1. Introduction
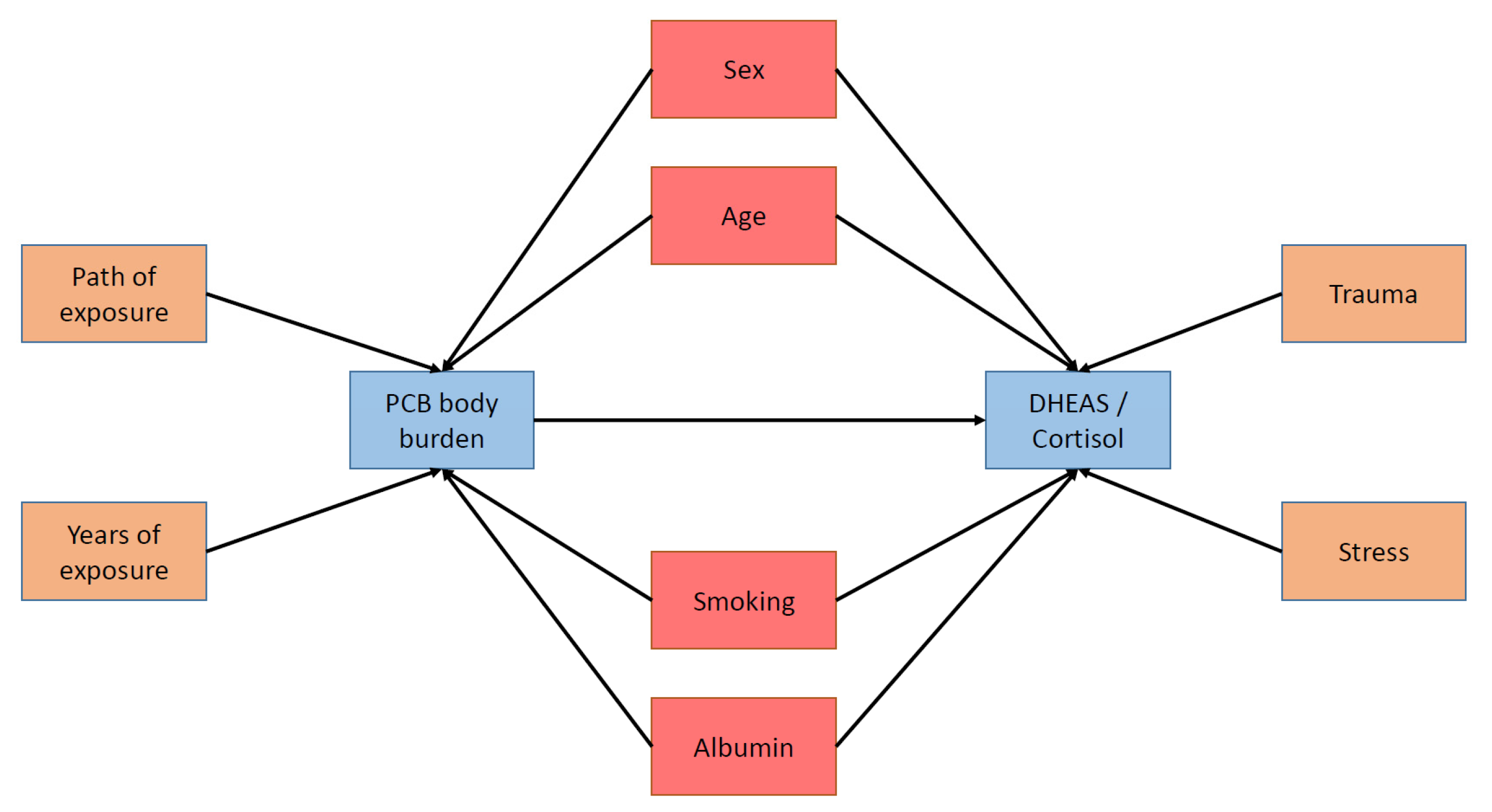
Polychlorinated biphenyls (PCBs) are a group of synthetically produced hazardous chemicals, whose negative consequences on human health and the environment have become visible decades after their intensive production and use [
1]. The present study analyzes potential adverse health effects of PCBs on the individuals’ stress response system and focuses on the influence of PCBs on human stress hormones. Prior studies mostly report positive associations between PCBs and dehydroepiandrosterone sulfate (DHEAS) in humans, but no or inconsistent findings with cortisol [
2]. This longitudinal study addresses both, DHEAS and cortisol to find causal relations.
