Assessment of Radiation Dose from the Consumption of Bottled Drinking Water in Japan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Chemical Characteristics of Bottled Water
2.2. Radionuclide Determination
2.2.1. Tritium and Carbon-14 Determination
2.2.2. Radon Determination
2.2.3. Potassium and Caesium Determination
2.2.4. Uranium Determination
2.2.5. Radium Determination
2.2.6. Polonium and Lead Determination
2.3. Determination of the Annual Effective Ingestion Doses
3. Results and Discussion
3.1. pH and Water Hardness
3.2. Radionuclide Activity Concentrations
3.2.1. Polonium and Lead activity Concentrations
3.2.2. Radium Isotope Activity Concentrations
3.2.3. Uranium Isotope Activity Concentrations
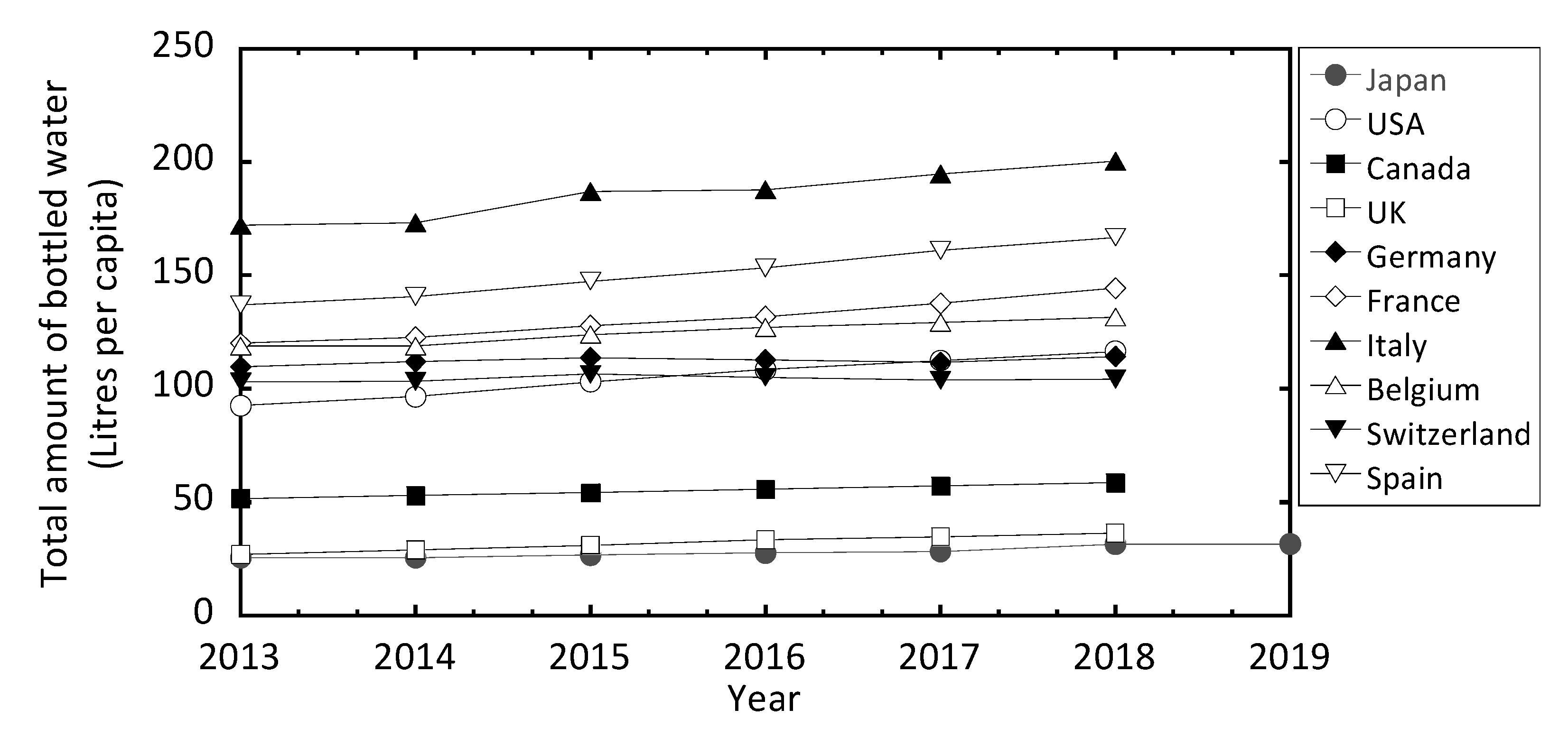
