Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions
Abstract
1. Introduction
2. Nutrient Content of Poultry Feed and Excreta
3. Significance of Poultry Manure
4. Microbial Content of Poultry Litter
5. Soil Amendment
5.1. As Animal Feed
5.2. As Fuel
6. Composting Process of Poultry Manure
7. Factors Affecting Composting Process
8. Antibiotics Used in Poultry
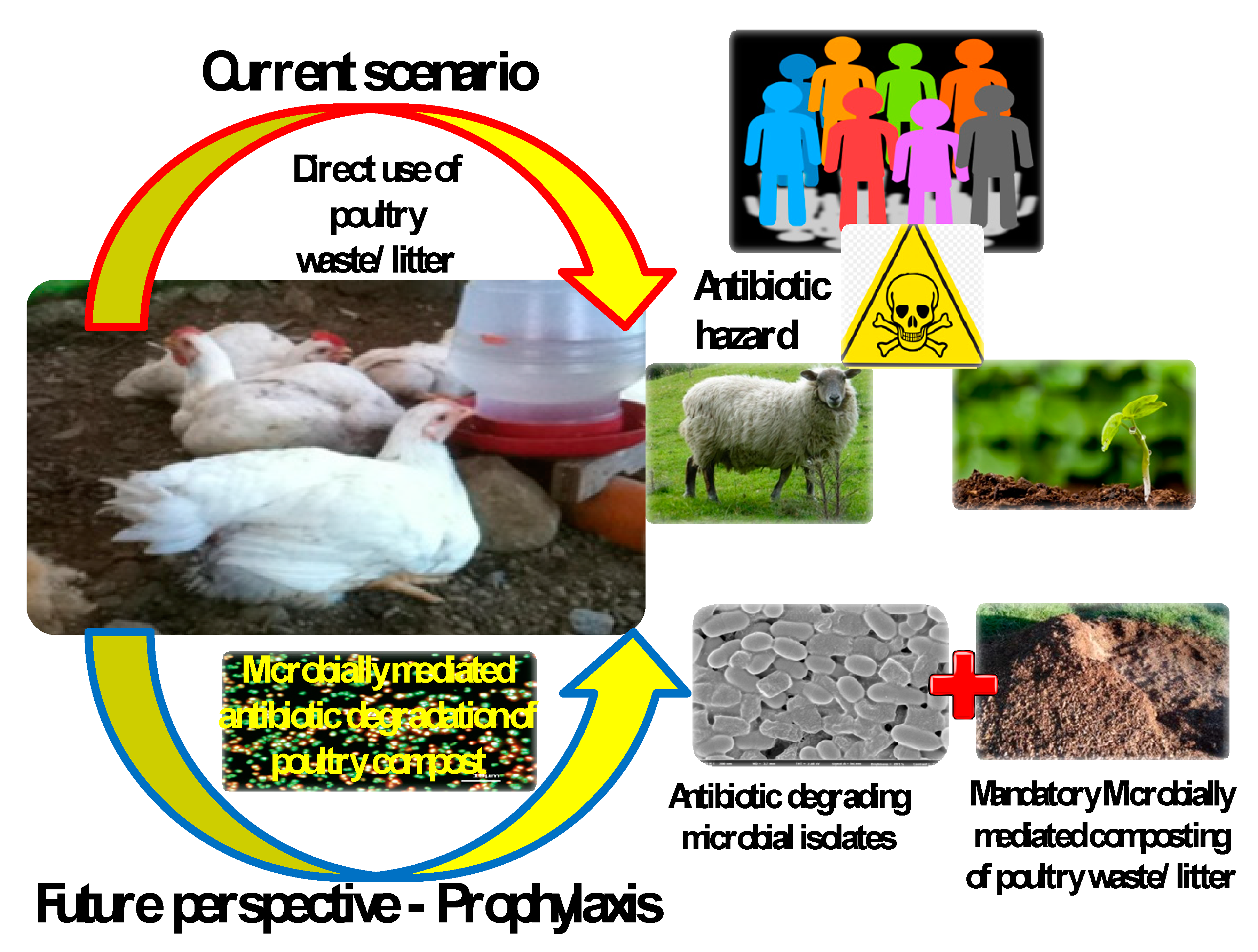
9. Future Prospects
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethical Approval
References
- Upali Wickramasinghe, G.A. Meat and Meat products. In Food Outlook, Food and Agricultural Organization; United Nations FAO Investment Centre Division: Rome, Italy, 2018; pp. 48–53. [Google Scholar]
- Jones, B.E.H.; Haynes, R.J.; Phillips, I.R. Effect of amendment of bauxite processing sand with organic materials on its chemical, physical and microbial properties. J. Environ. Manag. 2010, 91, 2281–2288. [Google Scholar]
- Castanon, J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [PubMed]
- Collins, E.R.; Barker, J.C.; Carr, L.E.; Brodie, H.L.; Martin, J.H. National Resource, Agriculture, and Engineering Service. In Poultry Waste Management Handbook; Cornell University: Ithaca, NY, USA, 1999. [Google Scholar]
- Williams, C.M.; Barker, J.C.; Sims, J.T. Management and utilization of poultry wastes. Rev. Environ. Contam. Toxicol. 1999, 162, 105–157. [Google Scholar]
- Mitchell, C.C.; Donald, J.O.; Martin, J. The Value and Use of Poultry Waste as Fertilizer; Alabama Cooperative Extension System: Auburn, AL, USA, 1995. [Google Scholar]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology—A review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar]
- Qin, C.; Yi, K.K.; Wu, P. Ammonium affects cell viability to inhibit root growth in Arabidopsis. J. Zhejiang Univ. Sci. B 2011, 12, 477–484. [Google Scholar] [PubMed]
- Zucconi, F.; De Bertoldi, M. Compost specifications for the production and characterization of compost from municipal solid waste. In Compost: Production, Quality and Use; De Bertoldi, M., Ferranti, M.P., L’Hermite, M.P., Zucconi, F., Eds.; Elsevier: London, UK, 1987; pp. 276–295. [Google Scholar]
- Garces, A.; Afonso, S.M.S.; Chilundo, A.; Jairoce, C.T.S. Evaluation of different litter materials for broiler production in a hot and humid environment: 1. Litter characteristics and quality. J. Appl. Poult. Res. 2013, 22, 168–176. [Google Scholar]
- Sweeten, J.M. Composting manure and sludge. In Proceedings of the National Poultry Waste Manage Symposium, Columbus, OH, USA, 18–19 April 1988; pp. 38–44. [Google Scholar]
- Kithome, M.; Paul, J.W.; Bomke, A.A. Reducing nitrogen losses during simulated composting of poultry manure using adsorbents or chemical amendments. J. Environ. Qual. 1999, 28, 194–201. [Google Scholar]
- Gao, M.C.; Li, B.; Yu, A.; Liang, F.Y.; Yang, L.J.; Sun, Y.X. The effect of aeration rate on forced-aeration composting of chicken manure and sawdust. Bioresour. Technol. 2010, 101, 1899–1903. [Google Scholar] [PubMed]
- Gao, M.C.; Liang, F.Y.; Yu, A.; Li, B.; Yang, L.J. Evaluation of stability and maturity during forced-aeration composting of chicken manure and sawdust at different C/N ratios. Chemosphere 2010, 78, 614–619. [Google Scholar]
- Tiquia, S.M.; Tam, N.F.Y. Fate of nitrogen during composting of chicken litter. Environ. Pollut. 2000, 110, 535–541. [Google Scholar] [PubMed]
- Shen, Y.; Ren, L.; Li, G.; Chen, T.; Guo, R. Influence of aeration on CH4, N2O and NH3 emissions during aerobic composting of a chicken manure and high C/N waste mixture. Waste Manag. 2011, 31, 33–38. [Google Scholar] [PubMed]
- Mahimairaja, S.; Bolan, N.S.; Hedley, M.J.; Macgregor, A.N. Losses and transformation of nitrogen during composting of poultry manure with different amendments: An incubation experiment. Bioresour. Technol. 1994, 47, 265–273. [Google Scholar]
