Inhalation Exposure to Gaseous and Particulate Bound Mercury Present in the Ambient Air over the Polluted Area of Southern Poland
Abstract
1. Introduction
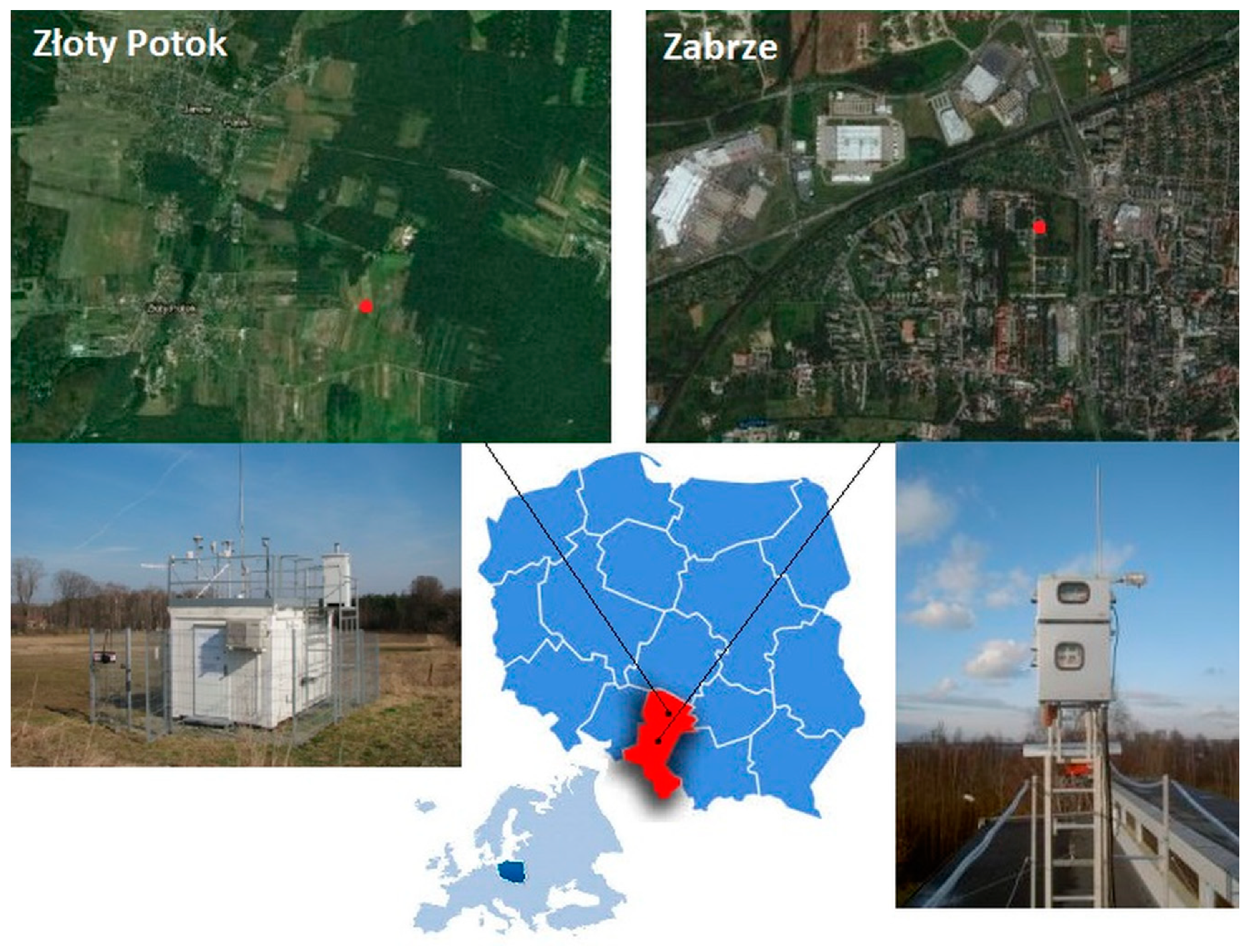
2. Study Area
3. Measurement Methods
3.1. Measurements of Gas-Phase Mercury
3.2. PM2.5 Sampling and Determination of Particulate Mercury
4. Inhalation Exposure and Health Risk Assessment
- CA [mg m−3] = 24 h concentrations of mercury species in the air (TGM or PBM);
- ET [hours day−1] = exposure time, here: 24 h day−1;
- EF [days year−1] = exposure frequency, here: 365 days year−1;
- ED [years] = exposure duration, here: lifespan 70 years;
- AT [hours] = averaging time calculated as the product of ED, EF and ET, here: (70 years × 365 days year−1 × 24 h day−1).
