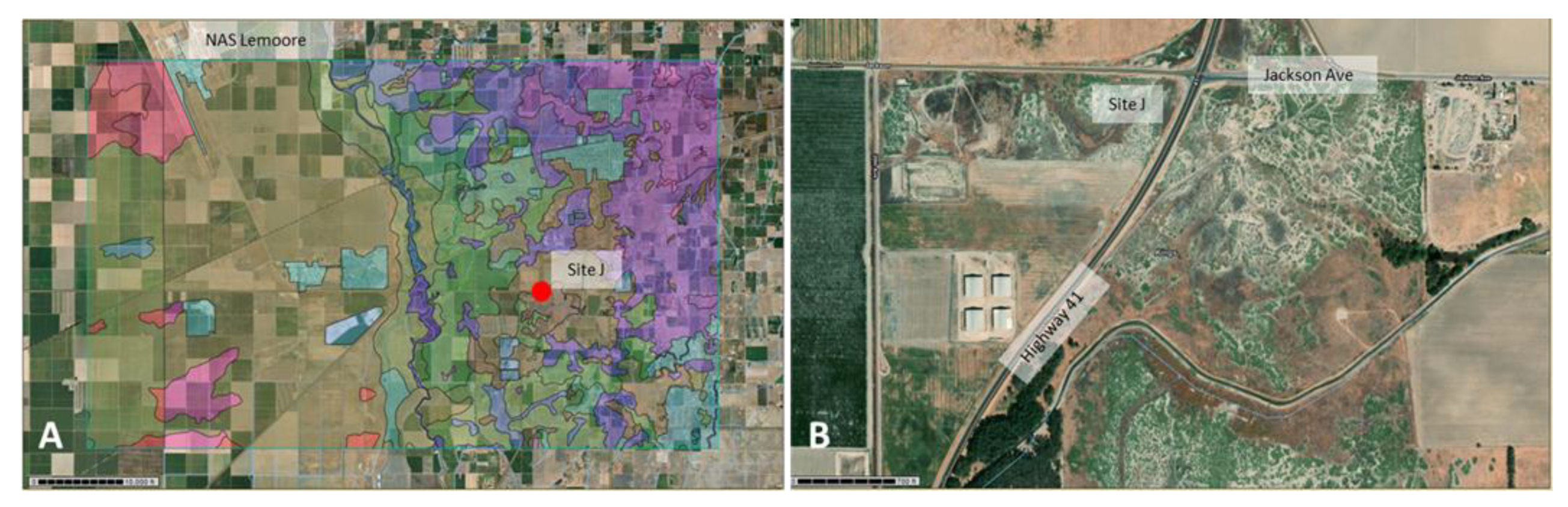
2.1. Site Selection
2.2. Soil and Dust Sampling
2.3. Detection of Coccidioides spp.
2.4. Soil Analyses
2.5. Statistical Analyses
3. Results
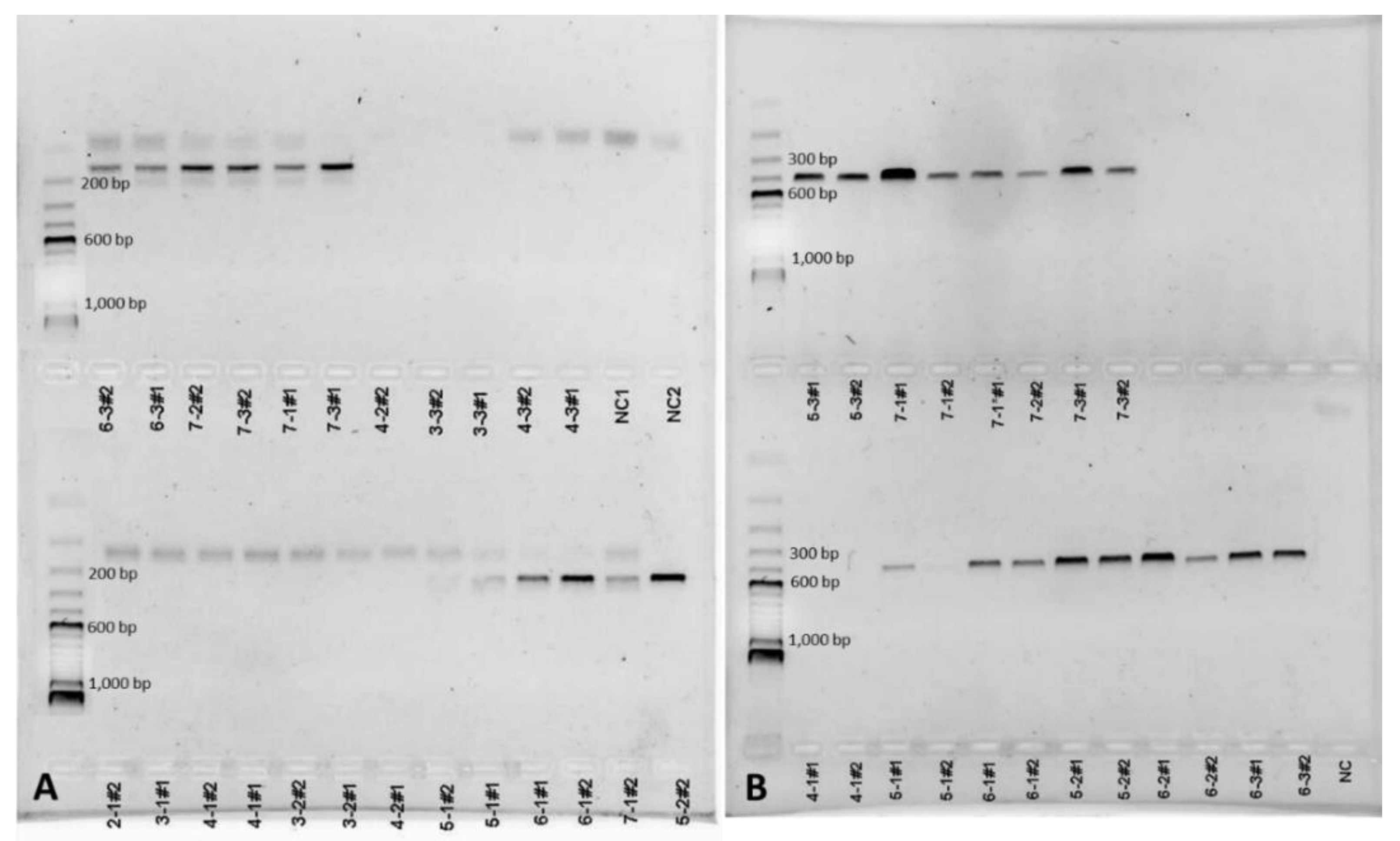
3.1. Detection of Coccidioides in Soil and Dust Samples
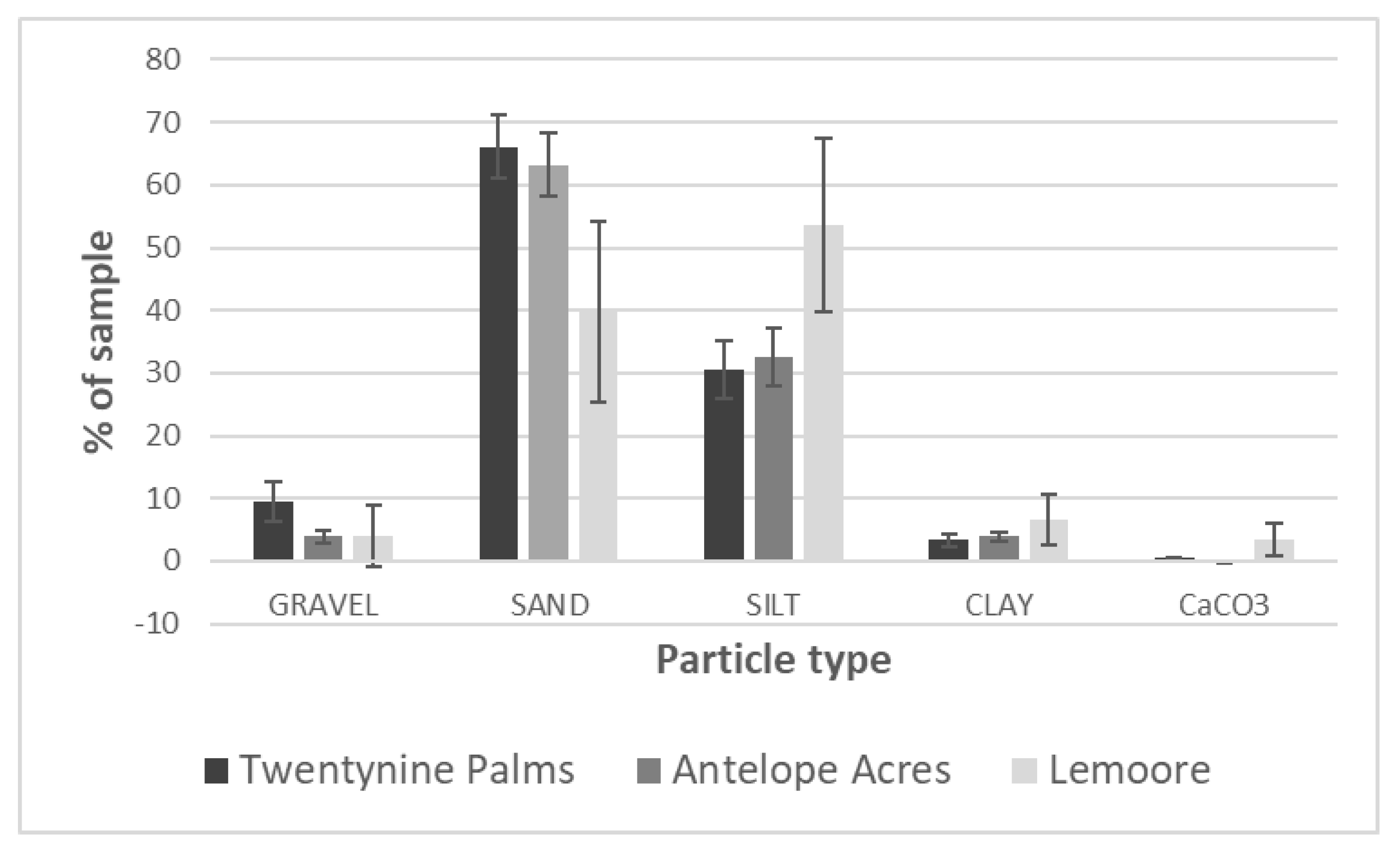
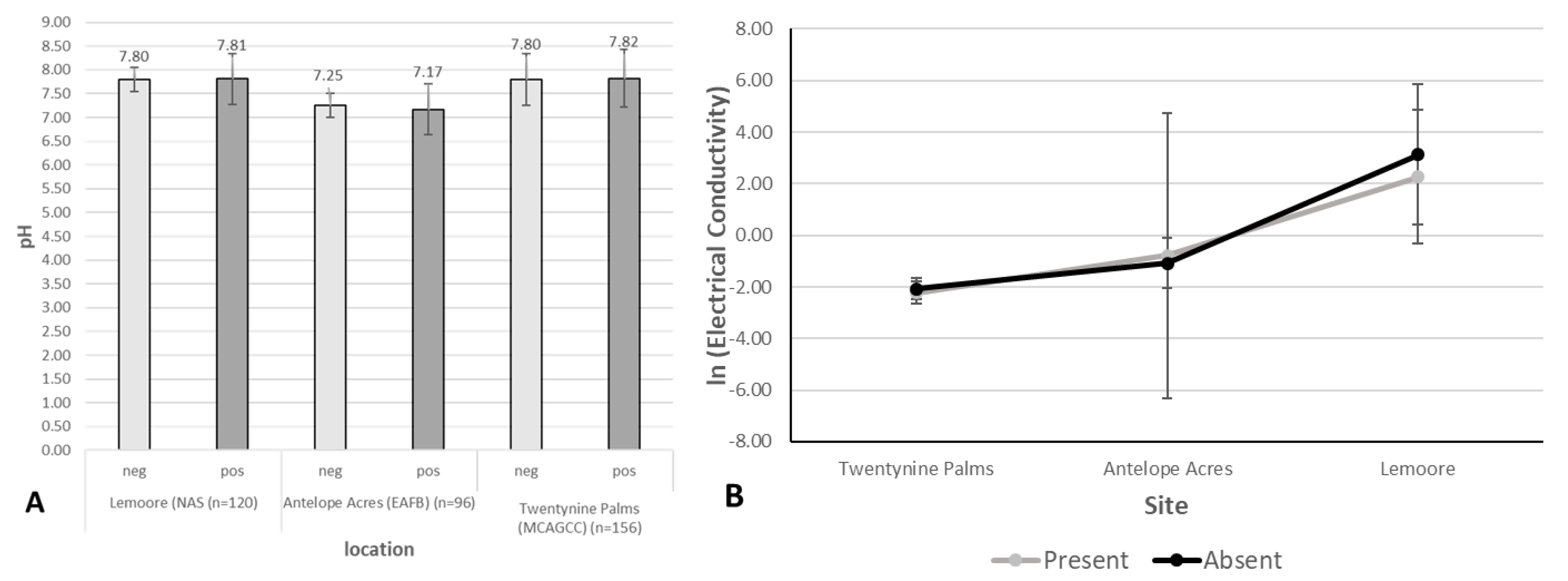
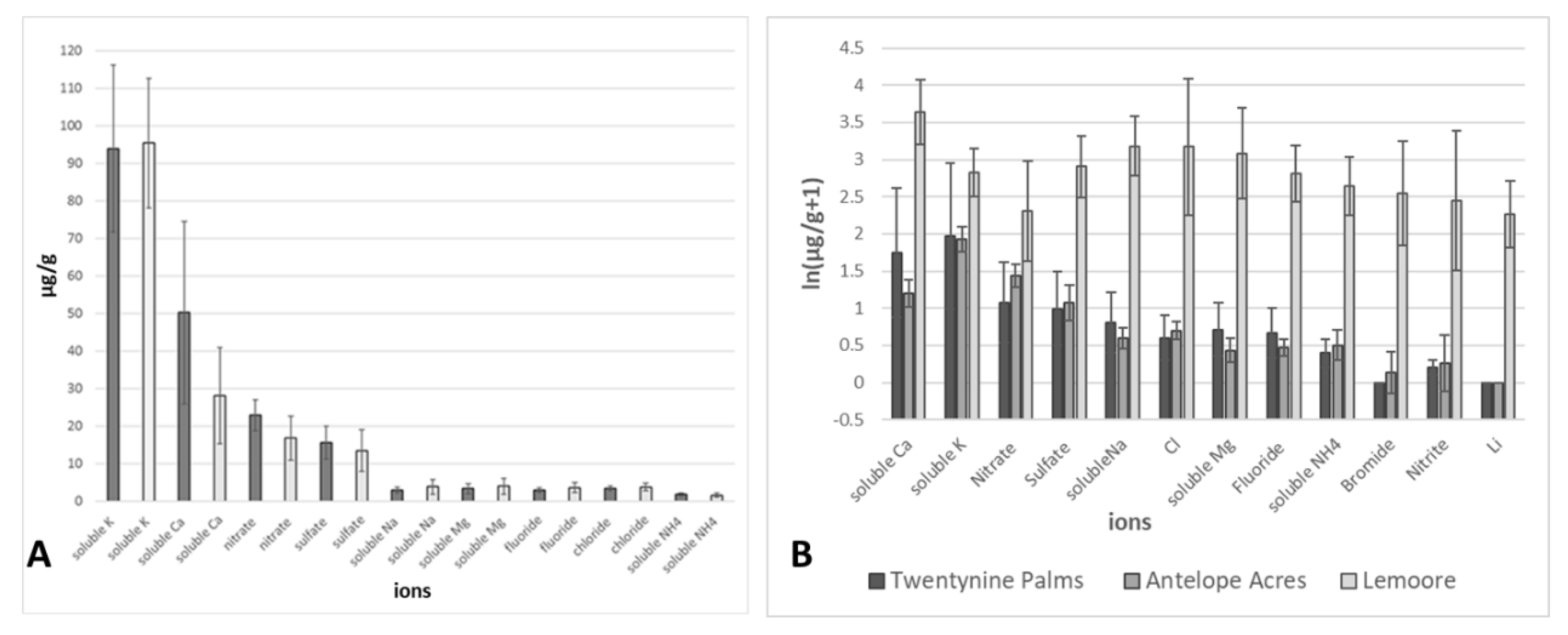
3.2. Environmental Analyses
4. Discussion
- Verify that the maximum exposure hazard is at the nexus of the somewhat ubiquitous (but highly dispersed) growth sites and activities that result in physical resuspension (e.g., earth-moving activities) or Aeolian resuspension. An accurate depth profile of Coccidioides is likely to be very informative for this purpose.
- Use large spatial datasets to define elevated conditions for exposure with the focus on the development of a Coccidioides early warning system, which, if implemented, will have a direct impact on public health.
- Investigate how climate-related events like drought and wildfires which are increasing in Coccidioides-endemic areas, are linked to fugitive dust development and coccidioidomycosis outbreaks in endemic and non-endemic areas of the pathogen.
