Exposure to New Emerging Bisphenols Among Young Children in Switzerland
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Artificial Urine
2.3. Urine Sample Extraction
2.4. Chemical Analysis of BPs
2.5. Calibration and Blank Assessment
2.6. Study Population
2.7. Statistical Analysis
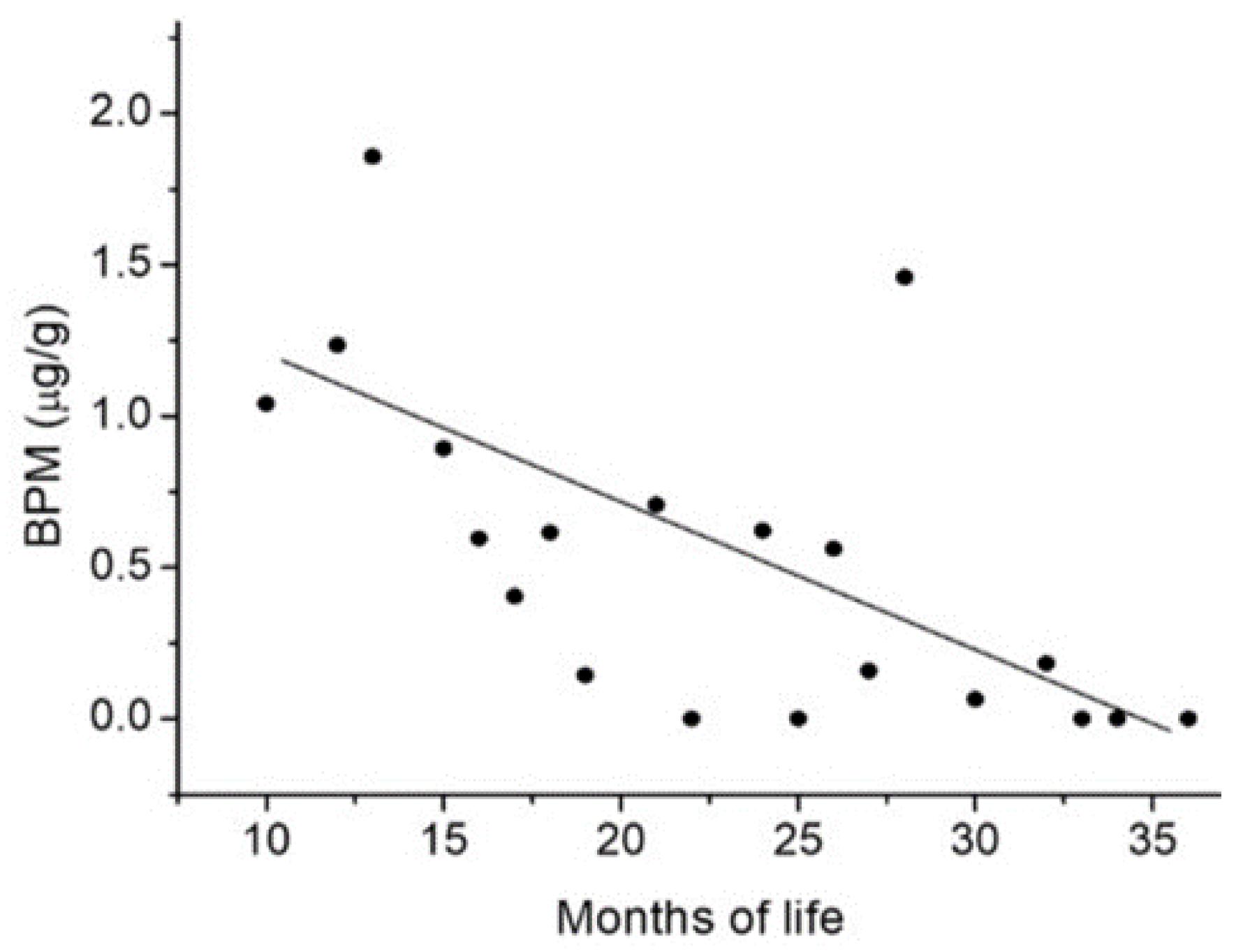
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Expert Industry. Bisphenol-A A Global Market Overview. Available online: https://industry-experts.com/verticals/files/articles/cp021-bisphenol-a-a-global-market-overview.pdf (accessed on 18 February 2020).