3.2.4. Comparison of Activity Concentrations in Bottled Mineral Waters for Sale in Other Countries
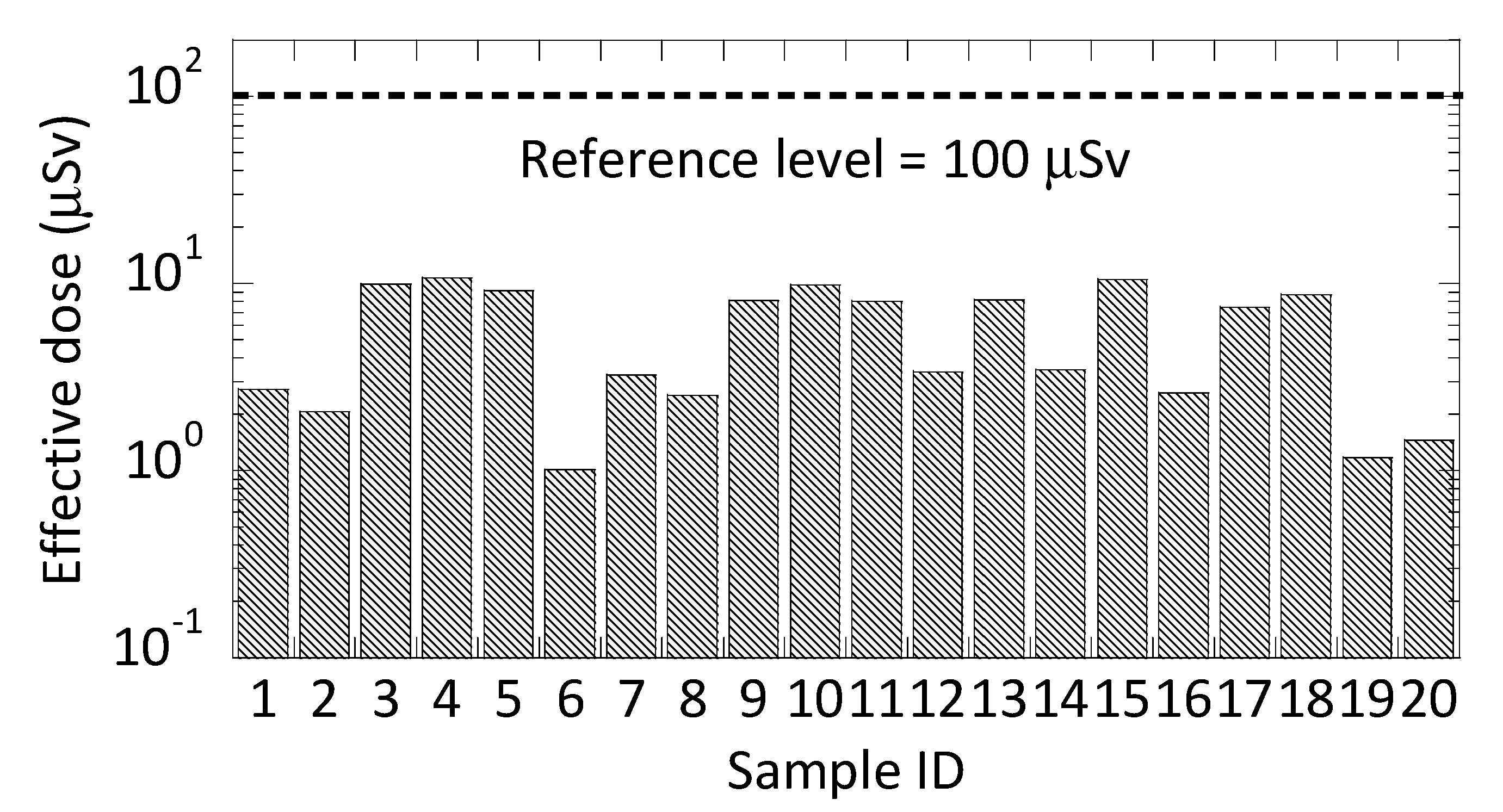
3.3. Dose Assessment of the Maximum Annual Effective Ingestion Doses from Bottled Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2000 Report to the General Assembly, with Scientific Annexes, Volume I: SOURCES; United Nations: New York, NY, USA, 2000. [Google Scholar]
- World Health Organization (WHO). Management of Radioactivity in Drinking-Water; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th edition, Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; pp. 203–218. [Google Scholar]
- The Mineral Water Association of Japan. Data Sheets—Various Statistics of Mineral Waters. Available online: https://minekyo.net/publics/index/5/ (accessed on 1 April 2020).