Due to their adverse health impact, in 1995, the manufacture, import, export and sale of PCBs and products and equipment containing PCBs have been banned worldwide by the UNEP (United Nations Environment Programme) [
3]. In Germany, the use of PCBs was already banned in 1989. Prior to the ban, these toxic chlorine compounds were widely used in electrical capacitors and transformers, as well as in hydraulic fluids, lubricants, pesticides and plasticizers [
4]. Of the 209 theoretically possible PCB congeners, 130 have been detected in the environment. The PCB congeners, which all have the same basic structure, differ from each other in the number and position of chlorine substituents [
5]. Based on their degree of chlorination (lower vs. higher chlorination) and their structural properties (non-dioxin-like vs. dioxin-like), PCB congeners can be classified into three categories: lower chlorinated PCBs (LPCBs; ≤ 5 chlorine atoms), higher chlorinated PCBs (HPCBs; >five chlorine atoms), and dioxin-like PCBs (dlPCBs) that include lower- as well as higher-chlorinated congeners but with no or only one chlorine atom in the ortho positions. Furthermore, this study also addresses a fourth group, the hydroxylated PCB metabolites (OH-PCBs). Due to the high persistence and bioaccumulative properties of the parent-PCB-congeners, PCBs remain in the environment and are still detectable in the blood of the general population [
6]. According to Carpenter, the majority of the population in developed countries is exposed to PCBs through food intake (i.e., HPCBs) [
7]. Furthermore, the place of living affects the exposure with PCBs, particularly in homes near hazardous waste sites [
7]. Work-related contact with PCB-contaminated material represents an additional exposure in the form of inhalation as well as cutaneous PCB intake (i.e., LPCBs and dlPCBs with lower degree of chlorination) [
8]. Past research has also described differential health effects for the different types of PCBs and OH-PCBs [
7,
9].
PCBs are attributed to reproductive toxicity [
10], immunotoxicity [
11] and neurotoxic effects [
12]. Moreover, hormonal changes in humans after PCB exposure were found in prior studies, for instance with thyroid hormones [
13] or the sex hormone free testosterone [
14]. In addition, a few studies focused on stress hormones in humans after PCB exposure, but these studies report inconsistent and sometimes contradictory results. For instance, Persky et al. found an inverse association between increased PCB body burden and the DHEAS concentration after the occupational PCB exposure of female workers during menopause [
15]. However, a subsequent study found a positive association between PCB exposure and the DHEAS concentration in male employees [
2]. Sun et al. also report a significant increase in DHEA concentration with increased PCB body burden in men [
16]. High DHEAS inhibits genetically programmed cell death [
17], and this enhances the risk for cancer. PCBs were classified as human carcinogens by the IARC (International Agency for Research on Cancer) in 2013 [
18]. Related to cortisol, no significant results for the effect of PCBs have been found in humans so far [
2,
15]. Possible reasons of these inconsistent findings may be the cross-sectional design of prior studies. PCBs may have different effects on human hormones depending on how long ago the exposure occurred. For example, in an earlier longitudinal study we found an interaction with time for the association between PCBs and free thyroxine [
13]. Cross-sectional study designs cannot detect changes in associations over time and report only a snapshot, which can result in inconsistent findings in various articles.
The aim of this study is therefore to enable a causal interpretation of the relationship between PCB body burden and the stress hormone concentration by conducting a longitudinal study with a repeated assessment of both PCBs and stress hormones. According to the above-mentioned studies, a positive association between PCB body burden and the DHEAS concentration is postulated. Furthermore, the association between the PCB body burden and the serum cortisol concentration is tested. This study consists of three parts to test the postulated hypothesis and the research question. In the first part, we compare categorized and mean stress hormone concentrations of higher exposed participants with those participants showing a PCB concentration at the level of background burden. We hypothesize that the higher exposed participants show a higher probability for DHEAS concentrations above the reference range (Hypothesis 1a). Research question 1a considers differences between higher and background-burdened participants according to abnormal cortisol concentrations above or under the reference range. We further expect a higher mean DHEAS concentration for the higher PCB-burdened participants (hypothesis 1b) and we investigate mean differences in cortisol (research question 1b). In the second part, for a better comparability with prior cross-sectional studies, the linear cross-sectional effects of PCB exposure on stress hormone concentrations are analyzed. Again, we expect a positive association between PCB body burden and DHEAS concentration (hypothesis 2). Furthermore, the association between PCBs and cortisol will be tested (research question 2). The third part analyzes the linear relation between PCB body burden and the stress hormone concentrations longitudinally while controlling for the different sampling time points. Hypothesis 3 postulates a positive association between PCBs and DHEAS continuously over all three sampling time points. Research question 3 focusses on the longitudinal associations between PCBs and cortisol.