- Huang, R.-P.; Liu, Z.-H.; Yuan, S.-F.; Yin, H.; Dang, Z.; Wu, P.-X. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ. Pollut. 2017, 230, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary Concentrations of Bisphenol A and 4-Nonylphenol in a Human Reference Population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Liu, Y.; Gong, X.; Zhang, T.; Sun, H. Widespread Occurrence of Bisphenol A in Daily Clothes and Its High Exposure Risk in Humans. Environ. Sci. Technol. 2019, 53, 7095–7102. [Google Scholar] [CrossRef] [PubMed]
- Covaci, A.; Den Hond, E.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Morck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef]
- Louro, H.; Heinala, M.; Bessems, J.; Buekers, J.; Vermeire, T.; Woutersen, M.; van Engelen, J.; Borges, T.; Rousselle, C.; Ougier, E.; et al. Human biomonitoring in health risk assessment in Europe: Current practices and recommendations for the future. Int. J. Hyg. Environ. Health 2019, 222, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010, 118, 1055–1070. [Google Scholar] [CrossRef]
- Lehmler, H.J.; Liu, B.; Gadogbe, M.; Bao, W. Exposure to Bisphenol A, Bisphenol F, and Bisphenol S in U.S. Adults and Children: The National Health and Nutrition Examination Survey 2013–2014. ACS Omega 2018, 3, 6523–6532. [Google Scholar] [CrossRef]
- French Agency for Food, Environmental and Occupational Health and Safety (ANSES). Reprotoxic Substances and Endocrine Disruptors. Compounds of the Bisphenol Family: Bisphenols M, S, B, AP, AF, F and BADGE. Expert Committee Report. 2013. Available online: https://www.anses.fr/fr/system/files/CHIM2009sa0331Ra-1.pdf (accessed on 18 February 2020).
- ANSES. Annex XV report. Proposal for identification of a substance of very high concern on the basis of the criteria set out in REACH Article 57. Substance Name(s): 4,4’-isopropylidenediphenol (Bisphenol A). EC Number: 201-245-8. CAS Number: 80-05-7. Available online: https://echa.europa.eu/documents/10162/93bf4be3-9af6-d7ca-8b07-4e8fb42bad11 (accessed on 18 February 2020).
- Environment Canada, Health Canada (ECHC). Screening Assessment for the Challenge Phenol, 4,4’ -(1-Methylethylidene)bis-(Bisphenol A), Chemical Abstracts Service Registry Number 80-05-7. 2008. Available online: http://www.ec.gc.ca/ese-ees/3C756383-BEB3-45D5-B8D3-E8C800F35243/batch2_80-05-7_en.pdf (accessed on 18 February 2020).
- European Chemicals Agency (ECHA). Opinion on an Annex XV Dossier Proposing Restrictions on Bisphenol, A. Compiled Version Prepared by the ECHA Secretariat of RAC’s Opinion (Adopted 5 June 2015) and SEAC’s Opinion (Adopted 4 December 2015). Available online: https://echa.europa.eu/documents/10162/9ce0977b-3540-4de0-af6d-16ad6e78ff20 (accessed on 18 February 2020).
- European Food Safety Authority (EFSA). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- EFSA; Croera, C.; Batke, M.; Corsini, E.; FitzGerald, R.E.; Gott, D.; Ntzani, E.; Gundert-Remy, U.; Halldorsson, T.; Schroeder, H.; et al. Testing the study appraisal methodology from the 2017 Bisphenol A (BPA) hazard assessment protocol. EFSA Supporting Publ. 2019, 16, 2397–8325. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration (USFDA). Bisphenol A (BPA): Use in Food Contact Application. 2018. Available online: https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application (accessed on 18 February 2020).