- The Federation of Electric Power Companies of Japan (FEPC). Nuclear Power Plants in Japan. Available online: https://www.fepc.or.jp/english/nuclear/power_generation/plants/index.html (accessed on 1 April 2020).
- Shiraishi, K.; Kimura, S.; Sahoo, S.K.; Arae, H. Dose effect for Japanese due to 232Th and 238U in imported drinking water. Health Phys. 2004, 86, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Shozugawa, K.; Saito, T.; Hori, M.; Matsuo, M. High-sensitivity determination of radioactive cesium in Japanese foodstuffs: 3 years after the Fukushima accident. J. Radioanal. Nucl. Chem. 2016, 307, 2117–2122. [Google Scholar] [CrossRef]
- Dávila Rangel, J.I.; López del Río, H.; García, F.M.; Torres, L.L.Q.; Villalba, M.L.; Sujo, L.C.; Cabrera, M.E.M. Radioactivity in bottled waters sold in Mexico. Appl. Radiat. Isot. 2002, 56, 931–936. [Google Scholar] [CrossRef]
- Amrani, D. Natural radioactivity in Algerian bottled mineral waters. J. Radioanal. Nucl. Chem. 2002, 252, 597–600. [Google Scholar] [CrossRef]
- Kralik, C.; Friedrich, M.; Vojir, F. Natural radionuclides in bottled water in Austria. J. Environ. Radioact. 2003, 65, 233–241. [Google Scholar] [CrossRef]
- Somlai, J.; Horváth, G.; Kanyár, B.; Kovács, T.; Bodrogi, E.; Kávási, N. Concentration of 226Ra in Hungarian bottled mineral water. J. Environ. Radioact. 2002, 62, 235–240. [Google Scholar] [CrossRef]
- Karamanis, D.; Stamoulis, K.; Ioannides, K.G. Natural radionuclides and heavy metals in bottled water in Greece. Desalination 2007, 213, 90–97. [Google Scholar] [CrossRef]
- Fatima, I.; Zaidi, J.H.; Arif, M.; Tahir, S.N.A. Measurement of natural radioactivity in bottled drinking water in Pakistan and consequent dose estimates. Radiat. Prot. Dosim. 2007, 123, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Desideri, D.; Meli, M.A.; Feduzi, L.; Roselli, C.; Rongoni, A.; Saetta, D. 238U, 234U, 226Ra, 210Po concentrations of bottled mineral waters in Italy and their dose contribution. J. Environ. Radioact. 2007, 94, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Torri, G. Estimation of radiation doses to members of the public in Italy from intakes of some important naturally occurring radionuclides (238U, 234U, 235U, 226Ra, 228Ra, 224Ra and 210Po) in drinking water. Appl. Radiat. Isot. 2007, 65, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, B.; Walencik, A.; Dorda, J.; Przylibski, T.A. Uranium, radium and 40K isotopes in bottled mineral waters from Outer Carpathians, Poland. Radiat. Meas. 2007, 42, 1380–1386. [Google Scholar] [CrossRef]
- Palomo, M.; Peñalver, A.; Borrull, F.; Aguilar, C. Measurement of radioactivity in bottled drinking water in Spain. Appl. Radiat. Isot. 2007, 65, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, F.; Baccouche, S.; Abdelli, W.; Samaali, M.; Oueslati, M.; Trabelsi, A. Uranium isotopes in Tunisian bottled mineral waters. J. Environ. Radioact. 2010, 101, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Wallner, G.; Jabbar, T. Natural radionuclides in Austrian bottled mineral waters. J. Radioanal. Nucl. Chem. 2010, 286, 329–334. [Google Scholar] [CrossRef]