- Characterize geographic risks, particularly in the context of environmental change, identifying further risk reduction strategies for high-risk groups.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daubenmire, R.F. Merriam’s life zones of North America. Quart. Rev. Biol. 1938, 13, 327–332. [Google Scholar] [CrossRef]
- Elconin, A.F.; Egeberg, R.O.; Egeberg, M.C. Significance of soil salinity on the ecology of Coccidioides immitis. J. Bacteriol. 1964, 87, 500–503. [Google Scholar] [CrossRef]
- Maddy, K.T.; Coccozza, J. The probable geographic distribution of Coccidioides immitis in Mexico. Bol. Oficina Sanit. Panama 1964, 57, 44–54. [Google Scholar]
- Maddy, K.T. Observations on Coccidioides immitis found growing naturally in soil. Ariz. Med. 1965, 22, 281–288. [Google Scholar] [PubMed]
- Lacy, G.H.; Swatek, F.E. Soil ecology of Coccidioides immitis at Amerindian middens in California. Appl. Environ. Microbiol. 1974, 27, 379–388. [Google Scholar] [CrossRef]
- Kolivras, K.N.; Johnson, P.S.; Comrie, A.C.; Yool, S.R. Environmental variability and coccidioidomycosis (Valley fever). Aerobiologia 2001, 17, 31–42. [Google Scholar] [CrossRef]
- De Hoog, S.; Zalar, P.; Van Den Ende, B.G.; Gunde-Cimerman, N. Relation of halotolerance to human-pathogenicity in the fungal tree of life: An overview of ecology and evolution under stress. In Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Springer: Dordrecht, Germany, 2005; pp. 371–395. [Google Scholar]
- Fisher, F.S.; Bultman, M.W.; Johnson, S.M.; Pappagianis, D.; Zaborsky, E. Coccidioides niches and habitat parameters in the southwestern United States. Ann. N. Y. Acad. Sci. 2007, 1111, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Rosas, R.L.; Hinojosa, A.; Riquelme, M. Ecological niche modeling of Coccidioides spp. in Western North American deserts. Ann. N. Y. Acad. Sci. 2007, 1111, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Baptista-Rosas, R.C.; Catalan-Dibene, J.; Romero-Olivares, A.L.; Hinojosa, A.; Cavazos, T.; Riquelme, M. Molecular detection of Coccidioides spp. from environmental samples in Baja California: Linking Valley fever to soil and climate conditions. Fung. Ecol. 2012, 5, 177–190. [Google Scholar] [CrossRef]
- Ampel, N.M. New perspectives on coccidioidomycosis. Proc. Am. Thor. Soc. 2010, 7, 181–185. [Google Scholar] [CrossRef]
- Kirkland, T.N.; Fierer, J. Coccidioidomycosis: A reemerging infectious disease. Emerg. Infect. Dis. 1996, 2, 192. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.W.; Barker, B.M. The endozoan, small-mammal reservoir hypothesis and the life cycle of Coccidioides species. Med. Mycol. 2019, 57, S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Barker, B.M.; Litvintseva, A.P.; Riquelme, M.; Vargas-Gastélum, L. Coccidioides ecology and genomics. Med. Mycol. 2019, 57, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Saubolle, M.A.; McKellar, P.P.; Sussland, D. Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J. Clin. Microbiol. 2007, 45, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Galgiani, J.N.; Ampel, N.M.; Blair, J.E.; Catanzaro, A.; Johnson, R.H.; Stevens, D.A.; Williams, P.L. Coccidioidomycosis. Clin. Infect Dis. 2005, 41, 1217–1223. [Google Scholar] [CrossRef]
- Louie, L.; Ng, S.; Hajjeh, R.; Johnson, R.; Vugia, D.; Werner, S.B.; Talbot, R.; Klitz, W. Influence of host genetics on the severity of coccidioidomycosis. Emerg. Infect. Dis. 1999, 5, 672. [Google Scholar] [CrossRef]
- Thompson, G.R., III; Stevens, D.A.; Clemons, K.V.; Fierer, J.; Johnson, R.H.; Sykes, J.; Rutherford, G.; Peterson, M.; Taylor, J.W.; Chaturvedi, V. Call for a California coccidioidomycosis consortium to face the top ten challenges posed by a recalcitrant regional disease. Mycopathologia 2015, 179, 1–9. [Google Scholar] [CrossRef]
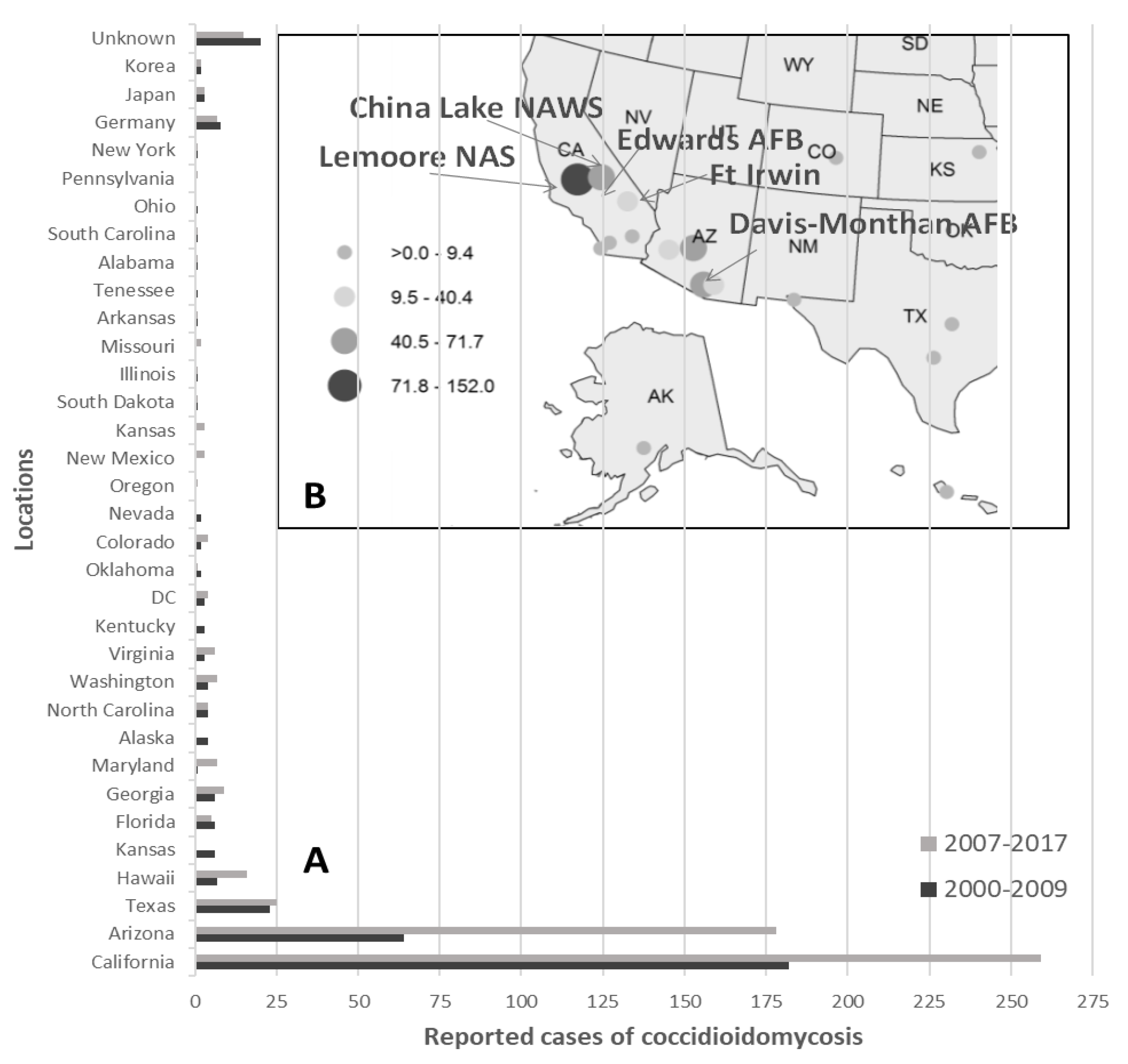
- Centers for Disease Control and Prevention (CDC). National Notifiable Disease Database. Available online: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/maps.html (accessed on 22 February 2019).