5. Results and Discussion
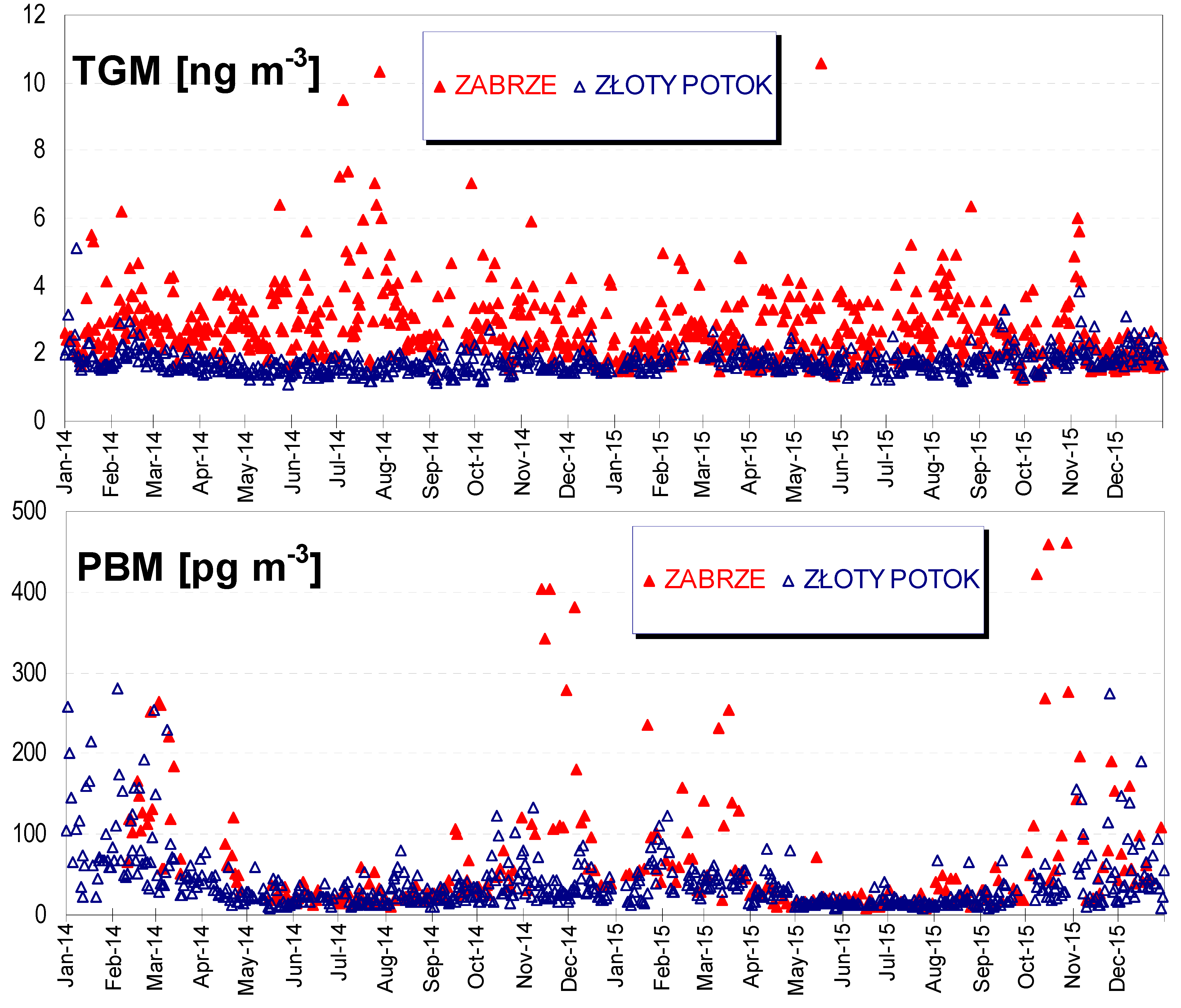
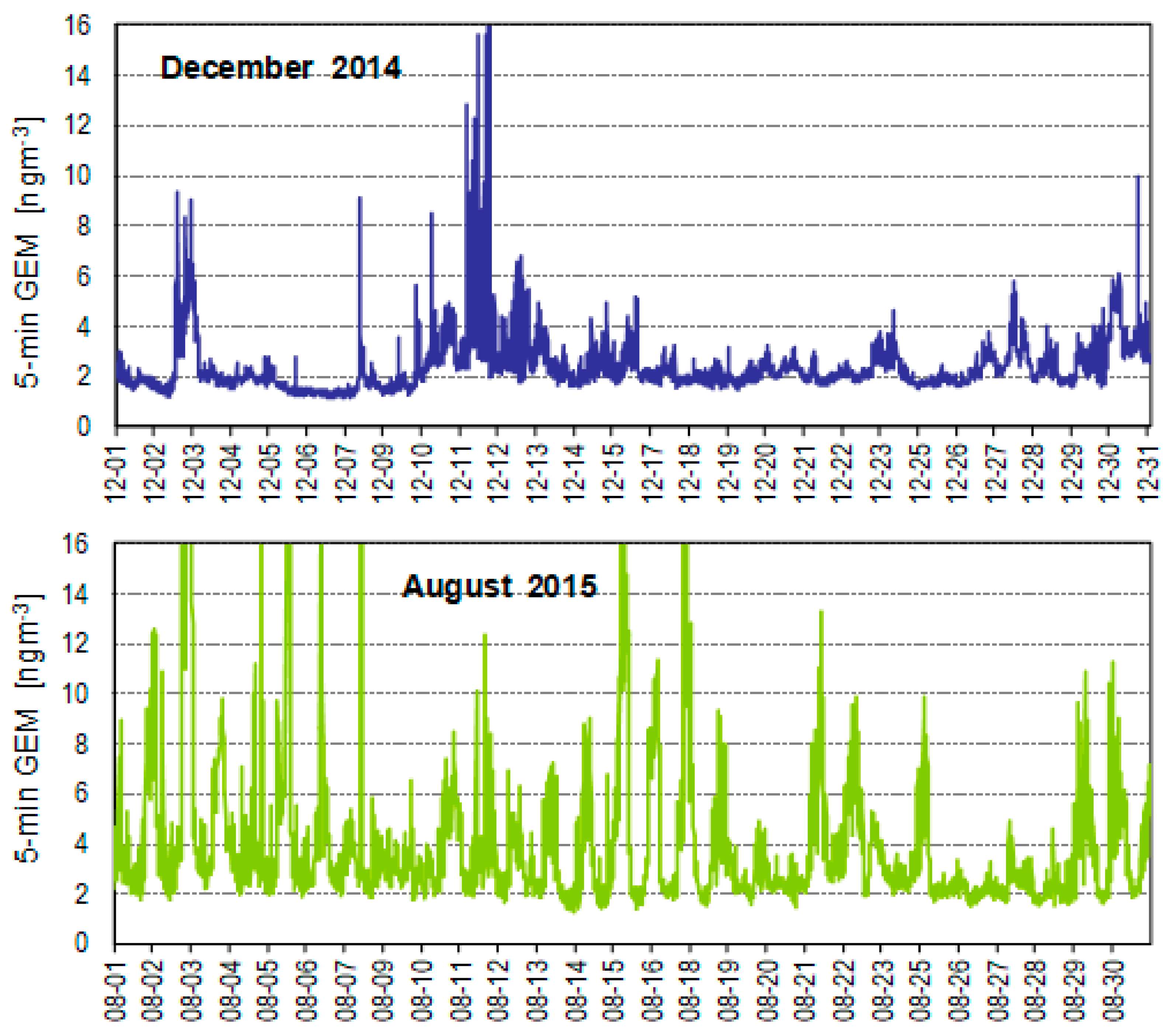
5.1. TGM and PBM Concentrations in the Air
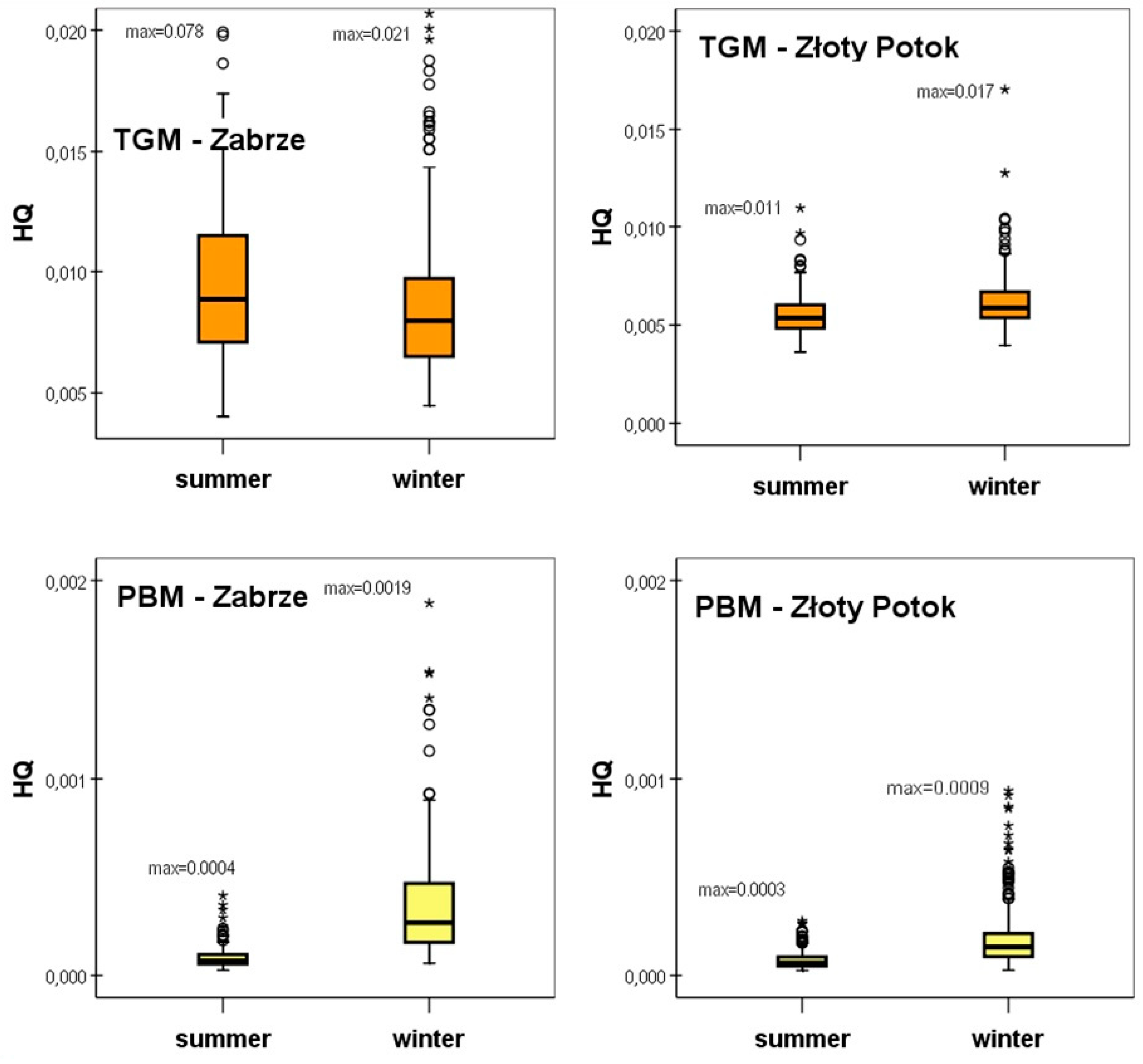
5.2. Health Risk Resulting from Inhalation of TGM and PBM
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selin, N.E. Global biogeochemical cycling of mercury: A review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Song, S.; Selin, N.E.; Soerensen, A.L.; Angot, H.; Artz, R.; Brooks, S.; Brunke, E.-G.; Conley, G.; Dommergue, A.; Ebinghaus, R.; et al. Top-down constraints on atmospheric mercury emissions and implications for global biogeochemical cycling. Atmos. Chem. Phys. 2015, 15, 7103–7125. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric mercury—An overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Lindberg, S.; Bullock, R.; Ebinghaus, R.; Engstrom, D.; Feng, X.; Fitzgerald, W.; Pirrone, N.; Prestbo, E.; Seigneur, C. A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 2007, 36, 19–32. [Google Scholar] [CrossRef]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmospheric Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- World Health Organization. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Concise International Chemical Assessment Document 50. Geneva, Switzerland, 2003. Available online: https://www.who.int/ipcs/publications/cicad/en/cicad50.pdf?ua=1 (accessed on 9 July 2020).
