Development of Novel Method for Immobilizing TMAH-Degrading Microbe into Pellet and Characterization Tool, for Verifying Its Robustness in Electronics Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Enrichment of TMAH Degrading Bacteria
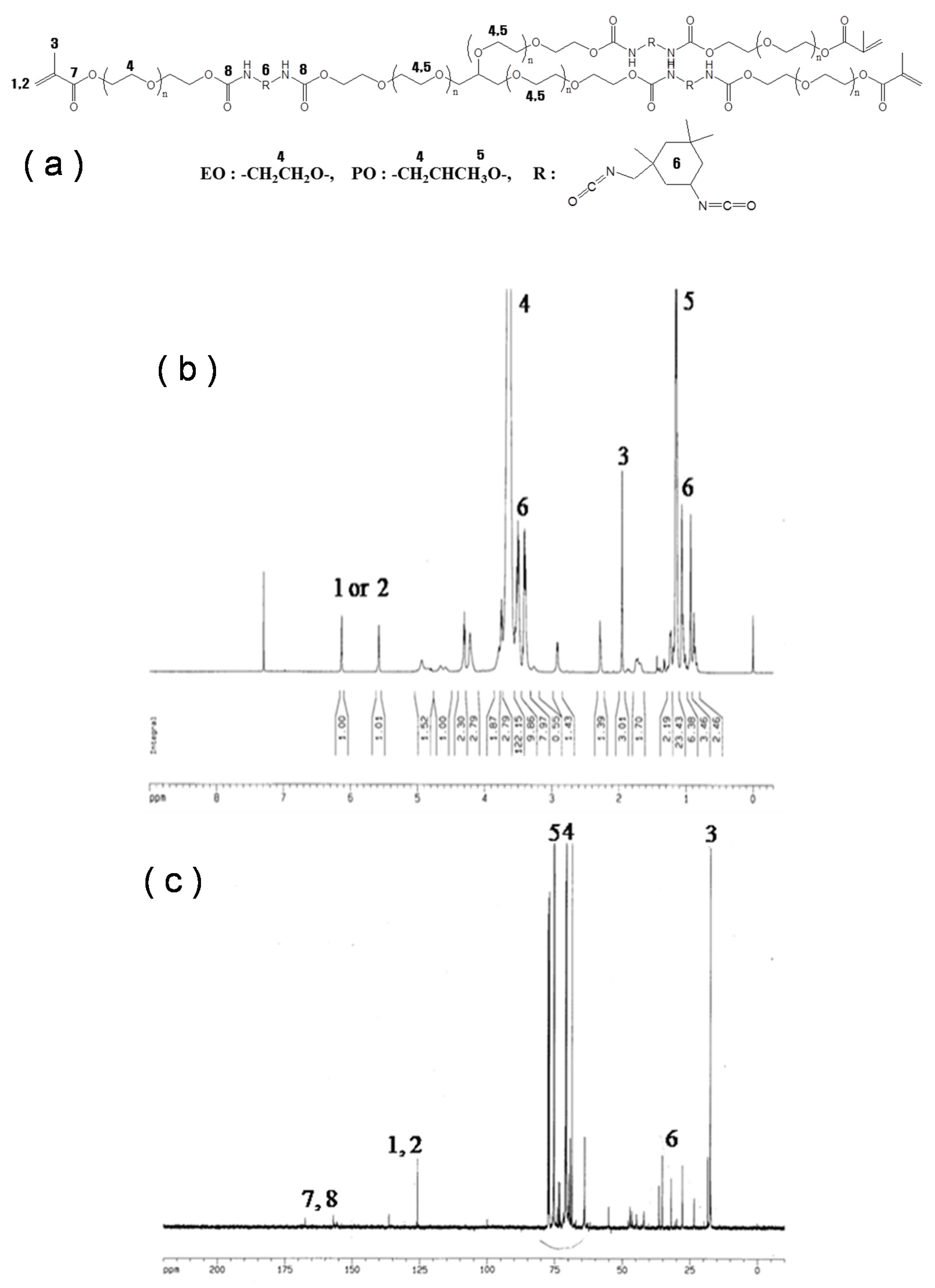
2.2. Polymerization Methods
2.3. Characterization of Pellets without Microorganism
2.4. Evaluation of Microorganism Immobilized Pellets
3. Results and Discussions