- U.S. National Toxycology Program (NTP). NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. CERHR, Ed.; National Toxicology Programm: Raleigh, NC, USA, 2020. Available online: https://ntp.niehs.nih.gov/ntp/ohat/bisphenol/bisphenol.pdf (accessed on 18 February 2020).
- Beausoleil, C.; Emond, C.; Cravedi, J.P.; Antignac, J.P.; Applanat, M.; Appenzeller, B.R.; Beaudouin, R.; Belzunces, L.P.; Canivenc-Lavier, M.C.; Chevalier, N.; et al. Regulatory identification of BPA as an endocrine disruptor: Context and methodology. Mol. Cell. Endocrinol. 2018, 475, 4–9. [Google Scholar] [CrossRef]
- Seachrist, D.D.; Bonk, K.W.; Ho, S.M.; Prins, G.S.; Soto, A.M.; Keri, R.A. A review of the carcinogenic potential of bisphenol A. Reprod. Toxicol. 2016, 59, 167–182. [Google Scholar] [CrossRef]
- Ejaredar, M.; Lee, Y.; Roberts, D.J.; Sauve, R.; Dewey, D. Bisphenol A exposure and children’s behavior: A systematic review. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L.; Kwiatkowski, C.F. Prenatal exposure to bisphenol A and hyperactivity in children: A systematic review and meta-analysis. Environ. Int. 2018, 114, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Lee, E.; Kim, Y. The Association between Bisphenol A Exposure and Obesity in Children—A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 2521. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EU). Bisphenol A: EU Ban on Baby Bottles to Enter into Force Tomorrow. 2011. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_11_664 (accessed on 18 February 2020).
- Pelch, K.; Wignall, J.A.; Goldstone, A.E.; Ross, P.K.; Blain, R.B.; Shapiro, A.J.; Holmgren, S.D.; Hsieh, J.H.; Svoboda, D.; Auerbach, S.S.; et al. A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives. Toxicology 2019, 424, 152235. [Google Scholar] [CrossRef]
- Serra, H.; Beausoleil, C.; Habert, R.; Minier, C.; Picard-Hagen, N.; Michel, C. Evidence for Bisphenol B Endocrine Properties: Scientific and Regulatory Perspectives. Environ. Health Perspect. 2019, 127, 106001. [Google Scholar] [CrossRef]
- Swedish Chemical Agency (KEMI). Bisfenoleren Kartläggning och Analys Rapport Från Ett Deluppdrag Inom Handlingsplanen för En Giftfri Vardag. 2017. Available online: https://www.kemi.se/en/global/rapporter/2017/rapport-5-17-bisfenoler-en-kartlaggning-och-analys.pdf (accessed on 18 February 2020).
- Cesen, M.; Lenarcic, K.; Mislej, V.; Levstek, M.; Kovacic, A.; Cimrmancic, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S.; et al. The occurrence and source identification of bisphenol compounds in wastewaters. Sci. Total Environ. 2018, 616, 744–752. [Google Scholar] [CrossRef]
- Ijaz, S.; Ullah, A.; Shaheen, G.; Jahan, S. Exposure of BPA and its alternatives like BPB, BPF, and BPS impair subsequent reproductive potentials in adult female Sprague Dawley rats. Toxicol. Mech. Methods 2020, 30, 60–72. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.L.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity-A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Bal’tser, A.E.; Zaitsev, D.A.; Zubritskaya, N.G.; Ivanova, T.V.; Babenko, T.G.; Barskova, E.N. Optimization of technological parameters of the process for obtaining bisphenols on cation-exchange catalyst in the presence of hydrogen sulfide. Russ. J. App. Chem. 2017, 89, 1421–1426. [Google Scholar] [CrossRef]
- Naderi, M.; Wong, M.Y.; Gholami, F. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquat. Toxicol. 2014, 148, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Hong, S.; Kho, Y.; Choi, K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environ. Sci. Technol. 2013, 47, 8793–8800. [Google Scholar] [CrossRef] [PubMed]
- Rosenmai, A.K.; Dybdahl, M.; Pedersen, M.; van Vugt-Lussenburg, B.M.A.; Wedebye, E.B.; Taxvig, C.; Vinggaard, A.M. Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci. 2014, 139, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.; Ahmad, M. From BPA to its analogues: Is it a safe journey? Chemosphere 2016, 158, 131–142. [Google Scholar] [CrossRef]