- Kajiya, Y.; Ishii, Y.; Fukuyama, K.; Idota, S. Precise optimization of poultry mnure composting using an experimental small-scale apparatus. J. Biol. Sci. 2015, 15, 40–44. [Google Scholar]
- Ravindran, V.; Blair, R. Feed resources for poultry production in Asia and the Pacific region. I. Energy sources. World Poult. Sci. J. 1991, 47, 213–231. [Google Scholar]
- Williams Beski, S.S.M.; Swick, R.A.; Iji, P.A. Specialized protein products in broiler chicken nutrition: A review. Anim. Nutr. 2015, 1, 47–53. [Google Scholar]
- Ravindran, V. Poultry feed availability and nutrition in developing countries. In Poultry Development Review; FAO: Rome, Italy, 2013; pp. 60–63. [Google Scholar]
- Dilger, R.N.; Adeola, O. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing chicks fed conventional and low-phytate soybean meals. Poult. Sci. 2006, 85, 661–668. [Google Scholar]
- Nahm, K.H. Feed formulations to reduce N excretion and ammonia emission from poultry manure. Bioresour. Technol. 2007, 98, 2282–2300. [Google Scholar]
- Gerber, P.; Opio, C.; Steinfeld, H. Poultry production and the environment—A review in Poultry in the 21st Century. In Proceedings of the International Conference Poultry in the Twenty-First Century: Avian Influenza and Beyond, Bangkok, Thailand, 5–7 November 2007; pp. 379–405. [Google Scholar]
- Arogo, J.; Westerman, P.W.; Heber, A.J.; Robarge, W.P.; Classen, J.J. Ammonia in Animal Production—A Review; Paper number 014089, ASAE Annual Meeting; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2001. [Google Scholar]
- Latshaw, J.D.; Zhao, L. Dietary protein effects on hen performance and nitrogen excretion. Poult. Sci. 2011, 90, 99–106. [Google Scholar]
- Leytem, A.B.; Plumstead, P.W.; Maguire, R.O.; Kwanyuen, P.; Brake, J. What aspect of dietary modification in broilers controls litter water-soluble phosphorus: Dietary phosphorus, phytase, or calcium? J. Environ. Qual. 2007, 36, 453–463. [Google Scholar]
- Nahm, K.H. Evaluation of the nitrogen content in poultry manure. World Poult. Sci. J. 2003, 59, 77–88. [Google Scholar]
- Chen, L.J.; Xing, L.; Han, L.J. Quantitative determination of nutrient content in poultry manure by near infrared spectroscopy based on artificial neural networks. Poult. Sci. 2009, 88, 2496–2503. [Google Scholar] [PubMed]
- Amanullah, M.M.; Sekar, S.; Muthukrishnan, P. Prospects and potential of poultry manure. Asian J. Plant. Sci. 2010, 9, 172–182. [Google Scholar]
- Nicholson, F.A.; Chambers, B.J.; Smith, K.A. Nutrient composition of poultry manures in England and Wales. Bioresour. Technol. 1996, 58, 279–284. [Google Scholar]
- Amanullah, M.M.; Vaiyapuri, K.; Sathyamoorthi, K. Poultry manure to crops—A review. Agric. Rev. 2007, 28, 216–222. [Google Scholar]
- Ravikumar, R.; Krishnamoorthy, K. Effect of recycling of organic wastes on plant growth and physical proper-ties of soil. In Annual Report of All Indian Coordinated Research Project for Improvement of Soil Physical Conditions to Increase Agricultural Production of Problem Soil; National Academy of Agricultural Sciences: Coimbatore, India, 1975; pp. 91–94. [Google Scholar]
- Diacono, M.; Montemurro, F. Effectiveness of organic wastes as fertilizers and amendments in salt-affected soils. Agriculture 2015, 5, 221–230. [Google Scholar]
- Mbagwu, J.S.C. Improving the productivity of a degraded ultisol in Nigeria using organic and inorganic amendments. Part 2: Changes in physical properties. Bioresour. Technol. 1992, 42, 167–175. [Google Scholar]
- Agbede, T.; Ojeniyi, S.; Adeyemo, A. Effect of poultry manure on soil physical and chemical properties, growth and grain yield of sorghum in southwest. Nigeria. Am. Eurasian J. Sustain. Agric. 2008, 2, 72–77. [Google Scholar]
- Acosta-Martinez, A.; Harmel, R.D. Soil microbial communities and enzyme activities under various poultry litter application rates. J. Environ. Qual. 2006, 35, 1309–1318. [Google Scholar]
