Breeding Habitat Preferences of Major Culicoides Species (Diptera: Ceratopogonidae) in Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Trapping Procedure
2.2. Biting Midge Identification
2.3. Data Analysis
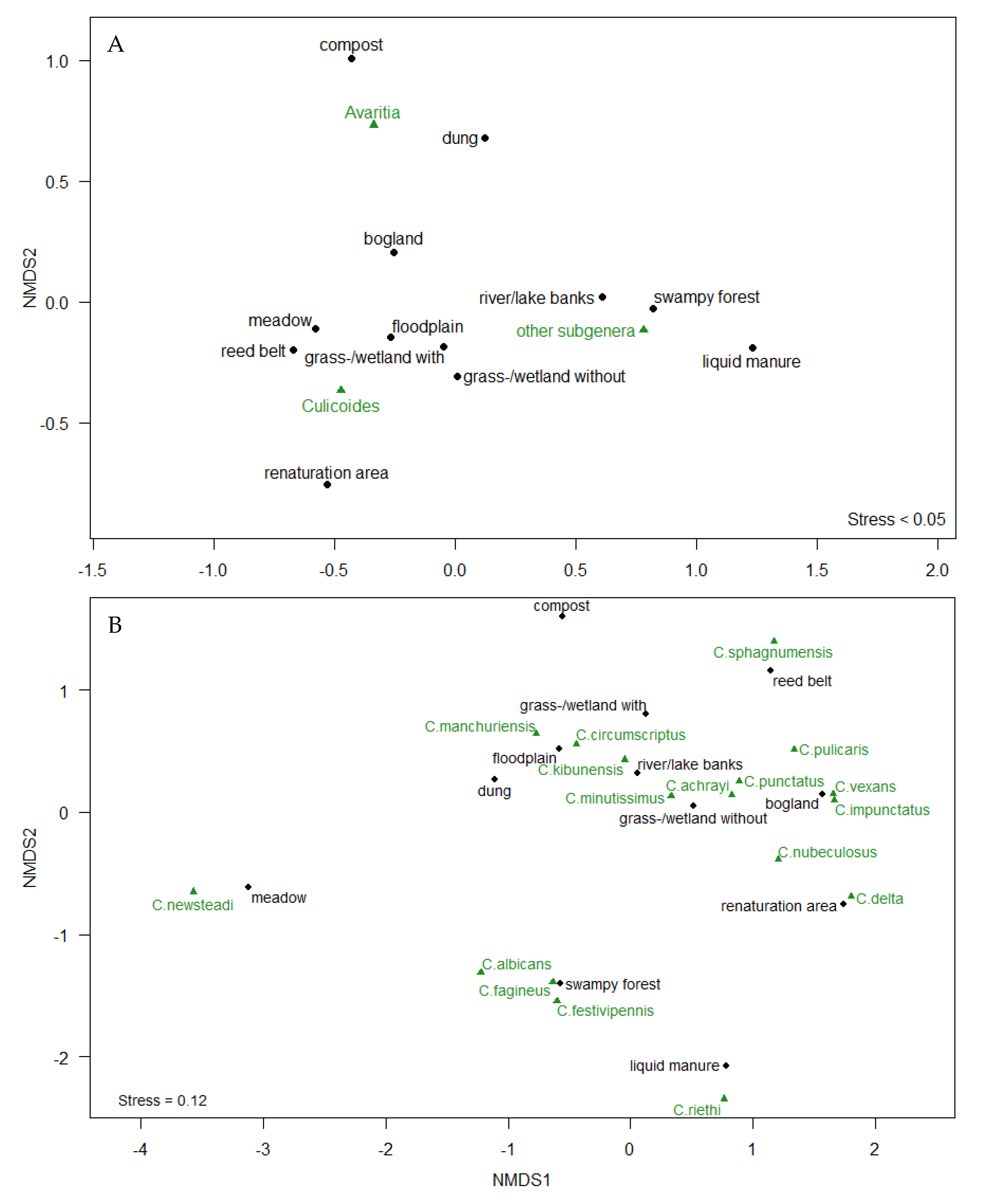
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hendry, G.A.F.; Godwin, G. Biting midges in Scottish forestry. A costly irritant or a trivial nuisance? Scott. For. 1988, 42, 113–119. [Google Scholar]
- Werner, D.; Kampen, H. Gnitzen (Diptera, Ceratopogonidae) und ihre medizinische Bedeutung. In Krank durch Arthropoden; Aspöck, H., Ed.; Biologiezentrum des Oberösterreichischen Landesmuseums: Linz, Austria, 2010; Volume 30, pp. 245–260. [Google Scholar]
- Meiswinkel, R.; Baldet, T.; De Deken, R.; Takken, W.; Delecolle, J.C.; Mellor, P.S. The 2006 outbreak of bluetongue in northern Europe–The entomological perspective. Prev. Vet. Med. 2008, 87, 55–63. [Google Scholar] [CrossRef]
- Tatem, A.J.; Huang, Z.; Das, A.; Qi, Q.; Roth, J.; Qiu, Y. Air-travel and vector-borne diseases movement. Parasitology 2012, 139, 1816–1830. [Google Scholar] [CrossRef] [PubMed]
- Sanders, C.J.; Shortall, C.R.; Gubbins, S.; Burgin, L.; Gloster, J.; Harrington, R.; Reynolds, D.R.; Mellor, P.S.; Carpenter, S. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J. Appl. Ecol. 2011, 48, 1355–1364. [Google Scholar] [CrossRef]
- Mellor, P.S.; Wittman, E.J. Bluetongue virus in the Mediterranean basin 1998–2001. Vet. J. 2002, 164, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; Van Rijn, P.; Leijs, P.; Goffredo, M. Potential new Culicoides vector of bluetongue virus in northern Europe. Vet. Rec. 2007, 161, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, L.; Purse, B.V. Culicoides biting midges, arbovirus and public health in Europe. Antivir. Res. 2013, 100, 102–113. [Google Scholar] [CrossRef]
- Afonso, A.; Abrahantes, J.C.; Conraths, F.; Veldhuis, A.; Elbers, A.; Roberts, H.; Van der Stede, Y.; Méroc, E.; Gache, K.; Richardson, J. The Schmallenberg virus epidemic in Europe-2011–2013. Prev. Vet. Med. 2014, 116, 391–403. [Google Scholar] [CrossRef]
- Wilson, A.; Mellor, P. Bluetongue in Europe: Vectors, epidemiology and climate change. Parasitol. Res. 2008, 103 (Suppl. 1), 69–77. [Google Scholar] [CrossRef]
- Braverman, Y.; Chizov-Ginzburg, A.; Eldor, H.; Mumcuoglu, K.Y. Overview and features of larval developmental sites of biting midge species associated with livestock in Israel with implications to their control. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 204–211. [Google Scholar] [CrossRef]
- Boorman, J. British Culicoides (Diptera: Ceratopogonidae): Notes on distribution and biology. Entomol. Gaz. 1986, 37, 253–266. [Google Scholar]