- Weaver, E.A.; Kolivras, K.N. Investigating the relationship between climate and Valley fever (coccidioidomycosis). EcoHealth 2018, 15, 840–852. [Google Scholar] [CrossRef]
- Hector, R.F.; Rutherford, G.W.; Tsang, C.A.; Erhart, L.M.; McCotter, O.; Anderson, S.M.; Komatsu, K.; Tabnak, F.; Vugia, D.J.; Yang, Y.; et al. The public health impact of coccidioidomycosis in Arizona and California. Int. J. Environ. Res. Public Health 2011, 8, 1150–1173. [Google Scholar] [CrossRef]
- Wilson, L.; Ting, J.; Lin, H.; Shah, R.; MacLean, M.; Peterson, M.W.; Stockamp, N.; Libke, R.; Brown, P. The rise of Valley fever: Prevalence and cost burden of coccidioidomycosis infection in California. Int. J. Environ. Res. Public Health 2019, 16, 1113. [Google Scholar] [CrossRef]
- Hugenholtz, P.G. Skin test survey at Williams Air Force Base, Arizona. In Symposium on Coccidioidomycosis; US Government Printing Office: Phoenix, AZ, USA, 1957; Volume 575, pp. 127–131. [Google Scholar]
- Crum-Cianflone, N. Coccidioidomycosis in the U.S. military: A review. Ann. N. Y. Acad. Sci. 2007, 1111, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Armed Forces Health Surveillance Center (AFHSC). Surveillance snapshot: Coccidioidomycosis diagnoses by location, active component, 2000–2009. MSMR 2010, 17, 13. [Google Scholar]
- Mease, L. Pulmonary and extrapulmonary coccidioidomycosis, active component, U.S. Armed Forces, 1999–2011. Armed Forces Health Surveillance Center (AFHSC). MSMR 2012, 19, 2–3. [Google Scholar]
- Armed Forces Health Surveillance Center (AFHSC). Historical perspective: Coccidioidomycosis in the U.S. military and military associated populations. MSMR 2012, 19, 5–6. [Google Scholar]
- Armed Forces Health Surveillance Center (AFHSC). Brief report: The geographic distribution of incident coccidioidomycosis among active component service members, 2000–2013. MSMR 2014, 21, 12–14. [Google Scholar]
- Williams, V.F.; Stahlman, S.; Oh, G.-T. Coccidioidomycosis, active component, U.S. Armed Forces, 2007–2017. Armed Forces Health Surveillance Center (AFHSC). MSMR 2018, 25, 2–5. [Google Scholar]
- Drips, W.; Smith, C.E. Epidemiology of coccidioidomycosis—A contemporary military experience. J. Am. Med. Assoc. 1964, 190, 1010–1012. [Google Scholar] [CrossRef]
- Hooper, R.; Curley, R.; Poppell, G.; Husted, S.; Schillaci, R. Coccidioidomycosis among military personnel in Southern California. Milit. Med. 1980, 145, 620–623. [Google Scholar] [CrossRef]
- Standaert, S.M.; Schaffner, W.; Galgiani, J.N.; Pinner, R.W.; Kaufman, L.; Durry, D.; Hutcheson, R.H. Coccidioidomycosis among visitors to a Coccidioides immitis-endemic area: An outbreak in a military reserve unit. J. Infect. Dis. 1995, 171, 1672–1675. [Google Scholar] [CrossRef]
- Gray, G.C.; Fogle, E.F.; Albright, K.L. Risk factors for primary pulmonary coccidioidomycosis hospitalizations among United States Navy and Marine Corps personnel, 1981–1994. Am. J. Trop. Med. Hyg. 1998, 58, 309–312. [Google Scholar] [CrossRef]
- Olivere, J.W.; Meier, P.A.; Fraser, S.L.; Morrison, W.B.; Parsons, T.W.; Drehner, D.M. Coccidioidomycosis—The airborne assault continues: An unusual presentation with a review of the history, epidemiology, and military relevance. Aviat. Space Environ. Med. 1999, 70, 790–796. [Google Scholar] [PubMed]
- Crum, N.F.; Lamb, C.; Utz, G.; Amundson, D.; Wallace, M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitis–endemic area—Coalinga, California. J. Infect. Dis. 2002, 186, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Crum, N.F.; Lederman, E.R.; Hale, B.R.; Lim, M.L.; Wallace, M.R. A cluster of disseminated coccidioidomycosis cases at a US military hospital. Mil. Med. 2003, 16, 460–464. [Google Scholar] [CrossRef]
- Crum, N.F.; Potter, M.; Pappagianis, D. Seroincidence of Coccidioidomycosis during military desert training exercises. J. Clin. Microbiol. 2004, 42, 4552–4555. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.K.; Kugblenu, R.K. Mortality from Fungal Disease in the US Air Force from 1970 to 2013. Public Health and Preventive Medicine Department, USAF School of Aerospace Medicine Wright-Patterson AFB United States. 2016. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/1018955.pdf (accessed on 6 August 2018).
- Sprigg, W.A.; Nickovic, S.; Galgiani, J.; Pejanovic, G.; Petkovic, S.; Vujadinovic, M.; Vukovic, A.; Dacic, M.; DiBiase, S.; Prasad, A.; et al. Regional dust storm modeling for health services: The case of Valley fever. Aeolian Res. 2014, 14, 53–73. [Google Scholar] [CrossRef]
- Huckabone, S.E.; Gulland, F.M.; Johnson, S.M.; Colegrove, K.M.; Dodd, E.M.; Pappagianis, D.; Dunkin, R.C.; Casper, D.; Carlson, E.L.; Sykes, J.E.; et al. Coccidioidomycosis and other systemic mycoses of marine mammals stranding along the central California, USA coast: 1998–2012. J. Wildlife Dis. 2015, 51, 295–308. [Google Scholar] [CrossRef]
- Cole, D.N. Trampling disturbance and recovery of cryptogamic soil crusts in Grand Canyon National Park. Great Basin Nat. 1990, 50, 321–325. Available online: https://www.jstor.org/stable/41712611 (accessed on 6 August 2018).
- Van Donk, S.J.; Huang, X.; Skidmore, E.L.; Anderson, A.B.; Gebhart, D.L.; Prehoda, V.E.; Kellogg, E.M. Wind erosion from military training lands in the Mojave Desert, California, USA. J. Arid Environ. 2003, 54, 687–703. [Google Scholar] [CrossRef]
- Anderson, A.B.; Palazzo, A.J.; Ayers, P.D.; Fehmi, J.S.; Shoop, S.; Sullivan, P. Assessing the impacts of military vehicle traffic on natural areas. Introduction to the special issue and review of the relevant military vehicle impact literature. J. Terramechanics. 2005, 42, 143–158. [Google Scholar] [CrossRef]
- Svendsen, N.G.; Koch, D.J.; Gertner, G.Z.; Howard, H.R.; Ayers, P.D. Vehicle impact analysis using vehicle tracking systems on military lands. Trans. ASABE 2017, 60, 1865–1872. [Google Scholar] [CrossRef]
- Prose, D.V.; Metzger, S.K. Recovery of Soils and Vegetation in World War II Military Base Camps, Mojave Desert. US Department of the Interior, Geological Survey; Geological Survey: Denver, CO, USA, 1985; pp. 85–234. Available online: https://pubs.usgs.gov/of/1985/0234/report.pdf (accessed on 8 August 2018).
- Kade, A.; Warren, S.D. Soil and plant recovery after historic military disturbances in the Sonoran Desert, USA. Arid Land Res. Manag. 2002, 16, 231–243. [Google Scholar] [CrossRef]
- Weber, B.; Bowker, M.; Zhang, Y.; Belnap, J. Natural recovery of biological soil crusts after disturbance. In Biological Soil Crusts: An Organizing Principle in Drylands; Springer: Berlin/Heidelberg, Germany, 2016; pp. 479–498. [Google Scholar]
- Bach, A.J.; Brazel, A.J.; Lancaster, N. Temporal and spatial aspects of blowing dust in the Mojave and Colorado deserts of southern California, 1973–1994. Phys. Geogr. 1996, 17, 329–353. [Google Scholar] [CrossRef]
- Lauer, A.; Talamantes, J.; Olivares, L.R.; Medina, L.J.; Baal, J.D.; Casimiro, K.N.; Shroff, N.; Emery, K.W. Combining forces-the use of landsat TM satellite imagery, soil parameter information, and multiplex PCR to detect Coccidioides immitis growth sites in Kern County, California. PLoS ONE 2014, 9, e111921. [Google Scholar] [CrossRef] [PubMed]
- Cooley, H.; Donnelly, K.; Phurisamban, R.; Subramanian, M. Impacts of California’s ongoing drought: Agriculture; Pacific Institute: Oakland, CA, USA, 24 August 2015; Available online: https://www.shrm.org/ResourcesAndTools/legal-and-compliance/state-and-local-updates/Documents/ImpactsOnCaliforniaDrought-Ag.pdf (accessed on 9 October 2018).
- Faunt, C.C.; Sneed, M.; Traum, J.; Brandt, J.T. Water availability and land subsidence in the Central Valley, California, USA. Hydrogeol. J. 2016, 24, 675–684. [Google Scholar] [CrossRef]
- Pokharel, A.; Kaplan, M. Dust climatology of the NASA Dryden Flight Research Center (DFRC) in Lancaster, California, USA. Climate 2017, 5, 15. [Google Scholar] [CrossRef]
- Das, R.; McNary, J.; Fitzsimmons, K.; Dobraca, D.; Cummings, K.; Mohle-Boetani, J.; Wheeler, C.; McDowell, A.; Iossifova, Y.; Baily, R.; et al. Occupational coccidioidomycosis in California—Outbreak investigation, respirator recommendations, and surveillance findings. J. Ocup. Environ. Med. 2012, 54, 564–571. [Google Scholar] [CrossRef]
- McCarty, J.M.; Demetral, L.C.; Dabrowski, L.; Khal, A.K.; Bowser, M.; Hahn, J.E. Pediatric coccidioidomycosis in Central California: A retrospective case series. Clin. Infect. Dis. 2013, 56, 1579–1585. [Google Scholar] [CrossRef]
- Pappagianis, D. Coccidioidomycosis in California state correctional institutions. Ann. N. Y. Acad. Sci. 2007, 1111, 103–111. [Google Scholar] [CrossRef]
- Burwell, L.A.; Park, B.J.; Wannemuehler, K.A.; Kendig, N.; Pelton, J.; Chaput, E.; Jinadu, B.A.; Emery, K.; Chavez, G.; Fridkin, S.K. Outcomes among inmates treated for coccidioidomycosis at a correctional institution during a community outbreak, Kern County, California, 2004. Clin. Infect. Dis. 2009, 49, e113–e119. [Google Scholar] [CrossRef]
- Sondermeyer, G.; Lee, L.; Gilliss, D.; Tabnak, F.; Vugia, D. Coccidioidomycosis-associated hospitalizations, California, USA, 2000–2011. Emerg. Infect. Dis. 2013, 19, 1590. [Google Scholar] [CrossRef]
- NOAA (National Oceanic and Atmospheric Administration). National Centers for Environmental Information. Available online: www.ncei.noaa.gov (accessed on 7 February 2016).