- Rožmarić, M.; Rogić, M.; Benedik, L.; Štrok, M. Natural radionuclides in bottled drinking waters produced in Croatia and their contribution to radiation dose. Sci. Total Environ. 2012, 437, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Currivan, L.; Kelleher, K.; Solodovnik, E.; McMahon, C. Radioactivity in Bottled Water Produced in Ireland; Technical Report; Radiological Protection Institute of Ireland: Dublin, Ireland, 2013. [Google Scholar]
- Khandaker, M.U.; Nasir, N.L.M.; Zakirin, N.S.; Kassim, H.A.; Asaduzzaman, K.; Bradley, D.A.; Zulkifli, M.Y.; Hayyan, A. Radiation dose to the Malaysian populace via the consumption of bottled mineral water. Radiat. Phys. Chem. 2017, 140, 173–179. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Hardness in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- International Organization for Standardization (ISO). ISO 17025 General Requirements for the Competence of Testing and Calibration Laboratories; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization (ISO). ISO 13164-4:2015—Water quality—Radon-222—Part 4: Test. Method Using Two-Phase Liquid Scintillation Counting; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- International Organization for Standardization (ISO). ISO 17294-2:2016—Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Hosoda, M.; Kelleher, K.; Murray, M.; McGinnity, P.; Hanley, O.; Wong, J.; Currivan, L. Generation and Migration of 222Rn in BaSO4 Precipitate Samples and Implications for their Analysis for 226Ra by Gamma Spectrometry. Radiat. Environ. Med. 2016, 5, 22–28. [Google Scholar]
- Flynn, W.W. The Determination of Low Levels of Polonium-210 in Environmental Materials. Anal. Chim. Acta 1968, 43, 221–227. [Google Scholar] [CrossRef]
- Ebaid, Y.Y.; Khater, A.E.M. Determination of 210 Pb in Environmental Samples. J. Radioanal. Nucl. Chem. 2006, 270, 609–619. [Google Scholar] [CrossRef]
- ICRP. ICRP Publication 72. Age-dependent Doses to the Members of the Public from Intake of Radionuclides—Part 5 Compilation of Ingestion and Inhalation Coefficients; ICRP: Oxford, UK, 1996. [Google Scholar]
- National Research Council (US). Risk Assessment of Radon in Drinking Water; National Academies Press (US): Washington, DC, USA, 1999; p. 76. [Google Scholar]
- Omori, Y.; Hosoda, M.; Takahashi, F.; Sanada, T.; Hirao, S.; Ono, K.; Furukawa, M. Japanese Population Dose from Natural Radiation. J. Radiol. Prot. 2020. Available online: https://iopscience.iop.org/article/10.1088/1361-6498/ab73b1/meta (accessed on 1 April 2020). [CrossRef] [PubMed]

| Radionuclide | Preparation | Analytical Techniques |
|---|---|---|
| 3H, 14C | 8:12 mL water:/Ultima Gold ™ LLT 7:1:12 mL water/spike/Ultima Gold ™ LLT | Liquid scintillation counting |
| 222Rn | 8:12 mL water/organic scintillation cocktail | Liquid scintillation counting |