- Bishop, A.L.; McKenzie, H.J.; Barchia, M.; Harris, A.M. Effect of temperature regimes on the development, survival and emergence of Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae) in bovine dung. Austr. J. Entomol. 1996, 35, 361–368. [Google Scholar] [CrossRef]
- Uslu, U.; Dik, B. Vertical distribution of Culicoides larvae and pupae. Med. Vet. Entomol. 2006, 20, 350–352. [Google Scholar] [CrossRef]
- Kettle, D.S.; Lawson, J.W.H. The early stages of British biting midges Culicoides Latreille (Diptera: Ceratopogonidae) and allied genera. Bull. Ent. Res. 1952, 43, 421–467. [Google Scholar] [CrossRef]
- Cannon, L.R.G.; Reye, E.J. A larval habitat of the biting midge Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae). Austr. J. Entomol. 1966, 5, 7–9. [Google Scholar] [CrossRef]
- Havelka, P.; Caspers, N. Die Gnitzen (Diptera, Nematocera, Ceratopogonidae) eines kleinen Waldbaches bei Bonn. Decheniana 1981, 25, 1–100. [Google Scholar]
- González, M.; López, S.; Mullens, A.; Baldet, T.; Goldarazena, A. A survey of Culicoides developmental sites on a farm in northern Spain, with a brief review of immature habitats of European species. Vet. Parasitol. 2013, 191, 81–93. [Google Scholar] [CrossRef]
- Conte, A.; Ippoliti, C.; Meiswinkel, R. Influence of biotic and abiotic factors on the distribution and abundance of Culicoides imicola and the Obsoletus Complex in Italy. Vet. Parasitol. 2007, 150, 333–344. [Google Scholar] [CrossRef]
- Ninio, C.; Augot, D.; Dufour, B.; Depaquit, J. Emergence of Culicoides obsoletus from indoor and outdoor breeding sites. Vet. Parasitol. 2011, 183, 125–129. [Google Scholar] [CrossRef]
- Steinke, S.; Lühken, R.; Balcun, C.; Kiel, E. Emergence of Culicoides obsoletus group species from farm-associated habitats in Germany. Med. Vet. Entomol. 2016, 30, 174–184. [Google Scholar] [CrossRef]
- Harrup, L.E.; Purse, B.V.; Golding, N.; Mellor, P.S.; Carpenter, S. Larval development and emergence sites of farm-associated Culicoides in the United Kingdom. Med. Vet. Entomol. 2013, 27, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Werner, D. Die Dipterenfauna verschiedener Mülldeponien und Kompostierungsanlagen in der Umgebung von Berlin unter besonderer Berücksichtigung ihrer Ökologie und Bionomie. Studia Dipterol. 1997, 1, 1–176. [Google Scholar]
- Meiswinkel, R.; Venter, G.J.; Nevill, E.M. Vectors: Culicoides spp. In Infectious Diseases of Livestock, 2nd ed.; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 2005; Volume 1, pp. 93–136. [Google Scholar]
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef] [PubMed]
- Lühken, R.; Kiel, E.; Steinke, S. Culicoides biting midge density in relation to the position and substrate temperature in a cattle dung heap. Parasitol. Res. 2014, 113, 4659–4662. [Google Scholar] [CrossRef]