- Tong, D.Q.; Wang, J.X.; Gill, T.E.; Lei, H.; Wang, B. Intensified dust storm activity and Valley fever infection in the southwestern United States. Geophys. Res. Lett. 2017, 44, 4304–4312. [Google Scholar] [CrossRef]
- Gorris, M.E.; Cat, L.A.; Zender, C.S.; Treseder, K.K.; Randerson, J.T. Coccidioidomycosis dynamics in relation to climate in the southwestern United States. GeoHealth 2018, 2, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Gorris, M.E.; Treseder, K.K.; Zender, C.S.; Randerson, J.T. Expansion of coccidioidomycosis endemic regions in the United States in response to climate change. GeoHealth 2019, 10, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Laws, R.L.; Cooksey, G.S.; Jain, S.; Wilken, J.; McNary, J.; Moreno, E.; Michie, K.; Mulkerin, C.; McDowell, A.; Vugia, D.; et al. Coccidioidomycosis outbreak among workers constructing a solar power farm—Monterey County, California, 2016–2017. MMWR 2018, 67, 931. [Google Scholar] [CrossRef] [PubMed]
- De Perio, M.A.; Materna, B.L.; Sondermeyer Cooksey, G.L.; Vugia, D.J.; Su, C.P.; Luckhaupt, S.E.; McNary, J.; Wilken, J.A. Occupational coccidioidomycosis surveillance and recent outbreaks in California. Med. Mycol. 2019, 57, S41–S45. [Google Scholar] [CrossRef]
- Lee, R.; Crum-Cianflone, N. Increasing incidence and severity of coccidioidomycosis at a naval air station. Milit. Med. 2008, 173, 769–775. [Google Scholar] [CrossRef]
- Guevara, R.E.; Motala, T.; Terashita, D. The changing epidemiology of coccidioidomycosis in Los Angeles (LA) County, California, 1973–2011. PLoS ONE 2015, 10, e0136753. [Google Scholar] [CrossRef]
- Colson, A.J.; Vredenburgh, L.; Guevara, R.E.; Rangel, N.P.; Kloock, C.T.; Lauer, A. Large-scale land development, fugitive dust, and increased coccidioidomycosis incidence in the antelope valley of California, 1999–2014. Mycopathologia 2017, 182, 439–458. [Google Scholar] [CrossRef]
- Etyemezian, V.; Kuhns, H.; Nikolich, G. Precision and repeatability of the TRAKER vehicle-based paved road dust emission measurement. Atmos. Environ. 2006, 40, 2953–2958. [Google Scholar] [CrossRef]
- Etyemezian, V.; Gillies, J.A.; Shinoda, M.; Nikolich, G.; King, J.; Bardis, A.R. Accounting for surface roughness on measurements conducted with PI-SWERL: Evaluation of a subjective visual approach and a photogrammetric technique. Aeol. Res. 2014, 13, 35–50. [Google Scholar] [CrossRef]
- Sweeney, M.; Etyemezian, V.; Macpherson, T.; Nickling, W.; Gillies, J.; Nikolich, G.; McDonald, E. Comparison of PI-SWERL with dust emission measurements from a straight-line field wind tunnel. J. Geophys. Res. Earth Surf. 2008, 113. [Google Scholar] [CrossRef]
- Sweeney, M.R.; McDonald, E.V.; Etyemezian, V. Quantifying dust emissions from desert landforms, eastern Mojave Desert, USA. Geomorphology 2011, 135, 21–34. [Google Scholar] [CrossRef]
- Kuhns, H.; Etyemezian, V.; Landwehr, D.; MacDougall, C.; Pitchford, M.; Green, M. Testing re-entrained aerosol kinetic emissions from roads: A new approach to infer silt loading on roadways. Atmos. Environ. 2001, 35, 2815–2825. [Google Scholar] [CrossRef]
- Kuhns, H.; Gillies, J.; Etyemezian, V.; Dubois, D.; Ahonen, S.; Nikolic, D.; Durham, C. Spatial variability of unpaved road dust PM10 emission factors near El Paso, Texas. J. Air Waste Manag. Assoc. 2005, 55, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Gastélum, L.; Romero-Olivares, A.L.; Escalante, A.E.; Rocha-Olivares, A.; Brizuela, C.; Riquelme, M. Impact of seasonal changes on fungal diversity of a semi-arid ecosystem revealed by 454 pyrosequencing. FEMS Microbiol. Ecol. 2015, 91, fiv044. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Carlson, E.L.; Fisher, F.S.; Pappagianis, D. Demonstration of Coccidioides immitis and Coccidioides posadasii DNA in soil samples collected from Dinosaur National Monument, Utah. Sabouraudia 2014, 52, 610–617. [Google Scholar] [CrossRef]
- Von Wintzingerode, F.; Göbel, U.B.; Stackebrandt, E. Determination of microbial diversity in environmental samples: Pitfalls of PCR-based rRNA analysis. FEMS Microbiol. Rev. 1997, 21, 213–229. [Google Scholar] [CrossRef]
- Benson, D.A.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 42, D32–D37. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, L.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2016, Vienna, Austria. Available online: https://www.r.project.org/ (accessed on 8 August 2018).
- Ugarte, M.D.; Militino, A.F.; Arnholt, A.T. Probability and Statistics with R. Chapman and Hall; CRC: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wheeler, C.; Lucas, K.D.; Mohle-Boetani, J.C. Rates and risk factors for coccidioidomycosis among prison inmates, California, USA, 2011. Emerg. Infect. Dis. 2015, 21, 70. [Google Scholar] [CrossRef]
- Germano, D.J.; Rathbun, G.B.; Saslaw, L.R.; Cypher, B.L.; Cypher, E.A.; Vredenburgh, L.M. The San Joaquin Desert of California: Ecologically misunderstood and overlooked. Nat. Areas J. 2011, 31, 138–147. [Google Scholar] [CrossRef]
- Emmons, C.W. Coccidioidomycosis. Mycologia 1942, 34, 452–463. [Google Scholar] [CrossRef]
- Garbeva, P.V.; Van Veen, J.A.; Van Elsas, J.D. Microbial diversity in soil: Selection of microbial populations by plant and soil type and implications for disease suppressiveness. Ann. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fert. Soils. 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Ding, G.C.; Piceno, Y.M.; Heuer, H.; Weinert, N.; Dohrmann, A.B.; Carrillo, A.; Andersen, G.L.; Castellanos, T.; Tebbe, C.C.; Smalla, K. Changes of soil bacterial diversity as a consequence of agricultural land use in a semi-arid ecosystem. PLoS ONE 2013, 8, e59497. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.R.; Schadt, C.W.; Carpenter-Boggs, L.; Kang, S.; Zhou, J.; Reganold, J.P. Effects of soil type and farm management on soil ecological functional genes and microbial activities. ISME J. 2010, 4, 1099. [Google Scholar] [CrossRef] [PubMed]