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.M.; Travnikov, O.; De Simone, F.; Hedgecock, I.M.; Sundseth, K.; Pacyna, E.G.; Steenhuisen, F.; Pirrone, N.; Munthe, J.; Kindbom, K. Current and future levels of mercury atmospheric pollution on a global scale. Atmospheric Chem. Phys. 2016, 16, 12495–12511. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013. [Google Scholar]
- Boerleider, R.Z.; Roeleveld, N.; Scheepers, P.T.J. Human biological monitoring of mercury for exposure assessment. AIMS Environ. Sci. 2017, 4, 251–276. [Google Scholar] [CrossRef]
- World Health Organization. Mercury and Health. 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 9 July 2020).
- Andreoli, V.; Sprovieri, F. Genetic aspects of susceptibility to mercury toxicity: An overview. Int. J. Environ. Res. Public Health 2017, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Sandborgh-Englund, G.; Elinder, C.G.; Johanson, G.; Lind, B.; Skare, I.; Ekstrand, J. The absorption, blood levels, and excretion of mercury after a single dose of mercury vapor in humans. Toxicol. Appl. Pharmacol. 1998, 150, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological profile for Mercury 1999. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp46.html (accessed on 9 July 2020).
- United Nations Environment Programme and World Health Organization. Guidelines for Identifying Populations at Risk from Mercury Exposure. UNEP Chemicals Branch and WHO, Department of Food Safety, Zoonoses and Foodborne Diseases: Geneva, Switzerland, 2008. Available online: http://www.who.int/foodsafety/publications/risk-mercury-exposure/en/ (accessed on 9 July 2020).
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Nordberg, F. Mercury, mercurials and mercaptans. In Inorganic Mercury—A Toxicological and Epidemiological Appraisal; Miller, M.W., Clarkson, T.W., Eds.; Charles C. Thomas: Springfield, IL, USA, 1973. [Google Scholar]
- Clarkson, T.W. Mercury. J. Am. Coll. Toxicol. 1989, 8, 1291–1296. [Google Scholar] [CrossRef]
- Boffetta, P.; Sällsten, G.; Garcia-Gómez, M.; Pompe-Kirn, V.; Zaridze, D.; Bulbulyan, M.; Caballero, J.D.; Ceccarelli, F.; Kobal, A.B.; Merler, E. Mortality from cardiovascular diseases and exposure to inorganic mercury. Occup. Environ. Med. 2001, 58, 461–466. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Mercury and mercury compounds. In Some Metals and Metal Compounds, and Occupational Exposures in the Glass Industry; IARC: Lyon, France, 1993; Volume 58. [Google Scholar]
- European Environment Agency. European Union Emission Inventory Report 1990–2017 Under the UNECE Convention on Long-range Transboundary Air Pollution (LRTAP). EEA Report No 8/2019. Copenhagen. Available online: https://www.eea.europa.eu//publications/european-union-emissions-inventory-report-2017 (accessed on 9 July 2020).