- Verhoef, A.A.; Venter, G.J.; Weldon, C.W. Thermal limits of two biting midges, Culicoides imicola Kieffer and C. bolitinos Meiswinkel (Diptera: Ceratopogonidae). Parasit. Vectors 2014, 7, 384. [Google Scholar] [CrossRef]
- Baetza, H.J. Eradication of bluetongue disease in Germany by vaccination. Vet. Immunol. Immunopathol. 2014, 158, 116–119. [Google Scholar] [CrossRef]
- Wernike, K.; Hoffmann, B.; Conraths, F.J.; Beer, M. Schmallenberg virus recurrence, Germany, 2014. Emerg. Infect. Dis. 2015, 21, 1202–1204. [Google Scholar] [CrossRef]
- Gache, K.; Touratier, A.; Bournez, L.; Zientara, S.; Bronner, A.; Dion, F.; Garin, E.; Calavas, D. Detection of Schmallenberg virus in France since 2012. Vet. Rec. 2017, 180, 24. [Google Scholar] [CrossRef]
- Wernike, K.; Beer, M. Schmallenberg virus: A novel virus of veterinary importance. Adv. Virus Res. 2017, 99, 39–60. [Google Scholar]
- Sailleau, C.; Bréard, E.; Viarouge, C.; Vitour, D.; Romey, A.; Garnier, A.; Fablet, A.; Lowenski, S.; Gorna, K.; Caignard, G.; et al. Re-emergence of bluetongue virus serotype 8 in France, 2015. Transbound. Emerg. Dis. 2017, 64, 998–1000. [Google Scholar] [CrossRef]
- Gethmann, J.; Probst, C.; Conraths, F.J. Economic Impact of a bluetongue serotype 8 epidemic in Germany. Front. Vet. Sci. 2020, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Bellini, R.; Zeller, H.; Van Bortel, W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit. Vectors 2014, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Caputo, B.; Chandre, F.; Drago, A.; della Torre, A.; Montarsi, F.; Rizzoli, A. Control methods against invasive Aedes mosquitoes in Europe: A review. Pest. Manag. Sci. 2015, 71, 1471–1485. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.A.; Pelham-Clinton, E.C. A taxonomic review of the British species of Culicoides Latreille (Diptera: Ceratopogonidae). Proc. R Soc. Edinb. 1960, 67, 181–302. [Google Scholar] [CrossRef]
- Delécolle, J.-C. Nouvelle contribution à l’étude systématique et iconographique des espèces du genre Culicoides (Diptera: Ceratopogonidae) du Nord-Est de la France. Ph.D. Thesis, Université Louis Pasteur, Strasbourg, France, 1985. [Google Scholar]
- Glukhova, V.M. Blood-sucking midges of the genera Culicoides and Forcipomyia (Ceratopogonidae). Fauna USSR 1989, 139, 1–408. [Google Scholar]
- Glukhova, V.M. Culicoides (Diptera, Ceratopogonidae) of Russia and adjacent lands. Int. J. Dipterol. Res. 2005, 16, 3–75. [Google Scholar]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the western Palaearctic region. Parasit. Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Mathieu, B.; Perrin, A.; Baldet, T.; Delécolle, J.C.; Albina, E.; Cêtre-Sossah, C. Molecular identification of western European species of Obsoletus Complex (Diptera: Ceratopogonidae) by an internal transcribed spacer-1 rDNA multiplex polymerase chain reaction assay. J. Med. Entomol. 2007, 44, 1019–1025. [Google Scholar] [CrossRef]