- Andra, S.S.; Charisiadis, P.; Arora, M.; van Vliet-Ostaptchouk, J.V.; Makris, K.C. Biomonitoring of human exposures to chlorinated derivatives and structural analogs of bisphenol A. Environ. Int. 2015, 85, 352–379. [Google Scholar] [CrossRef]
- Macczak, A.; Cyrkler, M.; Bukowska, B.; Michalowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef]
- Ding, Z.M.; Jiao, X.F.; Wu, D.; Zhang, J.Y.; Chen, F.; Wang, Y.S.; Huang, C.J.; Zhang, S.X.; Li, X.; Huo, L.J. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem. Biol. Interact. 2017, 278, 222–229. [Google Scholar] [CrossRef]
- DeFoor, W.; Asplin, J.; Jackson, E.; Jackson, C.; Reddy, P.; Sheldon, C.; Erhard, M.; Minevich, E. Urinary metabolic evaluations in normal and stone forming children. J. Urol. 2006, 176, 1793–1796. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Kitamura, S.; Suzuki, T.; Sanoh, S.; Kohta, R.; Jinno, N.; Sugihara, K.; Yoshihara, S.; Fujimoto, N.; Watanabe, H.; Ohta, S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol. Sci. 2005, 84, 249–259. [Google Scholar] [CrossRef]
- Molina-Molina, J.M.; Amaya, E.; Grimaldi, M.; Saenz, J.M.; Real, M.; Fernandez, M.F.; Balaguer, P.; Olea, N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol. Appl. Pharmacol. 2013, 272, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Choi, J.S.; Kim, W.K.; Lee, Y.J.; Park, J.W. Estrogenic potency of bisphenol S, polyethersulfone and their metabolites generated by the rat liver S9 fractions on a MVLN cell using a luciferase reporter gene assay. Reprod. Biol. Endocrinol. 2014, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zhang, H.M.; Cao, J.; Tang, B.P. Binding of a new bisphenol analogue, bisphenol S to bovine serum albumin and calf thymus DNA. J. Photochem. Photobiol. B 2014, 138, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Audebert, M.; Dolo, L.; Perdu, E.; Cravedi, J.P.; Zalko, D. Use of the gammaH2AX assay for assessing the genotoxicity of bisphenol A and bisphenol F in human cell lines. Arch. Toxicol. 2011, 85, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Durrani, T.S. Exposures to Endocrine Disrupting Chemicals in Consumer Products-A Guide for Pediatricians. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 107–118. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Wang, J.; Tong, Z.; Yan, J.; Zhang, T.; Qin, Y.; Jiang, T.; She, J.; Shen, H. Urinary sexual steroids associated with bisphenol A (BPA) exposure in the early infant stage: Preliminary results from a Daishan birth cohort. Sci. Total Environ. 2017, 601, 1733–1742. [Google Scholar] [CrossRef]
- Andaluri, G.; Manickavachagam, M.; Suri, R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities. Environ. Monit. Assess. 2018, 190, 65. [Google Scholar] [CrossRef]
- Gonzalez, N.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Marques, M.; Domingo, J.L.; Nadal, M. Quantification of eight bisphenol analogues in blood and urine samples of workers in a hazardous waste incinerator. Environ. Res. 2019, 176, 108576. [Google Scholar] [CrossRef]
- Owczarek, K.; Kubica, P.; Kudlak, B.; Rutkowska, A.; Konieczna, A.; Rachon, D.; Namiesnik, J.; Wasik, A. Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography-tandem mass spectrometry. Sci. Total Environ. 2018, 628, 1362–1368. [Google Scholar] [CrossRef]
- Rocha, B.A.; Asimakopoulos, A.G.; Honda, M.; da Costa, N.L.; Barbosa, R.M.; Barbosa, F., Jr.; Kannan, K. Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ. Int. 2018, 116, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulos, A.G.; Xue, J.; De Carvalho, B.P.; Iyer, A.; Abualnaja, K.O.; Yaghmoor, S.S.; Kumosani, T.A.; Kannan, K. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 2016, 150, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Valentin-Blasini, L.; Li, Z.; Mortensen, M.E.; Wong, L.Y. Co-exposure to non-persistent organic chemicals among American pre-school aged children: A pilot study. Int. J. Hyg. Environ. Health 2017, 220, 55–63. [Google Scholar] [CrossRef]