- National Centre for Emissions Management (KOBiZE). Poland’s Informative Inventory Report 2017. Submission under the UN ECE Convention on Long-Range Transboundary Air Pollution and the Directive (EU) 2016/2284; KOBiZE: Warsaw, Poland, 2017. [Google Scholar]
- Landis, M.; Stevens, R.K.; Schaedlich, F.; Prestbo, E.M. Development and characterization of an annular denuder methodology for the measurement of divalent inorganic reactive gaseous mercury in ambient air. Environ. Sci. Technol. 2002, 36, 3000–3009. [Google Scholar] [CrossRef]
- Gustin, M.S.; Amos, H.M.; Huang, J.; Miller, M.B.; Heidecorn, K. Measuring and modeling mercury in the atmosphere: A critical review. Atmos. Chem. Phys. 2015, 15, 5697–5713. [Google Scholar] [CrossRef]
- Tekran. Model 2537B Ambient Mercury Vapor Analyzer User Manual, Ver. 3.11; Tekran Instruments Corporation: Toronto, ON, Canada, 2008. [Google Scholar]
- Cole, A.S.; Steffen, A.; Eckley, C.S.; Narayan, J.; Pilote, M.; Tordon, R.; Graydon, J.A.; St. Louis, V.L.; Xu, X.; Branfireun, B.A. A survey of mercury in air and precipitation across Canada: Patterns and trends. Atmosphere 2014, 5, 635–668. [Google Scholar] [CrossRef]
- The Polish Committee for Standardization. Ambient air. Standard Gravimetric Measurement Method for the Determination of the PM10 or PM2,5 Mass Concentration of Suspended Particulate Matter; The Polish Committee for Standardization: Warsaw, Poland, 2014. [Google Scholar]
- Pyta, H.; Rogula-Kozłowska, W.; Mathews, B. Co-occurrence of PM2.5-bound mercury and carbon in rural areas affected by coal combustion. Atmos. Pollut. Res. 2017, 8, 127–135. [Google Scholar] [CrossRef]
- Pyta, H.; Rogula-Kozłowska, W. Determination of mercury in size-segregated ambient particulate matter using CVAAS. Microchem. J. 2016, 124, 76–81. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part. F, Supplemental Guidance for Inhalation Risk Assessment). Office of Superfund Remediation and Technology Innovation, US EPA: Washington, DC, USA, 2009. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/partf_200901_final.pdf (accessed on 9 July 2020).
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part. A). Office of Emergency and Remedial Response, U.S. EPA: Washington, DC, 1989. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 9 July 2020).
- Majewski, G.; Widziewicz, K.; Rogula-Kozłowska, W.; Rogula-Kopiec, P.; Kociszewska, K.; Rozbicki, T.; Majder-Łopatka, M.; Niemczyk, M. PM origin or exposure duration? Health hazards from PM-bound mercury and PM-bound PAHs among students and lecturers. Int. J. Environ. Res. Public Health 2018, 15, 316. [Google Scholar] [CrossRef]
- Lettmeier, B.; Boese-O’Reilly, S.; Drasch, G. Proposal for a Revised Reference Concentration (RfC) for mercury vapour in adults. Sci. Total Environ. 2010, 408, 3530–3535. [Google Scholar] [CrossRef]
- Higueras, P.; Oyarzun, R.; Kotnik, J.; Esbrí, J.M.; Martínez-Coronado, A.; Horvat, M.; López-Berdonces, M.A.; Llanos, W.; Vaselli, O.; Nisi, B.; et al. A compilation of field surveys on gaseous elemental mercury (GEM) from contrasting environmental settings in Europe, South America, South Africa, and China: Separating fads from facts. Environ. Geochem. Health 2014, 36, 713–734. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Cheng, I.; Zhang, L. Current understanding of the driving mechanisms for spatiotemporal variations of atmospheric speciated mercury: A review. Atmos. Chem. Phys. 2016, 16, 12897–12924. [Google Scholar] [CrossRef]
- Weigelt, A.; Temme, C.; Bieber, E.; Schwerin, A.; Schuetze, M.; Ebinghaus, R.; Kock, H.-H. Measurements of atmospheric mercury species at a German rural background site from 2009 to 2011—Methods and results. Environ. Chem. 2013, 10, 102–110. [Google Scholar] [CrossRef]
- Kentisbeer, J.; Leeson, S.; Malcolm, H.; Leith, I.; Braban, C.F.; Cape, J.N. Patterns and source analysis for atmospheric mercury at Auchencorth Moss, Scotland. Environ. Sci. Process. Imp. 2014, 16, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.C.; Goddard, S.L.; Butterfield, D.M.; Brown, A.S.; Robins, C.; Mustoe, C.L.; McGhee, E.A. Ten years of mercury measurement at urban and industrial air quality monitoring stations in the UK. Atmos. Environ. 2015, 109, 1–8. [Google Scholar] [CrossRef]