- Lehmann, K.; Hoffmann, B.; Kampen, H. PCR identification of culicoid biting midges (Diptera, Ceratopogonidae) of the Obsoletus Complex including putative bluetongue and Schmallenberg virus vectors. Parasit. Vectors 2012, 5, 213. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2018. Available online: https://www.R-project.org/ (accessed on 1 May 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: http://CRAN.R-project.org/package=vegan (accessed on 1 May 2020).
- European Food Safety Authority. Scientific Opinion of the Scientific Panel on Animal Health and Welfare on the EFSA Selfmandate on ‘Bluetongue origin and occurrence’. EFSA J. 2007, 408, 1–20. [Google Scholar]
- Probst, C.; Gethmann, J.; Kampen, H.; Beer, M.; Werner, D.; Conraths, F.J. A comparison of four light trap for collecting Culicoides biting midges. Parasitol. Res. 2015, 114, 4717–4727. [Google Scholar] [CrossRef] [PubMed]
- Baldet, T.; Delecolle, J.C.; Cetre-Sossah, C.; Mathieu, B.; Meiswinkel, R.; Gerbier, G. Indoor activity of Culicoides associated with livestock in the bluetongue virus (BTV) affected region of northern France during autumn 2006. Prev. Vet. Med. 2008, 87, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, M.; Meiswinkel, R. Entomological surveillance of bluetongue in Italy: Methods of capture, catch analysis and identification of Culicoides biting midges. Vet. Ital. 2004, 40, 260–265. [Google Scholar] [PubMed]
- Purse, B.V.; Caracappa, S.; Marino, A.M.; Tatem, A.J.; Rogers, D.J.; Mellor, P.S.; Baylis, M.; Torina, A. Modelling the distribution of outbreaks and Culicoides vectors in Sicily: Towards predictive risk maps for Italy. Vet. Ital. 2004, 40, 303–310. [Google Scholar]
- Miranda, M.A.; Rincón, C.; Borràs, D. Seasonal abundance of Culicoides imicola and C. obsoletus in the Balearic Islands. Vet. Ital. 2004, 40, 292–295. [Google Scholar]
- Werner, D.; Will, M.; Groschupp, S.; Dähn, O.; Kampen, H. Monitoring of biting midges (Diptera: Ceratopogonidae), potential vectors of bluetongue and Schmallenberg viruses, in Germany. Mitt. Dtsch. Ges. Allgem Angew. Ent. 2020, 22. in press. [Google Scholar]
- Havelka, P.; Aguilar, M. Ceratopogonidae. In Checkliste der Dipteren Deutschlands; Schumann, H., Bährmann, R., Stark, A., Eds.; Ampyx-Verlag: Halle, Germany, 1999; pp. 33–38. [Google Scholar]
- Ayllón, T.; Nijhof, A.M.; Weiher, W.; Bauer, B.; Allène, X.; Clausen, P.H. Feeding behaviour of Culicoides spp. (Diptera: Ceratopogonidae) on cattle and sheep in northeast Germany. Parasit. Vectors 2014, 7, 34. [Google Scholar] [CrossRef]
- Meiswinkel, R.; Gomulski, L.M.; Delécolle, J.C.; Goffredo, M.; Gasperi, G. The taxonomy of Culicoides vector complexes–unfinished business. Vet. Ital. 2004, 40, 151–159. [Google Scholar]