- Zhen, Z.; Liu, H.T.; Wang, N.; Guo, L.Y.; Meng, J.; Ding, N.; Wu, G.L.; Jiang, G.M. Effects of manure compost application on soil microbial community diversity and soil microenvironments in a temperate cropland in China. PLoS ONE 2014, 9, e108555. [Google Scholar]
- Lu, J.; Sanchez, S.; Hofacre, C.; Maurer, J.J.; Harmon, B.G.; Lee, M.D. Evaluation of Broiler Litter with Reference to the Microbial Composition as Assessed by Using 16S rRNA and Functional Gene Markers. Appl. Environ. Microbiol. 2003, 69, 901–908. [Google Scholar] [PubMed]
- Enticknap, J.J.; Nonogaki, H.; Place, A.R.; Hill, R.T. Microbial Diversity Associated with Odor Modification for Production of Fertilizers from Chicken Litter. Appl. Environ. Microbiol. 2006, 72, 4105–4114. [Google Scholar] [PubMed]
- Wadud, S.; Michaelsen, A.; Gallagher, E.; Parcsi, G.; Zemb, O.; Stuetz, R.; Manefield, M. Bacterial and fungal community composition over time in chicken litter with high or low moisture content. Br. Poult. Sci. 2012, 53, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kyakuwaire, M.; Olupot, G.; Amoding, A.; Nkedi-Kizza, P.; Ateenyi Basamba, T. How Safe is Chicken Litter for Land Application as an Organic Fertilizer?: A Review. Int. J. Environ. Res. Public Health 2019, 16, 3521. [Google Scholar]
- Carrera, L.M.; Buyer, J.S.; Vinyard, B.; Abdul-Baki, A.A.; Sikora, L.J.; Teasdale, J.R. Effects of cover crops, compost, and manure amendments on soil microbial community structure in tomato production systems. Appl. Soil Ecol. 2007, 37, 247–255. [Google Scholar]
- McGrath, S.; Maguire, R.O.; Tracy, B.F.; Fike, J.H. Improving soil nutrition with poultry litter application in low-input forage systems. Agron. J. 2010, 102, 48–54. [Google Scholar]
- Bolan, N.S.; Szogi, A.; Chuasavathi, T.; Seshadri, B.; Rothrock, M.; Panneerselvam, P. Uses and management of poultry litter. World Poult. Sci. J. 2010, 66, 673–698. [Google Scholar]
- Smith, L.W.; Fries, G.F. Dehydrated poultry manure as a crude protein supplement for lactating cows. J. Dairy Sci. 1973, 56, 668–672. [Google Scholar]
- Fontenot, J.P.; De Baca, R.C.; Glimp, H.A. Recycling Animal Wastes by Feeding to Enhance Environmental Quality. Prof. Anim. Sci. 1991, 7, 1–8. [Google Scholar]
- Sharma, M.; Joshi, C.; Saxena, N. Mineral toxicity in livestock: A review. Indian J. Anim. Sci. 2005, 75, 753–764. [Google Scholar]
- Turnell, J.R.; Faulkner, R.D.; Hinch, G.N. Recent advances in Australian broiler litter utilisation. World Poult. Sci. J. 2007, 63, 223–231. [Google Scholar]
- Finstein, M.S.; Morris, M.L. Microbiology of municipal solid waste composting. Adv. Appl. Microbiol. 1975, 19, 113–151. [Google Scholar] [PubMed]
- Chen, Z.; Jiang, X. Microbiological Safety of Chicken Litter or Chicken Litter-Based Organic Fertilizers: A Review. Agriculture 2014, 4, 1–29. [Google Scholar]
- Ahmed, Z.A.; Hussin, H.; Rohaim, M.; Nasr, S. Efficacy of composting dead poultry and farm wastes infected with avian influenza virus H5N1. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 588–596. [Google Scholar]
- Khalil, A.; Domeizel, M.; Prudent, P. Monitoring of green waste composting process based on redox potential. Bioresour. Technol. 2008, 99, 6037–6045. [Google Scholar] [CrossRef]
- Willson, G.B. Combining raw materials for composting. BioCycle 1989, 30, 82–83. [Google Scholar]
- Bueno, P.; Tapias, R.; Lopez, F.; Diaz, M.J. Optimizing composting parameters for nitrogen conservation in composting. Bioresour. Technol. 2008, 99, 5069–5077. [Google Scholar]
- Diaz, M.J.; Eugenio, M.E.; Jimenez, L.; Madejon, E.; Cabrera, F. Modelling vinasse/cotton waste ratio incubation for optimum composting. Chem. Engl. J. 2003, 93, 233–240. [Google Scholar]
- Gray, K.; Sherman, K.; AJ, B. A Review of Composting—Part 1. Process Biochem. 1971, 6, 32–36. [Google Scholar]