- Ebinghaus, R.; Jennings, S.G.; Kock, H.H.; Derwent, R.G.; Manning, A.J.; Spain, T.G. Decreasing trends in total gaseous mercury in baseline air at Mace Head, Ireland from 1996 to 2009. Atmos. Environ. 2011, 45, 3475–3480. [Google Scholar] [CrossRef][Green Version]
- Wängberg, I.; Nerentorp-Mastromonaco, M.G.; Munthe, J.; Gårdfeldt, K. Airborne mercury species at the Raö background monitoring site in Sweden: Distribution of mercury as an effect of long-range transport. Atmos. Chem. Phys. 2016, 16, 13379–13387. [Google Scholar] [CrossRef]
- Kock, H.-H.; Bieber, E.; Ebinghaus, R.; Spain, T.G.; Thees, B. Comparison of long-terms and seasonal variations of atmospheric mercury concentrations at the two European costal monitoring stations Mace Head, Ireland, and Zingst, Germany. Atmos. Environ. 2005, 39, 7549–7556. [Google Scholar] [CrossRef]
- Li, J.; Sommar, J.; Wangberg, I.; Lindqvist, O.; Wei, S.Q. Short-time variation of mercury speciation in the urban of Göteborg during GÖTE-2005. Atmos. Environ. 2008, 42, 8382–8388. [Google Scholar] [CrossRef]
- Albuquerque, M.; Coutinho, M.; Rodrigues, J.; Ginja, J.; Borrego, C. Long-term monitoring of trace metals in PM10 and total gaseous mercury in the atmosphere of Porto, Portugal. Atmos. Pollut. Res. 2017, 3, 535–544. [Google Scholar] [CrossRef]
- Stamenkovic, J.; Lyman, S.; Gustin, M.S. Seasonal and diel variation of atmospheric mercury concentrations in the Reno (Nevada, USA) airshed. Atmos. Environ. 2007, 31, 6662–6672. [Google Scholar] [CrossRef]
- Lyman, S.N.; Gustin, M.S. Determinants of atmospheric mercury concentrations in Reno, Nevada, USA. Sci. Total Environ. 2009, 408, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.A.; Schmeltz, D.; Prestbo, E.; Olson, M.; Sharac, T.; Tordon, R. The atmospheric mercury network: Measurement and initial examination of an ongoing atmospheric mercury record across North America. Atmos. Chem. Phys. 2013, 13, 11339–11349. [Google Scholar] [CrossRef]
- Cheng, I.; Zhang, L.; Mao, H.; Blanchard, P.; Tordon, R.; Dalziel, J. Seasonal and diurnal patterns of speciated atmospheric mercury at a coastal-rural and a coastal-urban site. Atmos. Environ. 2014, 82, 193–205. [Google Scholar] [CrossRef]
- Xu, L.; Chen, J.; Yang, L.; Niu, Z.; Tong, L.; Yin, L.; Chen, Y. Characteristics and sources of atmospheric mercury speciation in a coastal city, Xiamen, China. Chemosphere 2015, 119, 530–539. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Y.; Mao, H.; Wang, T.; Li, T.; Wu, Y.; Li, Y.; Hou, C.; Qie, G.; Feng, X.; et al. Atmospheric mercury in an eastern Chinese metropolis (Jinan). Ecotoxicol. Environ. Safety 2020, 15, 110541. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, C.; Zhang, X. Concentrations and gas-particle partitioning of atmospheric reactive mercury at an urban site in Beijing, China. Environ. Pollut. 2019, 249, 13–23. [Google Scholar] [CrossRef]
- Yin, X.; Zhou, W.; Kang, S.; de Foy, B.; Yu, Y.; Xie, J.; Sun, S.; Wu, K.; Zhang, Q. Latest observations of total gaseous mercury in a megacity (Lanzhou) in northwest China. Sci. Total Environ. 2020, 720, 137494. [Google Scholar] [CrossRef]
- Han, Y.-J.; Kim, J.-E.; Kim, P.-R.; Kim, W.-J.; Yi, S.-M.; Seo, Y.-S.; Kim, S.-H. General trends of atmospheric mercury concentrations in urban and rural areas in Korea and characteristics of high-concentration events. Atmospheric Environ. 2014, 94, 754–764. [Google Scholar] [CrossRef]
- Kim, K.-H.; Brown, R.J.C.; Kwon, E.; Kim, I.-S.; Sohn, J.-R. Atmospheric mercury at an urban station in Korea across three decades. Atmos. Environ. 2016, 131, 124–132. [Google Scholar] [CrossRef]
- Lyman, S.N.; Gustin, M.S. Speciation of atmospheric mercury at two sites in northern Nevada, USA. Atmospheric Environ. 2008, 42, 927–939. [Google Scholar] [CrossRef]
- Czaplicka, M.; Pyta, H. Transformations of mercury in processes of solid fuel combustion—Review. Arch. Environ. Prot. 2017, 43, 82–93. [Google Scholar] [CrossRef]