- Harrup, L.E.; Bellis, G.A.; Balenghien, T.; Garros, C. Culicoides Latreille (Diptera: Ceratopogonidae) taxonomy: Current challenges and future directions. Infect. Genet. Evol. 2015, 30, 249–266. [Google Scholar] [CrossRef]
- Schwenkenbecher, J.M.; Mordue, A.J.; Piertney, S.B. Phylogenetic analysis indicates that Culicoides dewulfi should not be considered part of the Culicoides obsoletus complex. Bull. Entomol. Res. 2009, 99, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Augot, D.; Mathieu, B.; Hadj-Henni, L.; Barriel, V.; Zapata Mena, S.; Smolis, S.; Slama, D.; Randrianambinintsoa, F.J.; Trueba, G.; Kaltenbach, M.; et al. Molecular phylogeny of 42 species of Culicoides (Diptera, Ceratopogonidae) from three continents. Parasite 2017, 24, 23. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.M.; Jess, S.; Murchie, A.K. Differential emergence of Culicoides (Diptera: Ceratopogonidae) from on-farm breeding substrates in Northern Ireland. Parasitology 2013, 140, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.Y.; Saegerman, C.; Losson, B.; Beckers, Y.; Haubruge, E.; Francis, F. Chemical composition of silage residues sustaining the larval development of the Culicoides obsoletus/Culicoides scoticus species (Diptera: Ceratopogonidae). Vet. Parasitol. 2013, 191, 197–201. [Google Scholar] [CrossRef]
- Kiel, E.; Liebisch, G.; Focke, R.; Liebisch, A. Monitoring of Culicoides at 20 locations in northwest Germany. Parasitol. Res. 2009, 105, 351–357. [Google Scholar] [CrossRef]
- Mehlhorn, H.; Wachendörfer, V.; Klimpel, S.; Schaub, G.; Kiel, E.; Focke, R.; Liebisch, G.; Liebisch, A.; Werner, D.; Geier, M.; et al. Bluetongue disease in Germany (2007–2008): Monitoring of entomological aspects. Parasitol. Res. 2009, 105, 313–319. [Google Scholar] [CrossRef]
- Vorsprach, B.; Meiser, C.K.; Werner, D.; Balczun, C.; Schaub, G.A. Monitoring of Ceratopogonidae in southwest Germany. Parasitol. Res. 2009, 105, 337–344. [Google Scholar] [CrossRef]
- Searle, K.R.; Blackwell, A.; Falconer, D.; Sullivan, M.; Butler, A.; Purse, B.V. Identifying environmental drivers of insect phenology across space and time: Culicoides in Scotland as a case study. Bull. Entomol. Res. 2013, 103, 155–170. [Google Scholar] [CrossRef]
- Searle, K.R.; Barber, J.; Stubbins, F.; Labuschagne, K.; Carpenter, S.; Butler, A.; Denison, E.; Sanders, C.; Mellor, P.S.; Wilson, A.; et al. Environmental drivers of Culicoides phenology: How important is species-specific variation when determining disease policy? PLoS ONE 2014, 9, e111876. [Google Scholar] [CrossRef]
- Zimmer, J.Y.; Brostaux, Y.; Haubruge, E.; Francis, F. Larval development sites of the main Culicoides species (Diptera: Ceratopogonidae) in northern Europe and distribution of coprophilic species larvae in Belgian pastures. Vet. Parasitol. 2014, 205, 676–686. [Google Scholar] [CrossRef]