- Nakasaki, K.; Ohtaki, A. A simple numerical model for predicting organic matter decomposition in a fed-batch composting operation. J. Environ. Qual. 2002, 31, 997–1003. [Google Scholar] [CrossRef]
- Eiland, F.; Klamer, M.; Lind, A.M.; Leth, M.; Baath, E. Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microbial Ecol. 2001, 41, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Sarma, U.S.; Das Ravindranath, A.; Radhakrishnan, S.; Ghosh, P. A novel method for accelerated composting of coir pith. Energy Fuels 2007, 21, 822–827. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itavaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, S.; Chtourou, M.; Medhioub, K.; Ammar, E. Compost of poultry manure and olive mill wastes as an alternative fertilizer. Agron. Sustain. Dev. 2006, 26, 135–142. [Google Scholar] [CrossRef]
- Ch’ng, H.Y.; Ahmed, O.H.; Kassim, S.; Ab Majid, N.M. Co-composting of pineapple leaves and chicken manure slurry. Int. J. Recycl. Org. Waste Agric. 2013, 2, 1–8. [Google Scholar] [CrossRef]
- Sakthivadivu, R.; Sivakumar, K.; Kumar, V.R.S.; Natarajan, A. Chemical changes during composting of poultry waste with coirpith waste and sugarcane top. Inter. J. Sci. Environ. Technol. 2015, 4, 40–49. [Google Scholar]
- Thomas, G.V.; Palaniswami, C.; Prabhu, S.R.; Gopal, M.; Gupta, A. Co-composting of coconut coir pith with solid poultry manure. Curr. Sci. 2013, 104, 245–250. [Google Scholar]
- Libby, D.A.; Schaible, P.J. Observations on growth responses to antibiotics and arsonic acids in poultry feeds. Science 1995, 121, 733–734. [Google Scholar] [CrossRef]
- Stokstad, E.L.; Jukes, T.H. Studies of the growth-promoting effect of antibiotics in chicks on a purified diet. Antibiot. Annu. 1958, 6, 998–1002. [Google Scholar]
- Waibel, P.E.; Abbott, O.; Baumann, C.; Bird, H. Disappearance of the growth response of chicks to dietary antibiotics in an “old” environment. Poult. Sci. 1954, 33, 1141–1146. [Google Scholar] [CrossRef]
- Moore, P.; Evenson, A.; Luckey, T.; McCoy, E.; Elvehjem, C.; Hart, E. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef]
- Graham, J.P.; Boland, J.J.; Silbergeld, E. Growth promoting antibiotics in food animal production: An economic analysis. Public Health Rep. 2007, 122, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Vieira, S.L.; Favero, A.; Taschetto, D.; Mascarello, N.C.; Cemin, H.S. Reassessing flavophospholipol effects on broiler performance. Revista Brasileira Zootecnia 2012, 41, 2458–2462. [Google Scholar] [CrossRef]
- Salmon, R.E.; Stevens, V.I. Effect of bambermycins (Flavomycin) in diets for growing turkeys. Poult. Sci. 1990, 69, 1133–1140. [Google Scholar] [CrossRef]
- McDougald, L.R. Intestinal protozoa important to poultry. Poult. Sci. 1998, 77, 1156–1158. [Google Scholar] [CrossRef]
- Elwinger, K.; Berndtson, E.; Engström, B.; Fossum, O.; Waldenstedt, L. Effect of antibiotic growth promoters and anticoccidials on growth of Clostridium perfringens in the caeca and on performance of broiler chickens. Acta Vet. Scand. 1998, 39, 433–441. [Google Scholar]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef]
- Koutoulis, K.C.; Kefalas, G.; Minos, E. Salinomycin toxicosis in broiler breeders and turkeys: Report of the first case. Am. J. Anim. Vet. Sci. 2013, 8, 190–196. [Google Scholar] [CrossRef]
- Logan, N.B.; McKenzie, M.E.; Conway, D.P.; Chappel, L.R.; Hammet, N.C. Anticoccidial efficacy of semduramicin.: 2. Evaluation against field isolates including comparisons with salinomycin, maduramicin, and monensin in battery tests. Poult. Sci. 1993, 72, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.; Romoser, G.; Shaffner, C. Nystatin (mycostatin) in rations for growing turkeys. Poult. Sci. 1958, 37, 896–898. [Google Scholar] [CrossRef]