- Tardy, V.; Spor, A.; Mathieu, O.; Lévèque, J.; Terrat, S.; Plassart, P.; Regnier, T.; Bardgett, R.D.; Van Der Putten, W.H.; Roggero, P.P.; et al. Shifts in microbial diversity through land use intensity as drivers of carbon mineralization in soil. Soil Biol. Biochem. 2015, 90, 204–213. [Google Scholar] [CrossRef]
- Lauer, A.; Baal, J.D.; Mendes, S.D.; Casimiro, K.N.; Passaglia, A.K.; Valenzuela, A.H.; Guibert, G. Valley fever on the rise—Searching for microbial antagonists to the fungal pathogen Coccidioides immitis. Microorganisms 2019, 7, 31. [Google Scholar] [CrossRef]
- Engelhardt, R.E.; Knebel, G.W. Characteristics of the dust environment in the vicinity of military activities (No. SWRI-AR-642). Southwest Res. Inst. San Antonio TX Dept. Automot. Res. 1968, 1–49. [Google Scholar] [CrossRef]
- Wilken, J.A.; Sondermeyer, G.; Shusterman, D.; McNary, J.; Vugia, D.J.; McDowell, A.; Borenstein, P.; Gilliss, D.; Ancock, B.; Prudhomme, J.; et al. Coccidioidomycosis among workers constructing solar power farms, California, USA, 2011–2014. Emerg. Infect. Dis. 2015, 21, 1997. [Google Scholar] [CrossRef]
- Diaz, J.H. Travel-related risk factors for coccidioidomycosis. J. Travel Med. 2018, 25, tay027. [Google Scholar] [CrossRef]
- Mazor, G.; Kidron, G.J.; Vonshak, A.; Abeliovich, A. The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol. Ecol. 1996, 21, 121–130. [Google Scholar] [CrossRef]
- Belnap, J.; Eldridge, D. Disturbance and recovery of biological soil crusts. In Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin/Heidelberg, Germany, 2003; pp. 363–383. [Google Scholar]
- Belnap, J.; Gillette, D.A. Vulnerability of desert biological soil crusts to wind erosion: The influences of crust development, soil texture, and disturbance. J. Arid. Environ. 1998, 39, 133–142. [Google Scholar] [CrossRef]
- Bowker, M.A. Biological soil crust rehabilitation in theory and practice: An underexploited opportunity. Restor. Ecol. 2007, 15, 13–23. [Google Scholar] [CrossRef]
- Ustin, S.L.; Valko, P.G.; Kefauver, S.C.; Santos, M.J.; Zimpfer, J.F.; Smith, S.D. Remote sensing of biological soil crust under simulated climate change manipulations in the Mojave Desert. Proc. SPIE 2009, 113, 317–328. [Google Scholar] [CrossRef]
- Sharpton, T.J.; Stajich, J.E.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chaturvedi, V. The powers and perils of PCR in the search for the natural reservoirs of Coccidioides species. Mycopathologia 2017, 182, 435–438. [Google Scholar] [CrossRef][Green Version]
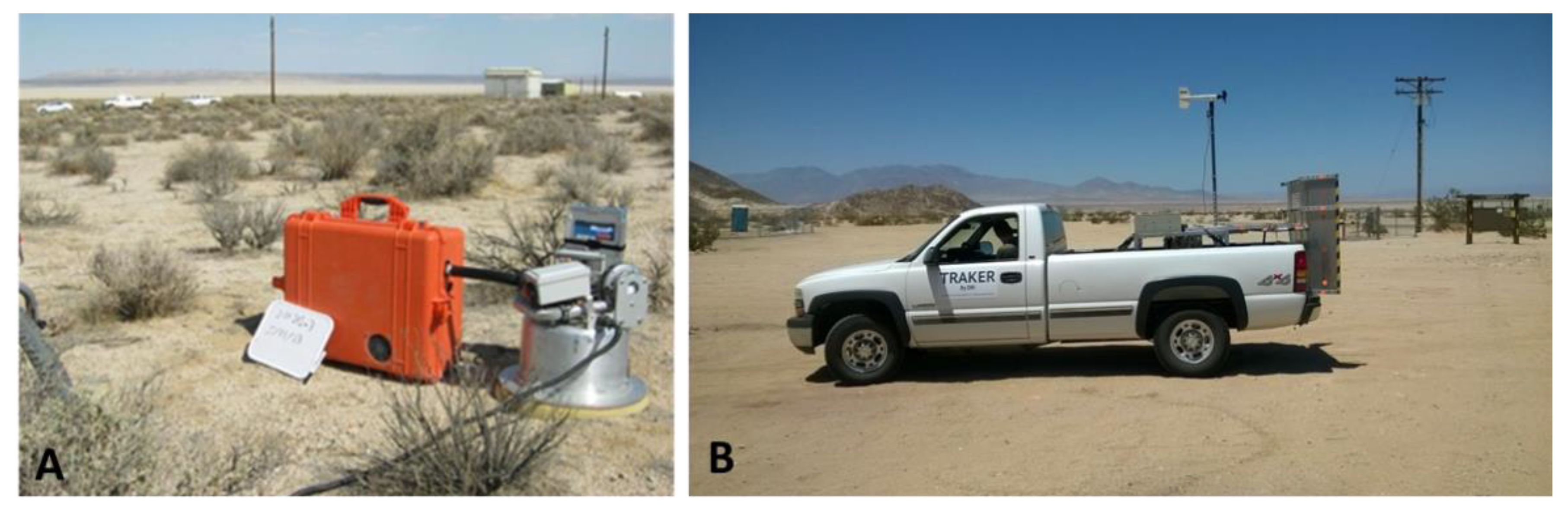


| Soil Sampling | Dust Sampling | |||||
|---|---|---|---|---|---|---|
| Study Area | Bulk Soil Sampling | Soil Core Sampling | PI-SWERL® | TRAKER™ | ||
| Winter | Spring/ Summer | Fall | Wind-Suspendable Dust Samples | Unpaved Road Dust Samples | ||
| Lemoore NAS | 1/17/17 and 1/18/17 56 bulk soil samples | 5/31/17 31 bulk soil samples | 9/15/2017 29 bulk soil samples | 5/31/17 (4 cores), 9/15/17 (3 cores) | 9/15/17 (unpaved areas and roads near sites 1 [N26 and salt pit] and J [including dirt road west of site J]) (13 samples) | 9/15/17 (unpaved roads around sites Lemoore [21 Str. north of Jackson Ave.], site 1 [N26], and 21 Str. to Bakersfield) (3 samples) |
| Edwards AFB | 2/2/17 36 bulk soil samples | 5/20/17 39 bulk soil samples | 9/12/17 19 bulk soil samples | 5/20/17 (5 cores), 9/12/17 (2 cores) | 9/12/17 (unpaved roads near sites 5, 7, and 8) (3 samples) | 9/12/17 (unpaved roads around sites 5 and 7) (3 samples) |
| 29 Palms MCAGCC | 1/28/17 43 bulk soil samples | 6/10/17 and 6/11/17 60 bulk soil samples | 10/13/17 and 10/14/17 76 bulk soil samples | 6/10/17 and 6/11/17 (7 cores), 10/13/17 and 10/14/17 (6 cores) | 10/13/17 (sites 5–10, and UT) (18 samples) | 10/13/17 (unpaved roads between sites 7 and 10) (1 sample) |
| Location | Soil Type and Soil Map Unit | Site Description | Coordinates | Coccidioides DNA * | |
|---|---|---|---|---|---|
| LM-1 (N26) | Panoche clay loam, saline-alkali (151) | dirt road between agricultural fields and eucalyptus forest towards a dry, salty lakebed | 36°22′38.96″ N | 119°55′56.82″ W | pos |
| LM-2 | Vanguard sandy loam, partially drained (168) | agricultural field | 36°19′42.28″ N | 119°52′06.31″ W | neg |
| LM-3 | water, either Vanguard sandy loam, partially drained (168), or Gepford clay, partially drained (115) | area with ponds near creek, salty soils, plenty of Salt Bush (Atriplex spp.), high animal activity (birds and small mammals) | 36°22′46.92″ N | 119°55′17.69″ W | pos |
| LM-4 | Gepford clay, partially drained (115) | dirt road along small canal, adjacent to new orchard | 36°21′17.57″ N | 119°54′18.29″ W | pos |
| LM-5 | Grangeville fine sandy loam, saline-alkali, partially drained (121) | orchard with young almond trees | 36°21′56.02″ N | 119°52′19.85″ W | neg |
| LM-482 | Calflax clay loam, saline-sodic, wet, 0–1% slope (482) | agricultural field | 36°20′33.22″ N | 120°02′55.25″ W | neg |
| LM-475 | Posochaet clay loam, saline-sodic, wet 0–2% slopes (479) | agricultural field | 36°19′41.27″ N | 120°02′29.11″ W | neg |
| LM-479-1 | Cerini clay loam, 0–2% slope (479) | agricultural field | 36°15′20.05″ N | 120°00′59.29″ W | neg |
| LM-479-2 | Cerini clay loam, 0–2% slope (479) | agricultural field | 36°17′24.04″ N | 120°06′11.88″ W | neg |
| LM-462 | Ciervo, wet Ciervo complex, saline-sodic, 0–1% slope (462) | meadow | 36°16′11.35″ N | 120°06′11.95″ W | neg |
| LM-477 | Westhaven clay loam, 0–2% slope (477) | almond orchard | 36°15′18.65″ N | 120°05′03.48″ W | neg |
| LM-435 | Lethenet clay loam, 0–2% slope (139) | agricultural field | 36°15′19.62″ N | 119°58′56.71″ W | neg |
| LM139-1 | Lethenet clay loam (139) | unused agricultural field, grasses, clay rich area | 36°14′04.20″ N | 119°53′13.60″ W | neg |
| LM139-2 | Lethenet clay loam (139) | abandoned agricultural field, high grasses and shrubs | 36°15′20.48″ N | 119°55′23.09″ W | neg |
| LM-115 | Gepford clay, partially drained (115) | unused agricultural fields with grass | 36°15′26.17″ N | 119°55′23.09″ W | neg |