- Xue, J.; Wu, Q.; Sakthivel, S.; Pavithran, P.V.; Vasukutty, J.R.; Kannan, K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ. Res. 2015, 137, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Ye, X.; Silva, M.J.; Kuklenyik, Z.; Needham, L.L. Human exposure assessment to environmental chemicals using biomonitoring. Int. J. Androl. 2006, 29, 166–171, discussion 181–165. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Exposure Factors Handbook 2011 Edition (Final Report). National Center for Environmental Assessment, Washington, DC.; EPA/600/R-09/052F. 2011. Available online: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252 (accessed on 22 June 2020).
- Cohen Hubal, E.A.; de Wet, T.; Du Toit, L.; Firestone, M.P.; Ruchirawat, M.; van Engelen, J.; Vickers, C. Identifying important life stages for monitoring and assessing risks from exposures to environmental contaminants: Results of a World Health Organization review. Regul. Toxicol. Pharmacol. 2014, 69, 113–124. [Google Scholar] [CrossRef]
- Liu, L.; Xia, T.; Zhang, X.; Barr, D.B.; Alamdar, A.; Zhang, J.; Tian, M.; Huang, Q.; Shen, H. Biomonitoring of infant exposure to phenolic endocrine disruptors using urine expressed from disposable gel diapers. Anal. Bioanal. Chem. 2014, 406, 5049–5054. [Google Scholar] [CrossRef]
- Hu, Y.; Beach, J.; Raymer, J.; Gardner, M. Disposable diaper to collect urine samples from young children for pyrethroid pesticide studies. J. Expo. Anal. Environ. Epidemiol. 2004, 14, 378–384. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Xia, T.; Guo, L.; Cao, L.; Zhao, B.; Zhang, J.; Dong, S.; Shen, H. Expressing urine from a gel disposable diaper for biomonitoring using phthalates as an example. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 625–631. [Google Scholar] [CrossRef][Green Version]
- Lock, J.Y.; Wyatt, E.; Upadhyayula, S.; Whall, A.; Nuñez, V.; Vullev, V.I.; Liu, H. Degradation and antibacterial properties of magnesium alloys in artificial urine for potential resorbable ureteral stent applications. J. Biomed. Mater. Res. A 2014, 102, 781–792. [Google Scholar] [CrossRef]
- Volkel, W.; Colnot, T.; Csanady, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gao, M.; Guo, X.; Ai, F.; Wang, Z. Enhanced degradation performance of bisphenol M using peroxymonosulfate activated by zero-valent iron in aqueous solution: Kinetic study and product identification. Chemosphere 2019, 221, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Deceuninck, Y.; Bichon, E.; Marchand, P.; Boquien, C.Y.; Legrand, A.; Boscher, C.; Antignac, J.P.; Le Bizec, B. Determination of bisphenol A and related substitutes/analogues in human breast milk using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2485–2497. [Google Scholar] [CrossRef] [PubMed]
- Myridakis, A.; Fthenou, E.; Balaska, E.; Vakinti, M.; Kogevinas, M.; Stephanou, E.G. Phthalate esters, parabens and bisphenol-A exposure among mothers and their children in Greece (Rhea cohort). Environ. Int. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Casas, M.; Valvi, D.; Luque, N.; Ballesteros-Gomez, A.; Carsin, A.E.; Fernandez, M.F.; Koch, H.M.; Mendez, M.A.; Sunyer, J.; Rubio, S.; et al. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ. Int. 2013, 56, 10–18. [Google Scholar] [CrossRef]
- Becker, K.; Goen, T.; Seiwert, M.; Conrad, A.; Pick-Fuss, H.; Muller, J.; Wittassek, M.; Schulz, C.; Kolossa-Gehring, M. GerES IV: Phthalate metabolites and bisphenol A in urine of German children. Int. J. Hyg. Environ. Health 2009, 212, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP). Research Concept: Bisphenol AF. 2008. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/bisphenolaf_093008_508.pdf (accessed on 18 February 2020).