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; de Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E.; et al. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2015, 22, 11255–11264. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Nagafuchi, O.; Kawakami, T.; Inoue, T.; Yokota, K.; Serikawa, Y.; Cyio, M.; Elvince, R. Human health risk assessment of mercury vapor around artisanal small-scale gold mining area, Palu city, Central Sulawesi, Indonesia. Ecotox. Environ. Safety 2015, 124, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-C.; Chen, Y.-C.; Lo, C.-T.; Cho, M.-H.; Zhuang, Y.-J.; Tsai, K.-H.; Huang, C.-Y.; Xiao, Y.-F. Concentrations and analysis of health risks of ambient air metallic elements at Longjing site in central Taiwan. Environ. Geochem. Health 2018, 40, 461–472. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Rodríguez-Cabo, A.; Fernández-Amado, M.; Piñeiro-Iglesias, M.; Muniategui-Lorenzo, S.; López-Mahía, P. Levels and sources of atmospheric particle-bound mercury in atmospheric particulate matter (PM10) at several sites of an Atlantic Coastal European Region. Atmosphere 2019, 11, 33. [Google Scholar] [CrossRef]
- Nazarpour, A.; Ghanavati, N.; Watts, M.J. Spatial distribution and human health risk assessment of mercury in street dust resulting from various land-use in Ahvaz, Iran. Environ. Geochem. Health 2018, 40, 693–704. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Liu, R.; Wang, M.; Zhang, Y. Spatial distribution, chemical speciation and health risk of heavy metals from settled dust in Qingdao urban area. Atmosphere 2019, 10, 73. [Google Scholar] [CrossRef]

| Statistical Parameter | Summer Season | Winter Season | Whole Period |
|---|---|---|---|
| TGM | |||
| Min – Max [ng m−3] | 1.21–23.26 | 1.34–6.21 | 1.21–23.26 |
| Mean ± SD [ng m−3] | 3.06 ± 2.02 | 2.57 ± 0.87 | 2.81 ± 1.56 |
| Median [ng m−3] | 2.66 | 2.40 | 2.48 |
| 95 percentile [ng m−3] | 5.17 | 4.26 | 4.76 |
| Number of measurements | 331 | 351 | 682 |
| PBM | |||
| Min – Max [pg m−3] | 7.64–121.06 | 18.78–565.67 | 7.64–565.67 |
| Mean ± SD [pg m−3] | 27.68 ± 18.62 | 118.74 ± 106.58 | 69.81 ± 86.53 |
| Median [pg m−3] | 21.58 | 80.21 | 37.87 |
| 95 percentile [pg m−3] | 62.40 | 385.15 | 255.89 |
| Number of measurements | 137 | 118 | 255 |
| Statistical Parameter | Summer Season | Winter Season | Whole Period |
|---|---|---|---|
| TGM | |||
| Min–Max [ng m−3] | 1.09–3.28 | 1.19–5.11 | 1.0–5.11 |
| Mean ± SD [ng m−3] | 1.65 ± 0.27 | 1.85 ± 0.39 | 1.74 ± 0.35 |
| Median [ng m−3] | 1.60 | 1.76 | 1.69 |
| 95 percentile [ng m−3] | 2.10 | 2.51 | 2.31 |
| Number of measurements | 358 | 326 | 684 |
| PBM | |||
| Min–Max [pg m−3] | 7.44–82.49 | 7.67–281.71 | 7.44–281.71 |
| Mean ± SD [pg m−3] | 22.84 ± 12.76 | 54.77 ± 44.27 | 38.48 ± 36.01 |
| Median [pg m−3] | 19.19 | 42.68 | 27.82 |
| 95 percentile [pg m−3] | 49.40 | 150.57 | 102.21 |
| Number of measurements | 330 | 317 | 647 |
| Country, Site of Measurements | Site Description | Period | TGM/GEM/ [ng m−3] * | PBM [pg m−3] * | Reference |
|---|---|---|---|---|---|
| Europe | |||||
| Germany, Waldhof | rural (Central- European background) | 2009–2011 | /GEM/ | [37] | |
| 1.61 | 6.30 | ||||
| 1.1–3.1 | 0.4–110 | ||||
| UK (Scotland), Auchencorth Moss | rural | 2009–2011 | /GEM/ | [38] | |
| 1.40 ± 0.19 | 3.11 ± 5.34 | ||||
| UK (England: London, Manchester Sheffield; Ireland: Belfast; Scotland: Motherwell; Wales: Cardiff, Swansea) | urban | 2004–2013 | 2.07 ± 0.03 (manual measurement) | - | [39] |
| Ireland, Mace Head (North Atlantic) | regional background, coastal site | 1996–2009 | 1.65 ± 0.13 | - | [40] |
| 2012–2015 | 1.48 1.48 ± 0.13 | - | [41] | ||
| Sweden, Råö (Baltic Sea) | rural, coastal site | 2012–2015 | /GEM/ | [41] | |
| 1.41 | 2.21 | ||||
| 1.42 ± 0.20 | 3.6 ± 4.5 | ||||
| Germany, Zingst (Baltic Sea) | rural, coastal site | 1998–2004 | 1.66 | - | [42] |
| Sweden, Göteborg (Baltic Sea) | urban, coastal site | 2005 | 1.96 ± 0.38 | 2.53 ± 4.09 | [43] |
| Portugal, Porto (Atlantic Ocean) | suburban coastal site | 2008–2014 | 1.93 (mean) 0.51–67.9 | - | [44] |