| LM-151 | Calflax clay loam, saline-sodic, 0 to 2% slopes (151) | agricultural field | 36°11′53.63″ N | 119°56′04.02″ W | neg |
| LM-119 | Grangeville sandy loam, saline-alkali (119) | along a dirt road between agricultural fields towards a meadow | 36°16′18.55″ N | 119°50′08.02″ W | pos |
| LM-J | Lemoore sandy loam, partially drained (137) Boggs sandy loam, partially drained (103) | salty environment with Iodine Bush (Allenrolfea occidentalis), was flooded in winter, rodent activity was observed | 36°15′16.63″ N | 119°48′40.00″ W | pos |
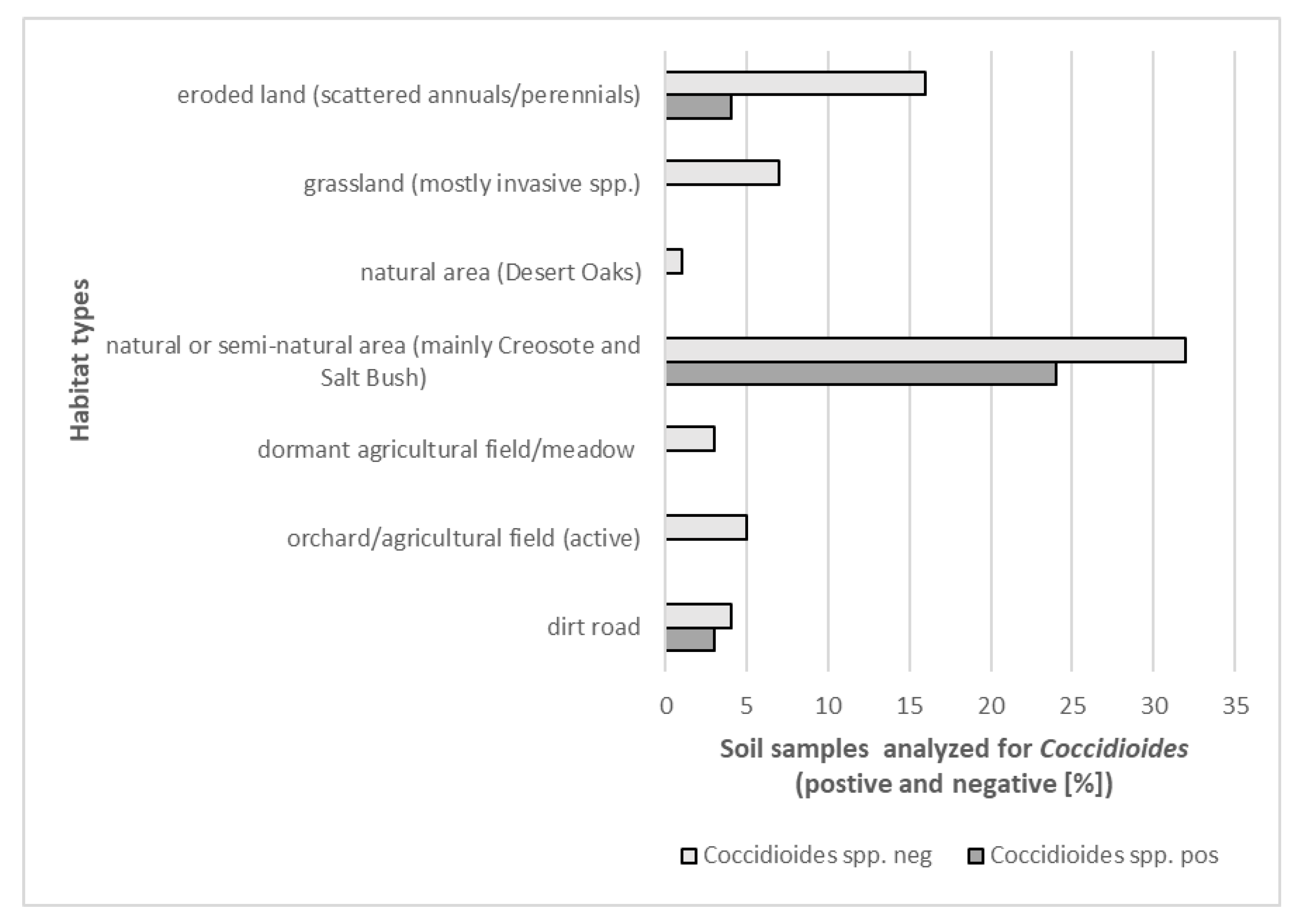
| Location | Soil Type and Soil Map Unit | Site Description | Coordinates | Detection of Coccidioides DNA * | |
|---|---|---|---|---|---|
| AA-1 | Adelanto coarse sandy loam, 2 to 5% slopes (AcA) | grassland, mostly invasive Bromus spp. and native and non-native annuals | 34°44′48.97″ N | 118°18′57.87″ W | neg |
| AA-2 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | grassland, mostly invasive Bromus spp. and native and non-native annuals | 34°44′23.01″ N | 118°18′59.69″ W | neg |
| AA-3 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | grassland, mostly invasive Bromus spp. and native and non-native annuals | 34°43′59.80″ N | 118°18′59.03″ W | neg |
| AA-4 | Cajon loamy sand, 0 to 2% slopes (CaA) | eroded landscape with scattered vegetation, mostly native and non-native annuals | 34°44′04.22″ N | 118°18′25.29″ W | neg |
| AA-5 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | eroded landscape with scattered vegetation, mostly native and non-native annuals | 34°44′20.40″ N | 118°18′24.36″ W | pos |
| AA-6 | Cajon loamy sand, 0 to 2% slopes (CaA) | eroded landscape with scattered vegetation, mostly native and non-native annuals | 34°44′34.60″ N | 118°18′24.82″ W | pos |
| AA-7 | Adelanto coarse sandy loam, 2 to 5% slopes (AcA) | eroded area dominated by rabbit brush (Ericameria nauseosa) | 34°44′42.86″ N | 118°18′25.24″ W | pos |
| AA-8 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | eroded area dominated by rabbit brush (Ericameria nauseosa) | 34°44′50.91″ N | 118°18′16.91″ W | neg |
| AA-9 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | eroded landscape with scattered vegetation, mostly native and non-native annuals | 34°44′48.56″ N | 118°18′03.94″ W | neg |
| AA-10 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | eroded landscape with scattered vegetation including tumble weeds (Salsola sp.) | 34°44′35.76″ N | 118°18′03.89″ W | neg |
| AA-11 | Cajon loamy sand, loamy substratum, 0 to 2% slopes (CbA) | abandoned agricultural field with scattered growth of invasive grasses (Bromus spp. and others) | 34°44′11.53″ N | 118°17′55.99″ W | neg |
| AA-12 | Cajon loamy sand, 0 to 2% slopes (CaA) | grassland, mostly invasive Bromus spp. and native and non-native annuals | 34°43′58.90″ N | 118°18′10.16″ W | neg |
| Location | Soil Type and Soil Map Unit | Site Description | Coordinates | Detection of Coccidioides DNA * | |
|---|---|---|---|---|---|
| 29P-1 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.) | 34°22′08.46″ N | 116°32′24.39″ W | pos |
| 29P-2 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.) | 34°21′40.37″ N | 116°31′55.75″ W | pos |
| 29P-3 | Not available && | area with large boulders, graffiti, littered with trash | 34°20′05.00″ N | 116°29′18.79″ W | pos |
| 29P-4 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.) including occasional Joshua trees (Yucca brevifolia) | 34°18′37.24″ N | 116°28′15.72″ W | pos |
| 29P-5 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.) including occasional Joshua trees (Yucca brevifolia), very rocky | 34°17′10.79″ N | 116°27′13.62″ W | pos |
| 29P-6 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.) including occasional Joshua trees (Yucca brevifolia), eroded soil | 34°15′35.74″ N | 116°26′23.01″ W | pos |
| 29P-7 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.), lots of ants | 34°08′11.17″ N | 116°01′04.85″ W | pos |
| 29P-8 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.), eroded soil, rodent activity high | 34°08′11.85″ N | 115°00′09.16″ W | pos |
| 29P-9 | Not available && | landscape with scattered Creosote (Larrea tridentata) and Salt Bush (Atriplex spp.), some Beavertail cacti (Opuntia basilaris) | 34°08′13.90″ N | 115°58′10.91″ W | pos |
| 29P-10 | Not available && | landscape with scattered Creosote (Larrea tridentata) and scattered Salt Bush (Atriplex spp.), lots of ants | 34°08′14.75″ N | 115°54′50.02″ W | pos |
| UT | Not available && | eroded landscape, scattered Salt Bush (Atriplex spp.), mostly unvegetated | 34°11′41.84″ N | 116°02′06.72″ W | pos |
| SR | Desertqueen-Jumborox-Rock outcrop association, 2 to 8% slopes, warm | Skull Rock trail, boulders, lots of organic matter, Desert Oaks (Quercus sp.) and many other shrubs | 33°59′50.28″ N | 116°03′37.44″ W | neg |
| HH | Morongo loamy sand, 2 to 4% slopes | Hall of Horrors Trail, scattered Joshua trees, Salt Bush (Atriplex spp.) and other shrubs | 34°00′02.16″ N | 116°08′45.96″ W | pos |
| HV | Rock outcrop | Hidden Valley, lots of organic matter, Desert oaks, shrubs and yucca plants | 34°00′12.00″ N | 116°10′25.32″ W | neg |
| Detection of Coccidioides spp. | NAS Lemoore | Antelope Acres (West of EAFB) | Twentynine Palms (South of MCAGCC) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| n (389) | 116 | 100 | 94 | 100 | 179 | 100 |
| positive (with at least one diagnostic primer pair) | 32 | 27.59 | 14 | 14.89 | 76 | 42.46 |
| positive (with both diagnostic primer pairs) | 11 | 9.48 | 11 | 11.7 | 42 | 23.46 |
| negative (with both diagnostic primer pairs) | 73 | 62.93 | 69 | 73.41 | 61 | 34.08 |
| Site | Sample Type | Soil Type and Map Unit | Coordinates | Detection of Coccidioides DNA. |
|---|---|---|---|---|
| Lemoore | ||||
| 21. Street to north of Jackson Ave., Lemoore | TRAKER | Panoche clay loam, saline-alkali (151) | neg | |