- Kruse, H.; Johansen, B.K.; Rorvik, L.M.; Schaller, G. The use of avoparcin as a growth promoter and the occurrence of vancomycin-resistant Enterococcus species in Norwegian poultry and swine production. Microb. Drug Resist. 1999, 5, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Bager, F.; Madsen, M.; Christensen, J.; Aarestrup, F.M. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev. Vet. Med. 1997, 31, 95–112. [Google Scholar] [CrossRef]
- Pantosti, A.; Del Grosso, A.; Tagliabue, S.; Macri, A.; Caprioli, A. Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet 1999, 354, 741–742. [Google Scholar] [CrossRef]
- Kieke, A.L.; Borchardt, M.A.; Kieke, B.A.; Spencer, S.K.; Vandermause, M.F.; Smith, K.E.; Jawahir, S.L.; Group MES. Use of streptogramin growth promoters in poultry and isolation of streptogramin-resistant Enterococcus faecium from humans. J. Infect. Dis. 2006, 194, 1200–1208. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Wellenreiter, R.H.; Mowrey, D.H.; Stobbs, L.A.; d’Assonville, J.A. Effects of avilamycin on performance of broiler chickens. Vet. Ther. 2000, 1, 118–124. [Google Scholar]
- Castillo, M.; Martin-Orue, S.M.; Roca, M.; Manzanilla, E.G.; Badiola, I.; Perez, J.F.; Gasa, J. The response of gastrointestinal microbiota to avilamycin, butyrate, and plant extracts in early-weaned pigs. J. Anim. Sci. 2006, 84, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Dumonceaux, T.J.; Hill, J.E.; Hemmingsen, S.M.; Van Kessel, A.G. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl. Environ. Microbiol. 2006, 72, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, A.A.; Menten, J.F.M.; Lambais, M.R.; Racanicci, A.M.C.; Longo, F.A.; Sorbara, J.O.B. Intestinal bacterial community and growth performance of chickens fed diets containing antibiotics. Poult. Sci. 2006, 85, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.; Shinde, P.; Choi, J.; Kim, J.; Seo, D.; Pak, J.; Chae, B.; Kwon, I. Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. Asian-Aust. J. Anim. Sci. 2010, 23, 521–529. [Google Scholar] [CrossRef]
- Islam, K.M.S.; Klein, U.; Burch, D.G.S. The activity and compatibility of the antibiotic tiamulin with other drugs in poultry medicine—A review. Poult. Sci. 2009, 88, 2353–2359. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Chopra, I.; Hawkey, P.; Hinton, M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemoth. 1992, 29, 245–277. [Google Scholar] [CrossRef]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Mingeot-Leclercq, M.-P.; Glupczynski, Y.; Tulkens, P.M. Aminoglycosides: Activity and resistance. Antimicrob. Agents Chemother. 1999, 43, 727–737. [Google Scholar] [CrossRef]
- Udumula, V.; Ham, Y.H.; Fosso, M.Y.; Chan, K.Y.; Rai, R.; Zhang, J.; Li, J.; Chang, C.-W. Investigation of antibacterial mode of action for traditional and amphiphilic aminoglycosides. Bioorgan. Med. Chem. Lett. 2013, 23, 1671–1675. [Google Scholar] [CrossRef]
- Javed, V.; Saleemi, M.; Khan, M.; Khan, A.; Javed, I. Pathological effects of gentamicin in growing broilers. In Proceedings of the 15th Congress of FAVA-OIE Joint Symposium on Emerging Diseases, Bangkok, Thailand, 27–30 October 2008; pp. 17–18. [Google Scholar]
- Morar, M.; Bhullar, K.; Hughes, D.W.; Junop, M.; Wright, G.D. Structure and mechanism of the lincosamide antibiotic adenylyltransferase LinB. Structure 2009, 17, 1649–1659. [Google Scholar] [CrossRef]
- Tenson, T.; Lovmar, M.; Ehrenberg, M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 2003, 330, 1005–1014. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Virgincar, N.; MacGowan, A. Glycopeptides (Dalbavancin, Oritavancin, Teicoplanin, Telavancin, Vancomycin). In Antimicrobial Therapy and Vaccines. Vol. II: Antimicrobial Agents 181–200; ESun Technologies: Pittsburgh, PA, USA, 2009. [Google Scholar]