| 40K, 134Cs, 137Cs | 500 mL Marinelli | Direct counting on HPGe |
| 40K | Acidified | ICP–MS (evaluated from stable potassium to 40K) |
| 210Po | 500 mL water, acidified, spontaneous deposition | Alpha spectrometry |
| 210Pb | 500 mL water, acidified, spontaneous deposition (after 6 months) | Alpha spectrometry |
| 226Ra, 228Ra | 1 to 4 L water, barium co-precipitation method, stored for one month prior to measurement | Gamma spectrometry (HPGe) |
| 234U, 235U, 238U | Acidified | ICP–MS (evaluated from total uranium) |
| Isotope | Dose Coefficient (nSv/Bq) |
|---|---|
| 222Rn [31] 1 | 3.5 × 100 |
| 3H | 1.8 × 10−2 |
| 14C | 5.8 × 10−1 |
| 134Cs | 1.9 × 101 |
| 137Cs | 1.3 × 101 |
| 210Po | 1.2× 103 |
| 210Pb | 6.9 × 102 |
| 226Ra | 2.8 × 102 |
| 228Ra | 6.9 × 102 |
| 234U | 4.9 × 101 |
| 235U | 4.7 × 101 |
| 238U | 4.5 × 101 |
| Sample | 210Po | 210Pb | 226Ra | 228Ra | 234U | 235U | 238U | 40K |
|---|---|---|---|---|---|---|---|---|
| 1 | <9.4 × 10−1 | (5.7 ± 0.5) × 100 | <1.9 × 102 | (2.6 ± 1.5) × 101 | (2.6 ± 0.5) × 101 | (1.2 ± 0.2) × 100 | (2.4 ± 0.5) × 101 | (1.3 ± 0.3) × 10−1 |
| 2 | <7.4 × 10−1 | (1.8 ± 0.2) × 100 | <1.1 × 102 | <3.7 × 101 | <1.3 × 10−1 | <5.8 × 10−3 | <1.2 × 10−1 | <7.7 × 10−3 |
| 3 | <1.3 × 100 | (2.0 ± 0.2) × 100 | <1.8 × 102 | <3.6 × 102 | (2.4 ± 0.5) × 100 | (1.0 ± 0.2) × 10−1 | (2.2 ± 0.5) × 100 | (1.5 ± 0.3) × 10−2 |
| 4 | (2.6 ± 0.4) × 100 | (7.4 ± 0.7) × 100 | <2.0 × 102 | <3.9 × 102 | (2.6 ± 0.5) × 10−1 | (1.2 ± 0.2) × 10−2 | (2.4 ± 0.5) × 10−1 | (1.9 ± 0.4) × 10−2 |
| 5 | <1.2 × 100 | < 1.7 × 100 | <1.7 × 102 | <3.4 × 102 | (1.3 ± 0.3) × 10−1 | (5.8 ± 0.1) × 10−3 | (1.2 ± 0.3) × 10−1 | (3.8 ± 0.8) × 10−2 |
| 6 | <1.3 × 100 | (5.3 ± 0.5) × 100 | (8.5 ± 3.9) × 100 | (2.5 ± 1.1) × 101 | (7.9 ± 0.2) × 10−1 | (3.5 ± 0.7) × 10−2 | (7.3 ± 0.2) × 10−1 | (6.2 ± 1.3) ± × 10−2 |
| 7 | <8.9 × 10−1 | (3.8 ± 0.3) × 100 | <2.3 × 102 | <4.2 × 101 | (5.2 ± 1.1) × 10−1 | (2.3 ± 0.5) × 10−2 | (4.8 ± 1.0) × 10−1 | (3.5 ± 0.7) × 10−2 |
| 8 | <1.2 × 100 | (5.3 ± 0.5) × 10−1 | <1.7 × 102 | <3.4 × 101 | (2.6 ± 0.5) × 10−1 | (1.2 ± 0.2) × 10−2 | (2.4 ± 0.5) × 10−1 | (4.9 ± 1.0) × 10−2 |
| 9 | <1.1 × 100 | (3.7 ± 0.3) × 100 | <6.0 × 102 | <1.1 × 102 | (1.3 ± 0.3) × 10−1 | (5.8 ± 1.2) × 10−3 | (1.2 ± 0.3) × 10−1 | (2.5 ± 0.5) × 10−2 |
| 10 | <1.1 × 100 | (8.1 ± 0.7) × 10−1 | <1.1 × 102 | <3.9 × 102 | (1.3 ± 0.3) × 10−1 | (5.8 ± 1.2) × 10−3 | (1.2 ± 0.3) × 10−1 | (2.5 ± 0.5) × 10−2 |
| 11 | <5.7 × 10−1 | (2.0 ± 0.2) × 100 | <1.5 × 102 | <2.9 × 102 | (3.9 ± 0.8) × 10−1 | (1.7 ± 0.4) × 10−2 | (3.6 ± 0.8) × 10−1 | (2.4 ± 0.5) × 10−2 |
| 12 | (1.0 ± 0.3) × 100 | (4.4 ± 0.4) × 100 | <2.4 × 102 | <4.3 × 101 | (5.2 ± 0.1) × 10−1 | (2.3 ± 0.5) × 10−2 | (4.8 ± 1.0) × 10−1 | (3.2 ± 0.7) × 10−2 |
| 13 | (4.9 ± 0.4) × 100 | (1.9 ± 0.2) × 101 | (1.2 ± 0.5) × 101 | <3.3 × 102 | (1.6 ± 0.3) × 100 | (6.9 ± 0.1) × 10−2 | (1.5 ± 0.3) × 100 | (5.1 ± 1.1) × 10−2 |
| 14 | (1.5 ± 0.6) × 100 | (4.3 ± 0.4) × 100 | <2.3 × 102 | (4.9 ± 1.8) × 101 | <1.3 × 10−1 | <5.8 × 10−3 | <1.2 × 10−1 | (2.9 ± 0.6) × 10−2 |