| Poland, Zabrze (Silesia) | urban | 2014–2015 | 2.48 2.81 ± 1.56 | 37.87 69.81 ± 86.53 | this study |
| Poland, Złoty Potok (Silesia) | rural, regional background | 2014–2015 | 1.69 1.74 ± 0.35 | 27.82 38.48 ± 36.01 | this study |
| North America | |||||
| USA, Reno NV | urban | 2002–2005 | 2.3 (mean) 2.1 (median) 0.9–8.6 | [45] | |
| 2007–2009 | 2.0 ± 0.7 | 7.0 ± 7.0 | [46] | ||
| USA, Birmingham AL, New York City, Rochester NY, Salt Lake City UT | urban (AMNet) | 2009–2011 | /GEM/ | [47] | |
| 1.51 | 4.97 | ||||
| 1.65 ± 1.36 | 8.46 ± 29.05 | ||||
| 0.4–237.7 | 0–3687 | ||||
| Canada, Halifax (North Atlantic) | urban, costal site | 2010–2011 | /GEM/ | [48] | |
| 1.7 ± 1.0 | 2.3 ± 3.1 | ||||
| Asia | |||||
| China (Southeast Ch.), Xiamen | urban | 2012–2013 | /GEM/ | [49] | |
| 3.50 ± 1.21 | 174.4 ± 280.6 | ||||
| China (Eastern Ch.), Jinan | urban | 2015–2016 | 4.91 ± 3.66 | 451.9 ± 433.4 | [50] |
| China, Beijing | urban | 2016–2017 | /GEM/ | [51] | |
| 4.70 ± 3.53 | 85.18 ± 95.34 | ||||
| China (Northwest Ch.), Lanzhou | urban | 2016–2017 | 4.48 ± 2.32 | - | [52] |
| South Korea, Seoul | urban | 2006–2009 | 3.72 ± 2.96 | 13.4 ± 12.0 | [53] |
| 0.19–149.84 | 2.1–64.3 | ||||
| South Korea, Seoul | urban | 2012–2013 | 2.34 ± 0.73 | - | [54] |
| Northern Hemisphere background | 1.5–1.7 | - | [5] | ||
| Statistical Parameter | HQ for TGM | HQ for PBM | HI for TGM and PBM | |||
|---|---|---|---|---|---|---|
| Zabrze | Złoty Potok | Zabrze | Złoty Potok | Zabrze | Złoty Potok | |
| Summer season | ||||||
| Median [unitless] | 0.00886 | 0.00535 | 0.00007 | 0.00006 | 0.00893 | 0.00541 |
| Mean [unitless] | 0.01020 | 0.00548 | 0.00009 | 0.00008 | 0.01029 | 0.00556 |
| Winter season | ||||||
| Median [unitless] | 0.00798 | 0.00587 | 0.00027 | 0.00014 | 0.00825 | 0.00601 |
| Mean [unitless] | 0.00856 | 0.00616 | 0.00040 | 0.00018 | 0.00896 | 0.00634 |
| Whole period | ||||||
| Median [unitless] | 0.00828 | 0.00562 | 0.00013 | 0.00009 | 0.00840 | 0.00571 |
| Mean [unitless] | 0.00936 | 0.00581 | 0.00023 | 0.00013 | 0.00959 | 0.00593 |
| Statistical Parameter | HQ for TGM | HQ for PBM | HI for TGM and PBM | |||
|---|---|---|---|---|---|---|
| Zabrze | Złoty Potok | Zabrze | Złoty Potok | Zabrze | Złoty Potok | |
| Summer season | ||||||
| Median [unitless] | 0.03798 | 0.02293 | 0.00031 | 0.00027 | 0.03829 | 0.02320 |
| Mean [unitless] | 0.04371 | 0.02350 | 0.00040 | 0.00033 | 0.04411 | 0.02383 |
| Winter season | ||||||
| Median [unitless] | 0.03422 | 0.02516 | 0.00115 | 0.00061 | 0.03536 | 0.02577 |
| Mean [unitless] | 0.03669 | 0.02640 | 0.00170 | 0.00078 | 0.03838 | 0.02718 |
| Whole period | ||||||
| Median [unitless] | 0.03548 | 0.02409 | 0.00054 | 0.00040 | 0.03602 | 0.02449 |
| Mean [unitless] | 0.04010 | 0.02488 | 0.00100 | 0.00055 | 0.04109 | 0.02543 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyta, H.; Widziewicz-Rzońca, K.; Słaby, K. Inhalation Exposure to Gaseous and Particulate Bound Mercury Present in the Ambient Air over the Polluted Area of Southern Poland. Int. J. Environ. Res. Public Health 2020, 17, 4999. https://doi.org/10.3390/ijerph17144999
Pyta H, Widziewicz-Rzońca K, Słaby K. Inhalation Exposure to Gaseous and Particulate Bound Mercury Present in the Ambient Air over the Polluted Area of Southern Poland. International Journal of Environmental Research and Public Health. 2020; 17(14):4999. https://doi.org/10.3390/ijerph17144999
Chicago/Turabian StylePyta, Halina, Kamila Widziewicz-Rzońca, and Krzysztof Słaby. 2020. "Inhalation Exposure to Gaseous and Particulate Bound Mercury Present in the Ambient Air over the Polluted Area of Southern Poland" International Journal of Environmental Research and Public Health 17, no. 14: 4999. https://doi.org/10.3390/ijerph17144999
APA StylePyta, H., Widziewicz-Rzońca, K., & Słaby, K. (2020). Inhalation Exposure to Gaseous and Particulate Bound Mercury Present in the Ambient Air over the Polluted Area of Southern Poland. International Journal of Environmental Research and Public Health, 17(14), 4999. https://doi.org/10.3390/ijerph17144999