- Uslu, U.; Dik, B. Description of breeding sites of Culicoides species (Diptera: Ceratopogonidae) in Turkey. Parasite 2007, 14, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Uslu, U.; Dik, B. Chemical characteristics of breeding sites of Culicoides species (Diptera: Ceratopogonidae). Vet. Parasitol. 2010, 169, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, S.; Morii, T. Observation on the breeding habitats of some biting midges and seasonal population dynamics in the life cycle of C. arakawae in Tokyo and its vicinity. Nat. Inst. Anim. Health Q 1963, 3, 198–208. [Google Scholar]
- Blackwell, A.; Young, M.R.; Mordue, W. The microhabitat of Culicoides impunctatus (Diptera: Ceratopogonidae) larvae in Scotland. Bull. Ent. Res. 1994, 84, 295–301. [Google Scholar] [CrossRef]
- Hoffmann, B.; Bauer, B.; Bauer, C.; Bätza, H.-J.; Beer, M.; Clausen, P.-H.; Geier, M.; Gethmann, J.M.; Kiel, E.; Liebisch, G.; et al. Monitoring of putative vectors of bluetongue virus serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 1481–1484. [Google Scholar] [CrossRef]
- Elbers, A.R.W.; Meiswinkel, R.; van Weezep, E.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Kooi, E.A. Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerg. Infect. Dis. 2013, 19, 106–109. [Google Scholar] [CrossRef]

| Species | Landscape/Habitat Type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Floodplain | Meadow | Renaturation Area/Floodplain | Mixed Grassland/Wetland without Bushes and Trees | Mixed Grassland/Wetland with Bushes and Trees | Swampy Forest Areas | Banks of Rivers and Lakes | Reed Belt | Bogland | Compost | Dung | Liquid Manure | |
| Genus Culicoides Subgenus Avaritia (Fox, 1955) | ||||||||||||
| C. chiopterus (Meigen, 1830) | +(3)/5 | +(2)/2 | – | +/2 | – | +(4)/1 | +/2 | +/3 1 | – | +/2 | +(3)/11 | – |
| C. obsoletus (Meigen, 1818) | +(1)/20 | +/9 | – | +(1)/9 | +(2)/4 | – | +(2)/10 | +(2)/8 1 | +(1)/8 | +(4)/32 | +(5)/354 | – |
| C. scoticus (Downes and Kettle, 1952) | +(2)/10 | – | – | +(5)/8 | +/4 | – | +/5 | +(4)/0 1 | – | – | +(1)/23 | – |
| C. dewulfi (Goetghebuer, 1935) | +(1)/2 | – | – | – | – | – | – | – | +/1 | – | +/3 | – |
| Total number of females/males belonging to subgenus Avaritia | 31/37 | 17/11 | 0/0 | 22/19 | 81/8 | 23/1 | 24/17 | 64/11 | 81/9 | 46/34 | 567/391 | 0/0 |
| Genus Culicoides Subgenus Culicoides (Latreille, 1809) | ||||||||||||
| C. delta (Edwards, 1939) | 1/1 | – | 116/8 | – | 1/0 | – | – | – | 2/0 | – | 5(1)/1 | – |
| C. pulicaris (Linnaeus, 1758) | 2/1 | – | – | 18(3)/0 | 24(3)/2 | 1/0 | 2/0 | 32/5 1,2 | 5(1)/6 | 3/0 | 2(8)/0 | – |
| C. punctatus(Meigen, 1804) | 14(2)/2 | – | – | 1/2 | 35(2)/4 | – | 4/3 | – | 1/1 | – | 8(1)/0 | – |