| site 1, N-26, along dirt road | TRAKER | Panoche clay loam, saline-alkali (151) | 36°22′37.02″ W, 119°55′56.89″ W | neg |
| Site 1, N-26, dirt road | PI-SWERL | Panoche clay loam, saline-alkali (151) | 36°22′37.02″ N, 119°55′56.89″ W | neg |
| Site 2, dry lake | PI-SWERL | Panoche clay loam, saline-alkali (151) | 36°22′18.19″ N, 119°55′54.16″ W | neg |
| Site 3, near creek, saltbush area | PI-SWERL | Vanguard sandy loam/Gepford clay (partially drained), (168/115) | 36°15′20.74” N, 119°50′04.34″ W | neg |
| Site J, iodine bush area | PI-SWERL | Lemoore sandy loam/Boggs sandy loam (partially drained), (137/103) | 36°15′16.81″ N, 119°48′35.82″ W | pos |
| 21. street, Lemoore to Bakersfield, near intersection to Hwy. 58 | TRAKER | neg | ||
| Antelope Acres | ||||
| Site 7 | PI-SWERL | Adelanto coarse sandy loam (CaA) | 34°44′48.12″ N, 118°18′24.80″ W | neg |
| Site 7 | TRAKER | Adelanto coarse sandy loam (CaA) | 34°44′48.12″ N, 118°18′24.80 W | neg |
| Site 5 | PI-SWERL | Cajon loamy sand (CaA) | 34°44′21.34″ N, 118°18′13.64″ W | pos |
| dust on car after sampling (site 8) | swab | 34°44′50.93″ N, 118°18′16.92″ W | neg | |
| Twentynine Palms | ||||
| Site 5 | PI-SWERL | no data | 34°15′56.66″ N, 116°26′45.20″ W | pos |
| Site 6 | PI-SWERL | no data | 34°15′33.01″ N, 116°26′26.41″ W | neg |
| Site 7 | PI-SWERL | no data | 34°08′12.34″ N, 116°01′0959″ W | neg |
| Site 8 | PI-SWERL | no data | 34°08′08.99″ W, 116°00′10.62″ W | pos |
| Site 9 | PI-SWERL | no data | 34°08′09.85″ N, 115°58′18.46″ W | pos |
| Site 10 | PI-SWERL | no data | 34°08′03.59″ N, 115°54′41.76″ W | pos |
| Dirt roads between sites 7 and 10 | TRAKER | no data | see coordinates above | pos |
| Site UT | PI-SWERL | no data | 34°11′44.84″ N, 116°02′11.04″ W | neg |
| Site UT | TRAKER | no data | 34°11′44.84″ N, 116°02′11.04″ W | neg |
| Soil Core Samples (a–f) and Depth (cm) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sites and Number of Cores | n | a 0–2 | b 5–7 | c 10–12 | d 18–20 | e 23–25 | f 28–30 | a 0–2 | b 5–7 | c 10–12 | d 18–20 | e 23–25 | f 28–30 |
| Indicated positive by one diagnostic primer pair (%) | Indicated positive with both diagnostic primer pairs in agreement (%) | ||||||||||||
| Lemoore, 5 cores | 26 | 11.5 | 11.5 | 7.7 | 7.7 | 0 | 3.9 | 7.7 | 3.9 | 3.9 | 3.9 | 0 | 0 |
| Antelope Acres, 7 cores | 40 | 0 | 0 | 2.5 | 0 | 0 | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 |
| Twentynine Palms, 13 cores | 80 | 11.3 | 6.3 | 5.0 | 5.0 | 1.3 | 2.5 | 5 | 2.5 | 2.5 | 2.5 | 1.3 | 1.3 |
| Locations | Soil Analyses (Parameter Averages or Ranges) | Detection of Coccidioides DNA (Both Diagnostic Primer Pairs Agreed) | Environment/Habitat | Exposure Risk | |||
|---|---|---|---|---|---|---|---|
| Site Description | Soil Chemical Analyses | Soil Physical Analyses | Soil | Dust | Landform | Vegetation | Presumed Risk of Coccidioides Exposure * |
| Lemoore: dominated by agricultural activities, ~6.4 in rainfall, soil types: clay loam, sandy loam, fine sandy loam, clay, many saline-sodic and alkaline | pH: 7.8–8.1, EC (mS/m3): 1 (winter)–13 (summer), TDS (g/L): 0.4–6.1 (winter–summer), CaCO3 (%): 3.52, high levels of SO42− | sand (39.7%), silt (53.7%), clay (6.6%) | 9.48% | site 4 (PI-SWERL) | alluvial fans | mostly agricultural fields and orchards, non-native trees, grasses, meadows, few semi-natural areas with Salt Bush and Iodine Bush | low |
| Antelope Acres (west of EAFB): western Mojave Desert, declining agricultural activities, former ranchland, increase in soil disturbance due to renewable energy construction, eroded soils, windy, ~7.4 in rainfall, soil types: coarse sandy loam, loamy sand | pH: 7.1–7.2, EC (mS/m3): <1 (winter–summer), TDS (g/L): <0.1 (winter–summer), CaCO3 (%): 0.05, high levels of K | sand (63%), silt (32.9%), clay (4.1%) | 11.70% | site 5 (PI-SWERL) | alluvial fans, dry lakes | invasive grasses, rabbit brush, Salt Bush, native and non-native herbs and wildflowers, eroded former farmland and non-vegetated areas (solar ranches) | high, increasing |
| Twentynine Palms: Southern Mojave Desert, semi-disturbed desert, windy, no agriculture, scattered settlements, ~4.4 in rainfall, outcrop associations, loamy sand, coarse loamy sand | pH: 7.5–8, EC (mS/m3): <1 (winter–summer), CaCO3 (%): 0.65, high levels of K and Ca | 29 Palms: sand (66.1%), silt (30.5%), clay (3.4%) | 23.46% | sites 5, 8, 10 (PI-SWERL), sites 7–10 TRAKER | fan aprons, alluvial fans | desert shrub, Creosote, Salt Bush, Joshua Trees, cacti, Desert Oak and pines, few invasive grasses, native and non-native herbs including wildflowers | high |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauer, A.; Etyemezian, V.; Nikolich, G.; Kloock, C.; Arzate, A.F.; Sadiq Batcha, F.; Kaur, M.; Garcia, E.; Mander, J.; Kayes Passaglia, A. Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California. Int. J. Environ. Res. Public Health 2020, 17, 5285. https://doi.org/10.3390/ijerph17155285
Lauer A, Etyemezian V, Nikolich G, Kloock C, Arzate AF, Sadiq Batcha F, Kaur M, Garcia E, Mander J, Kayes Passaglia A. Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California. International Journal of Environmental Research and Public Health. 2020; 17(15):5285. https://doi.org/10.3390/ijerph17155285
Chicago/Turabian StyleLauer, Antje, Vicken Etyemezian, George Nikolich, Carl Kloock, Angel Franco Arzate, Fazalath Sadiq Batcha, Manpreet Kaur, Eduardo Garcia, Jasleen Mander, and Alyce Kayes Passaglia. 2020. "Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California" International Journal of Environmental Research and Public Health 17, no. 15: 5285. https://doi.org/10.3390/ijerph17155285
APA StyleLauer, A., Etyemezian, V., Nikolich, G., Kloock, C., Arzate, A. F., Sadiq Batcha, F., Kaur, M., Garcia, E., Mander, J., & Kayes Passaglia, A. (2020). Valley Fever: Environmental Risk Factors and Exposure Pathways Deduced from Field Measurements in California. International Journal of Environmental Research and Public Health, 17(15), 5285. https://doi.org/10.3390/ijerph17155285