- Zavascki, A.P.; Goldani, L.Z.; Li, J.; Nation, R.L. Polymyxin B for the treatment of multidrug-resistant pathogens: A critical review. J. Antimicrob. Chemother. 2007, 60, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, P.M. Mechanisms of quinolone action and microbial response. J. Antimicrob. Chemother. 2003, 51, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C. Mechanisms of action of antimicrobials: Focus on fluoroquinolones. Clin. Infect. Dis. 2001, 32, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Carson, C.; Léger, D. Antimicrobial therapy of selected diseases in turkeys, laying hens, and minor poultry species in Canada. Can. Vet. J. 2013, 54, 1041–1052. [Google Scholar]
- Mubito, E.B.; Shahada, F.; Kimanya, M.E.; Buza, J.J. Sulfonamide residues in commercial layer chicken eggs in Dar-es-Salaam, Tanzania. Am. J. Res. Commun. 2014, 2, 124–132. [Google Scholar]
- Cheong, C.; Parvaneh, H.; Selamat, J.; Rashedi, M.; Fitry, I. Sulfonamides determination in chicken meat products from Malaysia. Int. Food. Res. J. 2010, 17, 885–892. [Google Scholar]
- Horton-Smith, C.; Long, P. Effect of sulfonamide medication on the life cycle of Eimeria meleagrimitis in turkeys. Exp. Parasitol. 1961, 11, 93–101. [Google Scholar] [CrossRef]
- Ungureanu, V. Macrolides, lincosamides, streptogramines (MLS): Mechanisms of action and resistance. Bacteriol. Virusol. Parazitol. Epidemiol. 2010, 55, 131–138. [Google Scholar]
- Shryock, T.; Mortensen, J.; Baumholtz, M. The effects of macrolides on the expression of bacterial virulence mechanisms. J. Antimicrob. Chemother. 1998, 41, 505–512. [Google Scholar] [CrossRef]
- Nakajima, Y. Mechanisms of bacterial resistance to macrolide antibiotics. J. Infect. Chemoth. 1999, 5, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Mubito, E.P.; Shahada, F.; Kimanya, M.E.; Buza, J.J. Antimicrobial use in the poultry industry in Dar-es-Salaam, Tanzania and public health implications. Am. J. Res. Commun. 2014, 2, 51–63. [Google Scholar]
- Vazquez, D. Studies on the mode of action of the streptogramin antibiotics. Microbiology 1966, 42, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.J.; Mukhtar, T.A.; Wright, G.D. Streptogramin antibiotics: Mode of action and resistance. Curr. Drug Targets 2002, 3, 335–344. [Google Scholar] [CrossRef]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef]
- Laue, H.; Weiss, L.; Bernardi, A.; Hawser, S.; Lociuro, S.; Islam, K. In vitro activity of the novel diaminopyrimidine, iclaprim, in combination with folate inhibitors and other antimicrobials with different mechanisms of action. J. Antimicrob. Chemoth. 2007, 60, 1391–1394. [Google Scholar] [CrossRef]
- Maasjost, J.; Mühldorfer, K.; de Jäckel, S.C.; Hafez, H. Antimicrobial susceptibility patterns of enterococcus faecalis and enterococcus faecium isolated from poultry flocks in germany. Avian Dis. 2015, 59, 143–148. [Google Scholar] [CrossRef]
- Williamson, R.; Collatz, E.; Gutmann, L. Mechanisms of action of beta-lactam antibiotics and mechanisms of non-enzymatic resistance. Presse Medicale 1986, 15, 2282–2289. [Google Scholar]
- Tipper, D.J. Mode of action of β-lactam antibiotics. Pharmacol. Ther. 1985, 27, 1–35. [Google Scholar] [CrossRef]
- Dierikx, C.M. Beta-Lactamases in Enterobacteriaceae in Broilers. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2013. [Google Scholar]
- Vinay Kant, P.S.; Verma, P.K.; Bais, I.; Parmar, M.S.; Gopal, A.; Gupta, V. Anticoccidial drugs used in the poultry: An overview. Sci. Inter. 2013, 1, 261–265. [Google Scholar] [CrossRef]
- Leme, I.L.; Ferreira, A.J.P.; Bottino, J.A.; Pignatari, A.C.C. Glycopeptides susceptibility among enterococci isolated from a poultry farm in São Paulo, Brazil (1996/1997). Braz. J. Microbiol. 2000, 31, 53–57. [Google Scholar] [CrossRef][Green Version]
- Kyriakis, S.C. The effect of avilamycin in the control of stress-induced post-weaning diarrhoea in piglets. J. Vet. Pharmacol. Ther. 1989, 12, 296–301. [Google Scholar] [CrossRef] [PubMed]