| C. newsteadi (Austen, 1921) | – | (4)/3 | – | – | – | – | – | – | – | – | (1)/0 | – |
| Total number of females/males belonging to subgenus Culicoides | 19/4 | 4/3 | 116/8 | 22/2 | 65/6 | 1/0 | 6/3 | 32/5 | 9/7 | 3/0 | 26/1 | 0/0 |
| Genus Culicoides Species of other subgenera | ||||||||||||
| C. achrayi (Kettle and Lawson, 1955) | – | – | 3/0 | 15/1 | 31(2)/0 | – | 27/0 | 1/0 1 | 1/0 | – | 74/0 | – |
| C. albicans (Winnertz, 1852) | – | (1)/0 | – | – | – | 1/0 | – | – | – | – | 16/0 | – |
| C. circumscriptus (Kieffer, 1918) | 4(2)/0 | – | – | – | – | – | 2(1)/0 | – | – | – | – | – |
| C. fagineus (Edwards, 1939) | – | – | – | – | 11/0 | 13/1 | 4/0 | – | – | – | 1/0 | – |
| C. festivipennis (Kieffer, 1914) | – | – | 1/0 | – | – | 31/2 | – | – | – | – | – | (1)/0 4 |
| C. impunctatus (Goetghebuer, 1920) | – | – | (2)/0 | – | – | – | – | – | 2/0 | – | – | – |
| C. kibunensis (Tokunaga, 1937) | – | – | – | – | – | – | (1)/0 | – | – | – | – | – |
| C. manchuriensis (Tokunaga, 1941) | (2)/0 | – | – | – | – | – | – | – | – | – | (1)/0 | – |
| C. minutissimus(Zetterstedt, 1855) | – | – | – | – | (1)/0 | – | – | – | – | – | (2)/0 | – |
| C. nubeculosus(Meigen, 1930) | 2/0 | – | – | – | 7/0 | – | – | – | 1/0 | – | 1/0 | 2/0 3,4 |
| C. riethi (Kieffer, 1914) | – | – | – | – | – | – | – | – | – | – | – | (1)/0 4 |
| C. sphagnumensis (Williams, 1955) | – | – | – | – | – | – | – | 1/0 2 | – | – | – | – |
| C. vexans (Staeger, 1839) | – | – | 2/0 | – | – | – | – | – | 5/0 | – | – | – |
| Total number of females/males of other subgenera | 10/0 | 1/0 | 8/0 | 15/1 | 52/0 | 45/3 | 35/0 | 2/0 | 9/0 | 0/0 | 95/0 | 4/0 |
| Atrichopogon spec. | – | – | – | – | + | – | – | + 1,2 | – | + | – | – |
| Forcipomyia spec. | + | – | – | + | + | + | + | + 1,2 | + | + | + | + |
| Dasyhelea spec. | – | – | – | – | – | – | – | – | – | – | + | – |
| Palomyia spec. | – | – | – | – | – | – | – | + 1 | + | – | – | – |
| Serromyia spec. | – | – | – | – | – | – | – | – | + | – | – | – |
| Species | Dung type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Llama/Alpaca | Chicken | Cattle | Cowpat | Deer | Horse | Duck | Rabbit | Sheep | Pig | Mixed Cattle/Horse | Mixed Sheep/Horse | Mixed Goat/Horse | |
| Genus Culicoides Subgenus Avaritia (Fox, 1955) | |||||||||||||
| C. chiopterus (Meigen, 1830) | – | – | +(1)/43 | +(2)/21 | +/2 | +(2)/19 | +/4 | – | – | – | – | +(2)/27 | – |
| C. obsoletus (Meigen, 1818) | (89)/9 | – | +(4)/50 | +/10 | – | +(1)/8 | – | +/5 | +(1)/13 | +(3)/0 | +(1)/24 | +/8 | +(2)/24 |
| C. scoticus (Downes and Kettle, 1952) | (1)/6 | – | – | +/2 | +(2)/5 | +/0 | – | – | +(1)/9 | – | +(2)/7 | +/16 | +(3)/0 |
| C. dewulfi (Goetghebuer, 1935) | – | +/4 | +/18 | +/28 | – | – | – | +/4 | +/4 | – | +(1)/0 | +/3 | +/18 |