3. Results
The correlations between all types of PCBs and the relevant outcome variables at each sampling time point are presented in
Table 3. There are positive correlations between LPCB and DHEAS at all sampling time points and between dlPCBs and OH-PCBs with DHEAS at t2. Between PCBs and cortisol there were no significant correlations. The correlations between the several PCB congeners with the relevant outcome variables are presented in the supplementary table,
Table A1.
In the first part of this study, participants with a higher PCB burden were compared with background-burdened participants. It was tested whether participants with higher a PCB burden had a higher probability for elevated DHEAS concentrations than background burdened (hypothesis 1a) and whether there are differences in the probability for elevated or reduced cortisol concentrations (research question 1a). Participants with higher exposure in LPCBs and dlPCBs had a higher risk of elevated DHEAS concentrations compared to the background-burdened participants, but only at t2 (
Table 4). With regard to cortisol, there was no higher risk of abnormal values, neither elevated nor reduced concentrations, in the higher PCB-exposed group compared to the background-burdened group (
Table 4). The comparison of the mean concentrations of DHEAS (hypothesis 1b) and cortisol (research question 1b) revealed that participants with higher LPCB exposure had a significantly higher mean DHEAS concentration than participants with a normal LPCB body burden at t2 and t3 (
Table 5). Furthermore, participants with a higher body burden of dlPCBs showed significant higher DHEAS concentrations at t2. Regarding the cortisol concentration, no significant mean differences between the higher and background-burdened participants can be found in all three sampling time points. According to these results, hypotheses 1a and 1b can be partially confirmed, but research questions 1a and 1b could not be answered.
The second part focused on the linear association between the PCB body burden and the concentrations of DHEAS (hypothesis 2) and cortisol (research question 2) for each sampling time point. These cross-sectional analyses showed one significant positive correlation between LPCB body burden and the DHEAS concentration for t2 (
Table 6). No significant correlations were found for HPCBs, dlPCBs and OH-PCBs and for all other sampling time points. The cortisol concentration was not significantly associated with any type of PCB body burden. The results partially support the postulated hypothesis and the research question could not be answered.
In the last part, the linear association between PCB body burden with DHEAS (hypothesis 3) and cortisol concentration (research question 3) were tested over time controlled for the influence of the sampling time point. The results of the mixed effect models confirmed the correlation between PCB body burden and the DHEAS concentration for LPCBs (
Table 7). For HPCBs, dlPCBs and OH-PCBs no significant correlations were found with DHEAS. As in the analyses before, no association could be found between any type of PCB and cortisol. The postulated hypotheses, again, were partially confirmed for DHEAS, but the research question according to cortisol could also not be answered under control for the sampling time points.
4. Discussion
The aim of this study was to investigate the effects of PCBs on the stress hormones DHEAS and cortisol. According to the literature, a positive association between PCB body burden and DHEAS concentration was postulated. Furthermore, undirected research questions were formulated for the associations between PCBs and cortisol because of prior inconsistent findings. To test the postulated hypotheses and research questions, this study was structured in three parts.
Higher-burdened participants in LPCBs and dlPCBs have an approximately two- and three-fold higher risk for elevated DHEAS concentrations compared to background-burdened participants. The mean DHEAS concentration was also higher in the higher exposed group, but only for LPCBs and dlPCBs. However, the findings concerning the mean differences must be interpreted with caution, because there was a variance inhomogeneity, which could have had an impact on the effect size. According to cortisol, no differences in risk or mean concentrations were found between the higher- and background-burdened group. The linear association between PCB exposure and stress hormone concentration was examined for each sampling time point. As in the first part, PCB exposure only affected the DHEAS concentration, but not the cortisol concentration. At t2 an increase of the DHEAS concentration could be observed with an increase of LPCB body burden. The same positive association between LPCB exposure and the DHEAS concentration was found in the third part when controlling for the sampling time points. As before, there was also no effect related to cortisol in the longitudinal analyses. The results of this study confirmed previous studies that also found a positive association between PCB exposure and DHEAS concentration, but none between PCB exposure and cortisol concentration [
2,
15,
16].