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Distribution and preliminary exposure assessment of bisphenol AF (BPAF) in various environmental matrices around a manufacturing plant in China. Environ. Sci. Technol. 2012, 46, 13136–13143. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, L.; Zhang, J.; Yang, Y.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef]
- Song, S.; Song, M.; Zeng, L.; Wang, T.; Liu, R.; Ruan, T.; Jiang, G. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ. Pollut. 2014, 186, 14–19. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, J.; Yin, J.; Shao, B.; Li, H. Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 2014, 112, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Hendryx, M.; Luo, J. Children’s environmental chemical exposures in the USA, NHANES 2003–2012. Environ. Sci. Pollut. Res. Int. 2018, 25, 5336–5343. [Google Scholar] [CrossRef]
- FitzGerald, R.E.; Wilks, M.F. Bisphenol A-Why an adverse outcome pathway framework needs to be applied. Toxicol. Lett. 2014, 230, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, L.S.; Bucher, J.R.; Collman, G.W.; Zeldin, D.C.; Johnson, A.F.; Schug, T.T.; Heindel, J.J. Consortium-based science: The NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ. Health Perspect. 2012, 120, 1640–1644. [Google Scholar] [CrossRef]
- Schug, T.T.; Heindel, J.J.; Camacho, L.; Delclos, K.B.; Howard, P.; Johnson, A.F.; Aungst, J.; Keefe, D.; Newbold, R.; Walker, N.J.; et al. A new approach to synergize academic and guideline-compliant research: The CLARITY-BPA research program. Reprod. Toxicol. 2013, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- NTP. Draft NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats. NTP Research Report 9, 1–249. 2018. Available online: https://ntp.niehs.nih.gov/ntp/about_ntp/rrprp/2018/april/rr09peerdraft.pdf (accessed on 20 February 2020).
- Carvaillo, J.C.; Barouki, R.; Coumoul, X.; Audouze, K. Linking Bisphenol S to Adverse Outcome Pathways Using a Combined Text Mining and Systems Biology Approach. Environ. Health Perspect. 2019, 127, 47005. [Google Scholar] [CrossRef] [PubMed]
- Rugard, M.; Coumoul, X.; Carvaillo, J.C.; Barouki, R.; Audouze, K. Deciphering Adverse Outcome Pathway Network Linked to Bisphenol F Using Text Mining and Systems Toxicology Approaches. Toxicol. Sci. 2020, 173, 32–40. [Google Scholar] [CrossRef]
- Teeguarden, J.G.; Tan, Y.M.; Edwards, S.W.; Leonard, J.A.; Anderson, K.A.; Corley, R.A.; Kile, M.L.; Simonich, S.M.; Stone, D.; Tanguay, R.L.; et al. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ. Sci. Technol. 2016, 50, 4579–4586. [Google Scholar] [CrossRef]
- Tan, Y.M.; Leonard, J.A.; Edwards, S.; Teeguarden, J.; Paini, A.; Egeghy, P. Aggregate Exposure Pathways in Support of Risk Assessment. Curr. Opin. Toxicol. 2018, 9, 8–13. [Google Scholar] [CrossRef]
- Aylward, L.L. Integration of biomonitoring data into risk assessment. Curr. Opin. Toxicol. 2018, 9, 14–20. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Bishop, A.M.; Calafat, A.M. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ. Health Perspect. 2011, 119, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Federal Department of Home Affairs (FDHA). Human Biomonitoring in Switzerland. Report of the Federal Council from November 18, 2009 on Human Biomonitoring in Switzerland in Response to the Moser Postulate 08.3223 on the Implementation of a Body Burden Analysis to Chemical Substances. 2009. Available online: https://www.bag.admin.ch/dam/bag/en/dokumente/chem/chemikalien-alltag/bundesratsbericht-von-2009-zum-human-biomonitoring.pdf (accessed on 20 February 2020).