- La-ongkhum, O.; Pungsungvorn, N.; Amornthewaphat, N.; Nitisinprasert, S. Effect of the antibiotic avilamycin on the structure of the microbial community in the jejunal intestinal tract of broiler chickens. Poult. Sci. 2011, 90, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Egger, H.; Reinshagen, H. New pleuromutilin derivatives with enhanced antimicrobial activity. II. Structure-activity correlations. J. Antibiot. 1976, 29, 923–927. [Google Scholar] [CrossRef]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Med. 2006, 119, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, F.X.R. Toxicological Evaluation of Certain Veterinary Drug Residues in Food; The Joint FAO⁄WHO Expert Committee on Food Additives: Rome, Italy, 1991. [Google Scholar]
- Lewicki, J.; Reeves, P.T.; Swan, G.E. Residue Evaluation of Certain Veterinary Drugs. In Proceedings of the 70th Meeting of the Joint FAO⁄WHO Expert Committee on Food Additives, Geneva, Switzerland, 21–29 October 2008; pp. 243–279. [Google Scholar]
- Goetting, V.; Lee, K.A.; Tell, L.A. Pharmacokinetics of Veterinary Drugs in Laying Hens and Residues in Eggs: A Review of the Literature. J. Vet. Pharmacol. Ther. 2011, 34, 521–556. [Google Scholar] [CrossRef] [PubMed]
- Muaz, K.; Riaz, M.; Akhtar, S.; Park, S.; Ismail, A. Antibiotic Residues in Chicken Meat: Global Prevalence, Threats, and Decontamination Strategies: A Review. J. Food Prot. 2018, 81, 619–627. [Google Scholar] [CrossRef]
- Aminov, R.I.; Mackie, R.I. Evolution and ecology of antibiotic resistance genes. FEMS Microbiol. Lett. 2007, 271, 147–161. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Beck, I.C. Effects of sulfonamide and tetracycline antibiotics on soil microbial activity and microbial biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef]
- Kotzerke, A.; Sharma, S.; Schauss, K.; Heuer, H.; Thiele-Bruhn, S.; Smalla, K.; Wilke, B.-M.; Schloter, M. Alterations in soil microbial activity and N-transformation processes due to sulfadiazine loads in pig-manure. Environ. Pollut. 2008, 153, 315–322. [Google Scholar] [CrossRef]
- Ding, C.; He, J. Effect of antibiotics in the environment on microbial populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941. [Google Scholar] [CrossRef] [PubMed]
| Category | Description | Moisture Content (%) | Crude Protein% | Crude Fiber% | Ash% | Feathers% |
|---|---|---|---|---|---|---|
| Dried poultry waste | Feces | >15 | <18 | >15 | >30 | >1 |
| Dried poultry waste | Void of part or all of crude protein and non-protein nitrogen, feces | >15 | <11 | >15 | >30 | >1 |
| Dried poultry litter | Feces and floor litter | >15 | <18 | >25 | >20 | >4 |
| Antibiotics Used in Poultry | Activity Against Microbial Pathogens | Mode of Action | Used for Poultry Species | Reference |
|---|---|---|---|---|
| Ionophores | ||||
| flavomycin/bambermycin/flavophospholipol, moenomycin | Gram-positive bacteria | Inhibition of cell wall synthesis | broiler chicken and turkeys | [75,76] |
| maduramicin, narasin and monensin | protozoan parasite (Eimeria sp.) | Cell wall disruption | broilers | [77,78] |
| lasalocid | protozoan parasite (Eimeria sp.) | Cell wall disruption | broilers and replacement pullets, turkeys, pheasants and quails. | [79] |
| salinomycin | protozoan parasite (Eimeria sp.) | Cell wall disruption | chicken and turkey | [80] |
| semduramicin | protozoan parasite (Eimeria sp.) | Cell wall disruption | chicken and turkey | [81] |
| nystatin | Candida albicans | Cell wall disruption | chicken and turkey | [82] |
| Glycopeptide antibiotic | ||||
| avoparcin | Gram-positive bacteria | Inhibition of cell wall synthesis | Broiler chicken and turkeys | [78,83,84] |
| vancomycin, teicoplanin, avoparcin, ardacin | Gram-positive bacteria and Actinomycetes such as Streptomyces orientalis (vancomycin), Nocardia actinoides (Actinoidin) | Inhibition of cell wall synthesis | Broiler chickens and Turkey | [85,86,87] |
| Orthosomycin | ||||
| avilamycin | Gram-positive bacteria | Inhibition of protein synthesis | Broiler chicken and turkeys | [88,89,90,91,92] |