We found the clearest effects for LPCBs and DHEAS. Interestingly, LPCBs have a shorter half-life than HPCBs and dlPCBs, which is the reason why they have often been reported to cause less damage to health [
35]. However, studies of work-related exposures show increased interest in LPCBs [
36]. The relevance of LPCBs for work-related exposure results from the non-food-related intake of PCBs (i.e., dermal or inhalative), which is mainly the path of exposure of LPCBs, and from the exposure material itself. Many PCB mixtures commercially used in Germany, such as Clophen A30, Clophen A40 or Aroclor 1242, contained high proportions of LPCBs [
37]. Our study cohort consists of former workers of a recycling company with an occupational PCB exposure. In this study, LPCBs consist of congeners with less than six chlorine atoms. It may be that the degree of chlorination is important for stress-hormone-related health effects. Additional analyses in this study support this. As can be seen in
Table A2 (
Appendix D) in the supplement, there are positive associations of all considered PCB congeners with less than six chlorine atoms and DHEAS. According to these associations, it can be concluded that the degree of chlorination may be important in the elevation of DHEAS levels after PCB exposure. This may also be an explanation for the inconsistent findings about the associations of PCB exposure and DHEAS in research. Many studies use different types of PCBs for testing the effects of PCBs on stress hormones and thus result in inconsistent findings, as different PCB congeners might have different effects on stress hormones.
As reported before, in this study, PCBs show an effect on plasma DHEAS concentration, but not on cortisol concentration. This result is in line with prior research, but the mechanism is not clearly described. Both steroid hormones are synthesized and released in the adrenal cortex. However, they are produced within the adrenal cortex in two different regions, cortisol in the zona fasciculata and DHEA as well as DHEAS in the zona reticularis [
38]. The difference of origin concerning the morphological zones could be a possible explanation that only DHEAS is affected by PCBs and not cortisol. It might be that PCBs only affect the zona reticularis in which DHEAS is produced.
The health consequences of an elevated DHEAS concentration after PCB exposure are difficult to predict due to the not well-known physiological mechanisms and pathological effects of DHEAS abnormalities [
17]. However, DHEAS, as an antagonist of cortisol, is mostly attributed with positive effects, such as the improvement of immune function or the stimulation of muscle and bone formation [
39]. Maninger et al. described that DHEAS inhibits genetically programmed cell death (apoptosis), which is necessary for subsequent cell proliferation [
17]. However, if apoptosis does not occur after a genotoxic lesion, in vitro studies have shown a growth advantage for tumor-promoting and toxicologically influenced cells [
40]. A relevant effect of DHEAS might be its apoptosis-inhibiting property. Higher DHEAS concentrations after PCB exposure may be a mechanism for the development of tumors and cancer after PCB exposure [
18]. The results of this study give first hints of such an underlying mechanism and recommend further investigation of DHEAS and the general physiological mechanisms in the case of PCB-burdened cells focused on tumor development. Next to somatic consequences in case of higher DHEAS concentrations, mental health problems can also occur. Uh et al. found a non-linear correlation between the DHEAS concentration and the occurrence of depression of varying severity [
41]. Furthermore, Lee et al. considered DHEAS primarily as a biomarker for manic symptoms and cognitive performance [
42]. Future research should also investigate the role of elevated DHEAS as a pathophysiological mechanism for depression after PCB exposure [
43].
A particular strength of this work is its longitudinal design. This allows a causal interpretation of the results and strengthens the described associations. Furthermore, mixed effect models were used to maximize statistical power and to reduce potentially distorting, inter-individual changes by considering random effects of the sampling time points. Because of the male study cohort, the generalizability of the study results are limited in terms of their applicability to PCB-exposed women. However, by using a male study cohort, biases according to gender-differences in stress hormone concentrations were reduced. Gender does influence the amount of PCB burden as well as the concentrations of DHEAS and cortisol [
44,
45]. The sample of 112 persons represents a well-founded size considering the fact of a longitudinal design and the strict selection criteria used, such as occupational PCB exposure and the exclusion of participants taking cortisol effective drugs. In addition, the use of mixed effect models result in a higher statistical power that reduces biases of the sample size.