| Total number of females/males belonging to subgenus Avaritia | 90/15 | 8/4 | 56/111 | 34/61 | 12/7 | 65/27 | 13/4 | 21/9 | 21/26 | 3/0 | 57/31 | 58/54 | 129/42 |
| Genus Culicoides Subgenus Culicoides (Latreille, 1809) | |||||||||||||
| C. delta (Edwards, 1939) | – | – | – | – | – | – | – | – | (1)/0 | – | – | – | – |
| C. pulicaris (Linnaeus, 1758) | – | – | − | − | (2)/0 | – | – | 1/0 | 3/0 | – | – | (1)/1 | 1/0 |
| C. punctatus(Meigen, 1804) | – | – | – | – | – | – | 5/0 | - | – | – | − | 6/0 | – |
| C. newsteadi (Austen, 1921) | – | – | − | – | – | (6)/0 | – | – | – | – | – | – | – |
| Total number of females/males belonging to subgenus Culicoides | 0/0 | 0/0 | 0/0 | 0/0 | 2/0 | 6/0 | 5/0 | 1/0 | 4/0 | 0/0 | 0/0 | 7/1 | 1/0 |
| Genus Culicoides Species of other subgenera | |||||||||||||
| C. achrayi (Kettle and Lawson, 1955) | 50(1)/0 | – | – | – | – | – | – | – | 8/0 | – | – | – | – |
| C. albicans(Winnertz, 1852) | (16)/0 | – | – | – | – | – | – | – | – | – | – | – | – |
| C. fagineus (Edwards, 1939) | – | – | 2/0 | – | – | – | – | – | – | – | – | – | – |
| C. minutissimus(Zetterstedt, 1855) | (1)/0 | – | – | – | – | – | – | – | – | – | – | (1)/0 | – |
| C. nubeculosus(Meigen, 1930) | 1/0 | 1/0 | – | – | – | – | – | – | 2(1)/0 | 3/0 | 4/0 | – | 4/0 |
| Total number of females/males of other subgenera | 69/0 | 1/0 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 11/0 | 3/0 | 4/0 | 1/0 | 4/0 |
| Dasyhelea spec. | + | – | – | – | – | – | – | – | – | – | – | – | – |
| Atrichopogon spec. | – | – | – | – | – | – | + | – | – | – | – | – | – |
| Forcipomyia spec. | + | + | + | + | – | + | + | + | + | + | + | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werner, D.; Groschupp, S.; Bauer, C.; Kampen, H. Breeding Habitat Preferences of Major Culicoides Species (Diptera: Ceratopogonidae) in Germany. Int. J. Environ. Res. Public Health 2020, 17, 5000. https://doi.org/10.3390/ijerph17145000
Werner D, Groschupp S, Bauer C, Kampen H. Breeding Habitat Preferences of Major Culicoides Species (Diptera: Ceratopogonidae) in Germany. International Journal of Environmental Research and Public Health. 2020; 17(14):5000. https://doi.org/10.3390/ijerph17145000
Chicago/Turabian StyleWerner, Doreen, Sarah Groschupp, Christian Bauer, and Helge Kampen. 2020. "Breeding Habitat Preferences of Major Culicoides Species (Diptera: Ceratopogonidae) in Germany" International Journal of Environmental Research and Public Health 17, no. 14: 5000. https://doi.org/10.3390/ijerph17145000
APA StyleWerner, D., Groschupp, S., Bauer, C., & Kampen, H. (2020). Breeding Habitat Preferences of Major Culicoides Species (Diptera: Ceratopogonidae) in Germany. International Journal of Environmental Research and Public Health, 17(14), 5000. https://doi.org/10.3390/ijerph17145000