| Diterpene antibiotics | ||||
| tiamulin hydrogen | Mycoplasma and Brachyspira | inhibition protein synthesis | Broilers chickens | [93] |
| Tetracyclines | ||||
| chlortetracycline, doxycycline, oxytetracycline, tetracycline | Gram-positive and Gram-negative bacteria, Mycobacterium, Mycoplasma, Nocardia, Streptomyces and Ureaplasma | inhibition protein synthesis | Turkeys, Pigeons, Ostriches, ducks | [94,95,96] |
| Aminoglycosides | ||||
| gentamicin, tobramycin, amikacin, streptomycin, kanamycin, neomycin, spectinomycin | Gram-positive and negative bacteria | Tetracyclines | Broiler chickens, Ducks and Turkeys | [97,98,99] |
| Cyclic peptide | ||||
| bacitracin/zinc bacitracin | Gram-positive and Gram-negative Bacteria | Inhibition of Cell wall and protein synthesis | Layers, Turkeys, Ducks, pigeons | [3] |
| Lincosamides | ||||
| Lincomycin, Clindamycin and Pirlimycin | Mycoplasma and Gram-positive anaerobes | Inhibition of protein synthesis | Chickens | [3,100,101] |
| Polymixins | ||||
| polymixin B, colistin (polymyxin E) | Gram-negative bacteria | Inhibition of cell membrane function | Chickens | [102,103] |
| Fluoroquinolones | ||||
| ciprofloxacin, ofloxacin. trimethoprim enrofloxacin marbofloxacin and difloxacin | Gram-negative bacteria | Inhibition of DNA replication | Chickens, Turkey and Ducks | [104,105,106] |
| sulfonamides | ||||
| sulfadimidine, sulfamethoxazole. | Gram-positive and Gram-negative bacteria and Protozoa | Inhibition DNA replication | Broiler and Layer Chickens and Turkeys | [107,108,109] |
| Macrolides | ||||
| azithromycin, clarithromycin, clindamycin, erythromycin, roxithromycin, spiramycin, tylosin, vancomycin | Gram-positive bacteria, Mycoplasma, Mycobacteria, some Rickettsia and Chlamydia | Inhibition of protein synthesis | Layer and broilers Chickens | [110,111,112,113] |
| Streptogramins | ||||
| virginiamycin | Gram-positive bacteria | Inhibition of protein synthesis. | Chickens and Turkeys | [86,114,115,116] |
| nosiheptide | Inhibit Protein synthesis | Broiler chickens | [86,114,115,116] | |
| Quinolones | ||||
| quinoxalines | Gram-positive Organisms | Inhibition of DNA replication | Chickens and Turkey | [116] |
| Diaminopyrimidines | ||||
| Trimethoprim, aditoprim, baquiloprim, ormetoprim | Gram-positive and many Gram-negative bacteria | Inhibition of DNA replication | Chickens | [117] |
| β-Lactams | ||||
| Penicillins: amoxicillin, ampicillin, benzylpenicillin, cloxacillin, dicloxacillin, flucloxacillin, methicillin, mezlocillin, nafcillin, oxacillin, piperacillin, phenoxymethylpenicillin | Gram-positive and many Gram-negative bacteria | Inhibition of cell wall synthesis | Fattening Turkeys Broiler Chickens | [118,119,120,121] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikandan, M.; Chun, S.; Kazibwe, Z.; Gopal, J.; Singh, U.B.; Oh, J.-W. Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. Int. J. Environ. Res. Public Health 2020, 17, 5053. https://doi.org/10.3390/ijerph17145053
Manikandan M, Chun S, Kazibwe Z, Gopal J, Singh UB, Oh J-W. Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. International Journal of Environmental Research and Public Health. 2020; 17(14):5053. https://doi.org/10.3390/ijerph17145053
Chicago/Turabian StyleManikandan, Muthu, Sechul Chun, Zakayo Kazibwe, Judy Gopal, Udai Bhan Singh, and Jae-Wook Oh. 2020. "Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions" International Journal of Environmental Research and Public Health 17, no. 14: 5053. https://doi.org/10.3390/ijerph17145053
APA StyleManikandan, M., Chun, S., Kazibwe, Z., Gopal, J., Singh, U. B., & Oh, J.-W. (2020). Phenomenal Bombardment of Antibiotic in Poultry: Contemplating the Environmental Repercussions. International Journal of Environmental Research and Public Health, 17(14), 5053. https://doi.org/10.3390/ijerph17145053