| 15 | <1.4 × 100 | (3.0 ± 0.3) × 100 | <1.9 × 102 | <3.9 × 102 | (3.0 ± 0.6) × 100 | (1.3 ± 0.3) × 10−1 | (2.8 ± 0.6) × 100 | (2.5 ± 0.5) × 10−1 |
| 16 | <9.7 × 10−1 | (4.5 ± 0.4) × 100 | <1.9 × 102 | (1.9 ± 1.0) × 101 | (1.4 ± 0.3) × 10−1 | (6.3 ± 1.3) × 10−2 | (1.3 ± 0.3) × 100 | (8.4 ± 1.7) × 10−2 |
| 17 | <8.3 × 10−1 | (2.4 ± 0.2) × 100 | (1.3 ± 0.6) × 101 | <3.2 × 102 | (2.6 ± 0.5) × 10−1 | (1.2 ± 0.2) × 10−2 | (2.4 ± 0.5) × 10−1 | (1.4 ± 0.3) × 10−1 |
| 18 | <1.4 × 100 | (2.6 ± 0.2) ×100 | <1.6 × 102 | <3.2 × 102 | (2.8 ± 0.6) × 100 | (1.2 ± 0.2) × 10−1 | (2.5 ± 0.5) × 100 | (2.4 ± 0.5) × 10−1 |
| 19 | (1.9 ± 1.5) × 100 | (6.9 ± 0.6) × 100 | (1.2 ± 0.4) × 101 | <2.5 × 101 | (1.7 ± 0.4) × 10−1 | (7.5 ± 1.5) × 10−2 | (1.6 ± 0.3) × 100 | (7.2 ± 1.5) × 10−2 |
| 20 | (1.7 ± 1.5) × 100 | (5.9 ± 0.5) × 100 | <7.1 × 102 | (1.6 ± 0.8) × 101 | (1.3 ± 0.3) × 10−1 | (5.8 ± 1.2) × 10−3 | (1.2 ± 0.3) × 10−1 | (2.5 ± 0.5) × 10−2 |
| Isotope | Min | Max | Median | Maximum Annual Effective Dose 1 (nSv) |
|---|---|---|---|---|
| 210Po 2 | 1.0 ± 0.26 | 4.9 ± 0.39 | 1.7 ± 0.34 | 186 |
| 210Pb 3 | 0.53 ± 0.050 | 19 ± 1.8 | 3.8 ± 0.34 | 421 |
| Isotope | Min | Max | Median | Maximum Annual Effective Dose 1 (nSv) |
|---|---|---|---|---|
| 226Ra 2 | 0.85 ± 0.39 | 13 ± 5.5 | 12 ± 4.3 | 100 |
| 228Ra 3 | 16 ± 8.2 | 49 ± 18 | 25 ± 11 | 589 |
| Isotope | Min | Max | Median | Maximum Annual Effective Dose 1 (nSv) |
|---|---|---|---|---|
| 234U 2 | 0.13 ± 0.030 | 26 ± 5.4 | 0.52 ± 0.11 | 3.3 |
| 235U 3 | 0.005 ± 0.002 | 1.2 ± 0.24 | 0.023 ± 0.005 | 0.14 |
| 238U 4 | 0.24 ± 0.050 | 24 ± 5.0 | 0.48 ± 0.099 | 2.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinahan, A.; Hosoda, M.; Kelleher, K.; Tsujiguchi, T.; Akata, N.; Tokonami, S.; Currivan, L.; León Vintró, L. Assessment of Radiation Dose from the Consumption of Bottled Drinking Water in Japan. Int. J. Environ. Res. Public Health 2020, 17, 4992. https://doi.org/10.3390/ijerph17144992
Kinahan A, Hosoda M, Kelleher K, Tsujiguchi T, Akata N, Tokonami S, Currivan L, León Vintró L. Assessment of Radiation Dose from the Consumption of Bottled Drinking Water in Japan. International Journal of Environmental Research and Public Health. 2020; 17(14):4992. https://doi.org/10.3390/ijerph17144992
Chicago/Turabian StyleKinahan, Aoife, Masahiro Hosoda, Kevin Kelleher, Takakiyo Tsujiguchi, Naofumi Akata, Shinji Tokonami, Lorraine Currivan, and Luis León Vintró. 2020. "Assessment of Radiation Dose from the Consumption of Bottled Drinking Water in Japan" International Journal of Environmental Research and Public Health 17, no. 14: 4992. https://doi.org/10.3390/ijerph17144992
APA StyleKinahan, A., Hosoda, M., Kelleher, K., Tsujiguchi, T., Akata, N., Tokonami, S., Currivan, L., & León Vintró, L. (2020). Assessment of Radiation Dose from the Consumption of Bottled Drinking Water in Japan. International Journal of Environmental Research and Public Health, 17(14), 4992. https://doi.org/10.3390/ijerph17144992