- FDHA. Human Biomonitoring in Switzerland. Current Situation and Long-Term Prospects. Interim Report. [In German]. Available online: https://www.bag.admin.ch/bag/en/home/gesund-leben/umwelt-und-gesundheit/chemikalien/chemikalien-im-alltag/human-biomonitoring/human-biomonitoring-projekte-in-der-schweiz.html (accessed on 20 February 2020).
| BP CAS n° | Structural Formula | Log kow | Applications | Potential Exposure Sources | Ref. |
|---|---|---|---|---|---|
| BISPHENOL A (BPA) 80-05-7 | 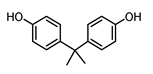 | 3.43 | Polycarbonate plastics, epoxy resins | Thermal papers, food, beverages, dust, personal care products, textile products | [26] [23] [9] [5] |
| BISPHENOL AF (BPAF) 1478-61-1 |  | 4.47 | Coating products, epoxy resins | Food, beverages, dust, dental materials, personal care products, textile products | [23] [26] [9] |
| BISPHENOL AP (BPAP) 1571-75-1 |  | 4.86 | Coating products, epoxy resins | Food, beverages, thermal papers, dust, textile products | [23] [26] [9] |
| BISPHENOL B (BPB) 77-40-7 | 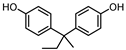 | 4.13 | Coating products, epoxy resins | Food, beverages, dust, textile products | [23] [26] [27] |
| BISPHENOL BP (BPBP) 1844-01-5 | 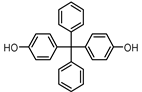 | 6.08 | Flame retardants, polycarbonate plastics | Plastic products, furniture | [26] |
| BISPHENOL C (BPC) 14868-03-2 |  | 3.74 | Flame retardants, polycarbonate plastics | Thermal papers, textile products | [26] [23] |
| BISPHENOL E (BPE) 2081-08-5 | 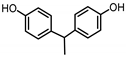 | 3.19 | Coating products, polycarbonate plastics | Food, beverages, textile products | [26] [23] |
| BISPHENOL F (BPF) 620-92-8 | 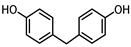 | 3.06 | Coating products, lacquers | Food, adhesives, varnishes, textile products, dust | [23] [26] [27] |
| BISPHENOL G (BPG) 127-54-8 | 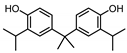 | 6.55 | Polycarbonate plastics, epoxy resins | Dental materials | [26] |
| BISPHENOL M (BPM) 13595-25-0 | 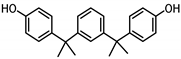 | 6.25 | Thermoplastics, polycarbonate plastics | Plastic products, dental materials | [28] |
| BISPHENOL P (BPP) 2167-51-3 | 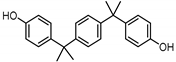 | 6.25 | Polycarbonate plastics, epoxy resins | Food, dust | [23] |
| BISPHENOL PH (BPPH) 24038-68-4 |  | 7.17 | Polycarbonate plastics, epoxy resins | Food, beverages | [28] |
| BISPHENOL S (BPS) 80-09-1 | 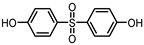 | 1.65 | Coating products, dyes, leather tanning agents | Food, beverages, thermal papers, dust, textile products | [23] [26] [27] |
| BISPHENOL TMC (BPTMC) 129188-99-4 |  | 6.29 | Polycarbonate plastics, epoxyr esins, polyesters | Dental materials, dust | [29] [28] |
| BISPHENOL Z (BPZ) 843-55-0 | 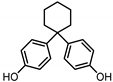 | 5.00 | Coating products, polycarbonate plastics | Food, personal care products, textile products | [23] [26] |
| Derivatized Bisphenols | Retention Time (min) | Monitored Ions (m/z) |
|---|---|---|
| BPAF | 18.85 | 480, 411, 225 |
| BPF | 20.26 | 344, 179, 157 |
| BPE | 20.52 | 358, 372 |
| BPA | 20.84 | 372, 357, 207 |
| BPB | 21.53 | 371, 357, 191 |
| BPG | 21.78 | 456, 441, 249 |
| BPC | 22.95 | 424, 354 |
| BPZ | 23.93 | 412, 369 |
| BTMC | 24.20 | 454, 397, 383 |
| BPS | 24.55 | 394, 379, 229 |
| BPAP | 24.83 | 434, 419, 269 |
| BPM | 26.12 | 490, 475, 207 |
| BPP | 27.40 | 490, 475, 207 |
| BPBF | 28.23 | 496, 419, 331 |
| BPPH | 28.40 | 509, 267, 247 |
| Bisphenols Derivatized | Detection Frequency % (Case Numbers) | Mean μg/g Creatinine (μg/L) | Range (μg/g Creatinine (μg/L) |
|---|---|---|---|
| BPF | 2 (2) | 0.07 (2.7) | 0.06–0.08 (1.80–3.61) |
| BPE | 2 (2) | 0.12 (5.85) | 0.07–0.17 (2.7–9.00) |
| BPA | 7 (8) | 0.06 (2.40) | 0.01–0.11 (1.22–3.30) |
| BPC | 23 (25) | 0.25 (11.22) | 0.03–1.03 (1.84–52.34) |
| BPS | 3 (3) | 0.15 (6.41) | 0.11–0.19 (2.60–12.02) |
| BPM | 25 (27) | 1.31 (53.07) | 0.13–8.56 (6.18–273.48) |
| BPP | 4 (4) | 0.58 (20.54) | 0.04–1.85 (4.38–49.36) |
| BPAF | <LOD | n.a. | n.a. |
| BPB | <LOD | n.a. | n.a. |
| BPG | <LOD | n.a. | n.a. |
| BPZ | <LOD | n.a. | n.a. |
| BPTMC | <LOD | n.a. | n.a. |
| BPAP | <LOD | n.a. | n.a. |
| BPBF | <LOD | n.a. | n.a. |
| BPPH | <LOD | n.a. | n.a. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, F.; Krasniqi, T.; Bailat Rosset, G.; Roth, N.; Hopf, N.B.; Broillet, M.-C.; Staedler, D. Exposure to New Emerging Bisphenols Among Young Children in Switzerland. Int. J. Environ. Res. Public Health 2020, 17, 4793. https://doi.org/10.3390/ijerph17134793
Lucarini F, Krasniqi T, Bailat Rosset G, Roth N, Hopf NB, Broillet M-C, Staedler D. Exposure to New Emerging Bisphenols Among Young Children in Switzerland. International Journal of Environmental Research and Public Health. 2020; 17(13):4793. https://doi.org/10.3390/ijerph17134793
Chicago/Turabian StyleLucarini, Fiorella, Tropoja Krasniqi, Gaëlle Bailat Rosset, Nicolas Roth, Nancy B Hopf, Marie-Christine Broillet, and Davide Staedler. 2020. "Exposure to New Emerging Bisphenols Among Young Children in Switzerland" International Journal of Environmental Research and Public Health 17, no. 13: 4793. https://doi.org/10.3390/ijerph17134793
APA StyleLucarini, F., Krasniqi, T., Bailat Rosset, G., Roth, N., Hopf, N. B., Broillet, M.-C., & Staedler, D. (2020). Exposure to New Emerging Bisphenols Among Young Children in Switzerland. International Journal of Environmental Research and Public Health, 17(13), 4793. https://doi.org/10.3390/ijerph17134793