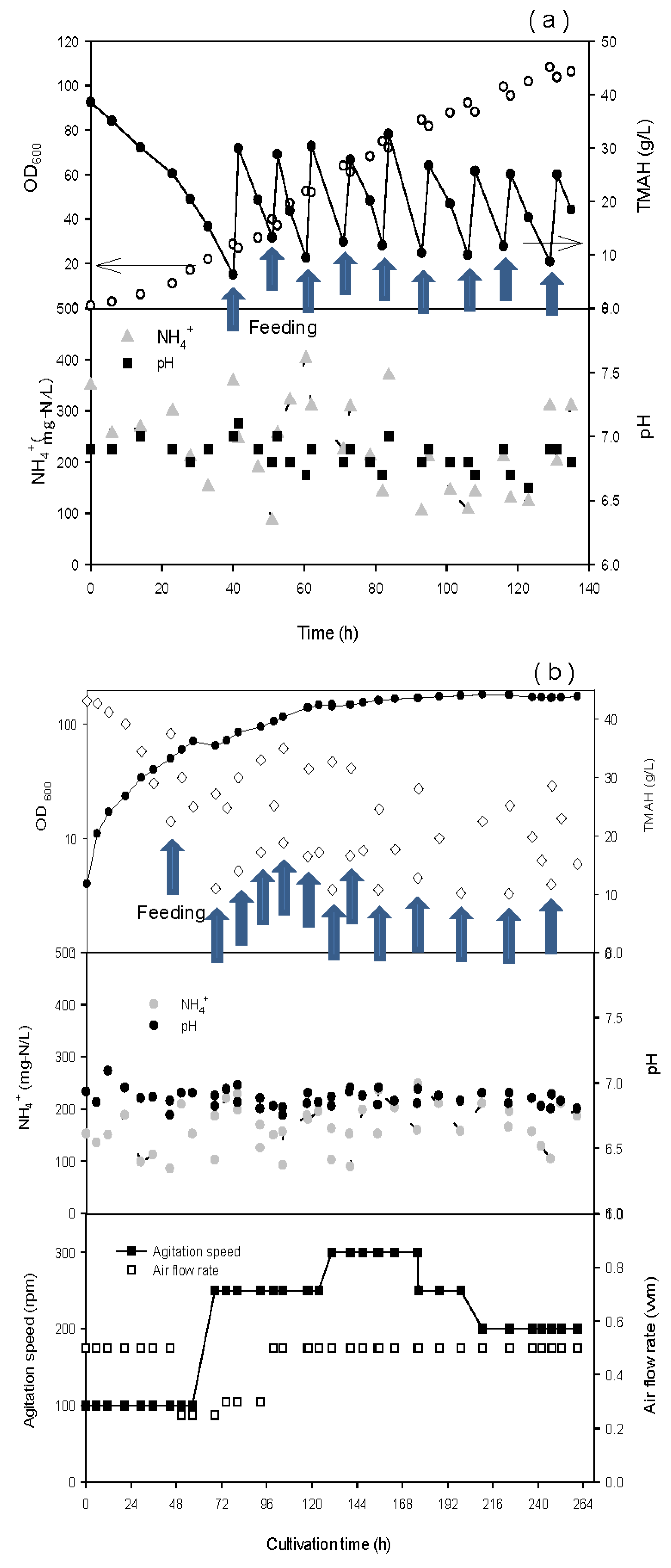
3.1. Enrichment of TMAH Degrading Bacteria
3.2. Preparation Conditions of Pellets
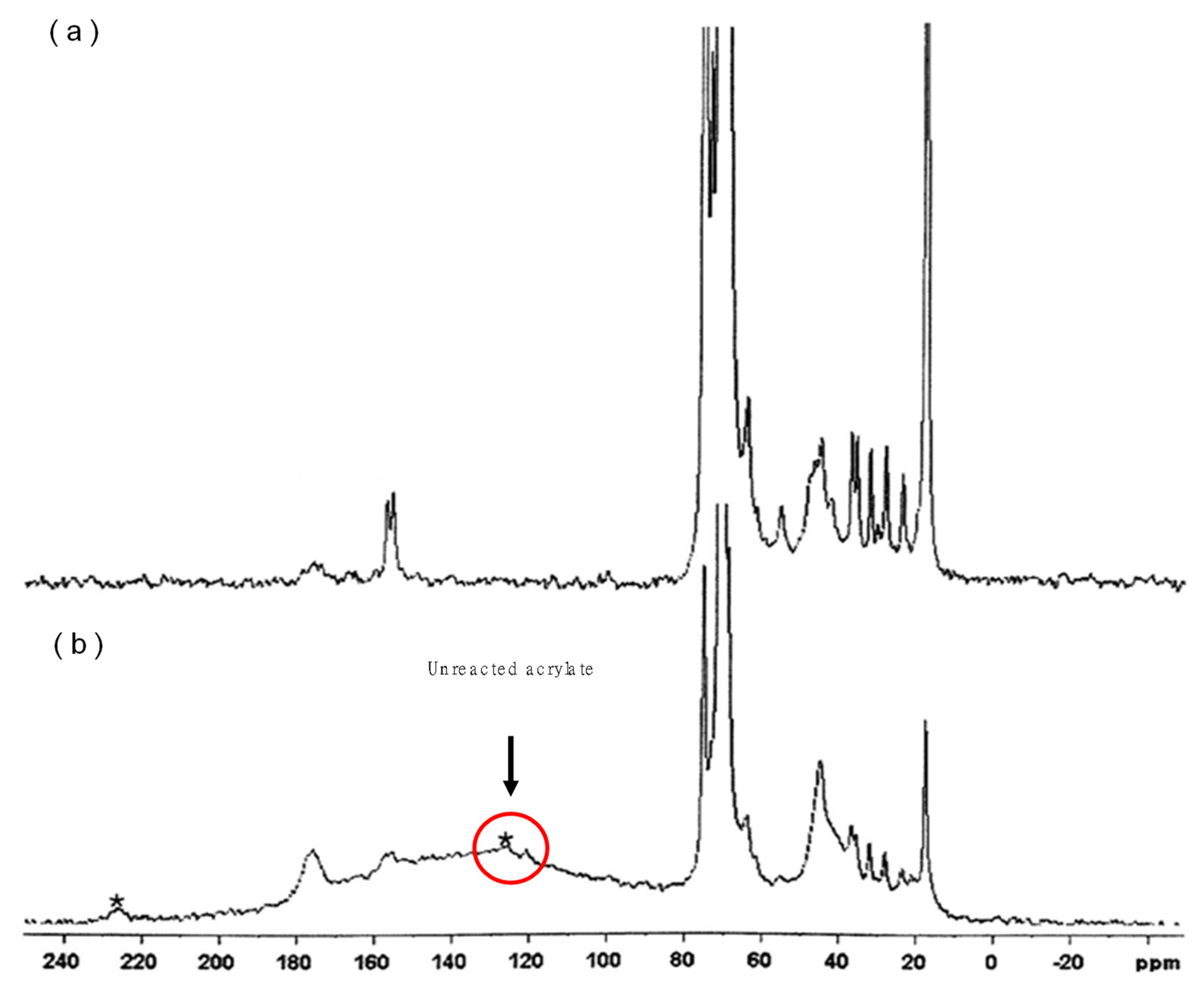
3.3. Characterization of Pellets
3.4. Characteristics of Wastewater
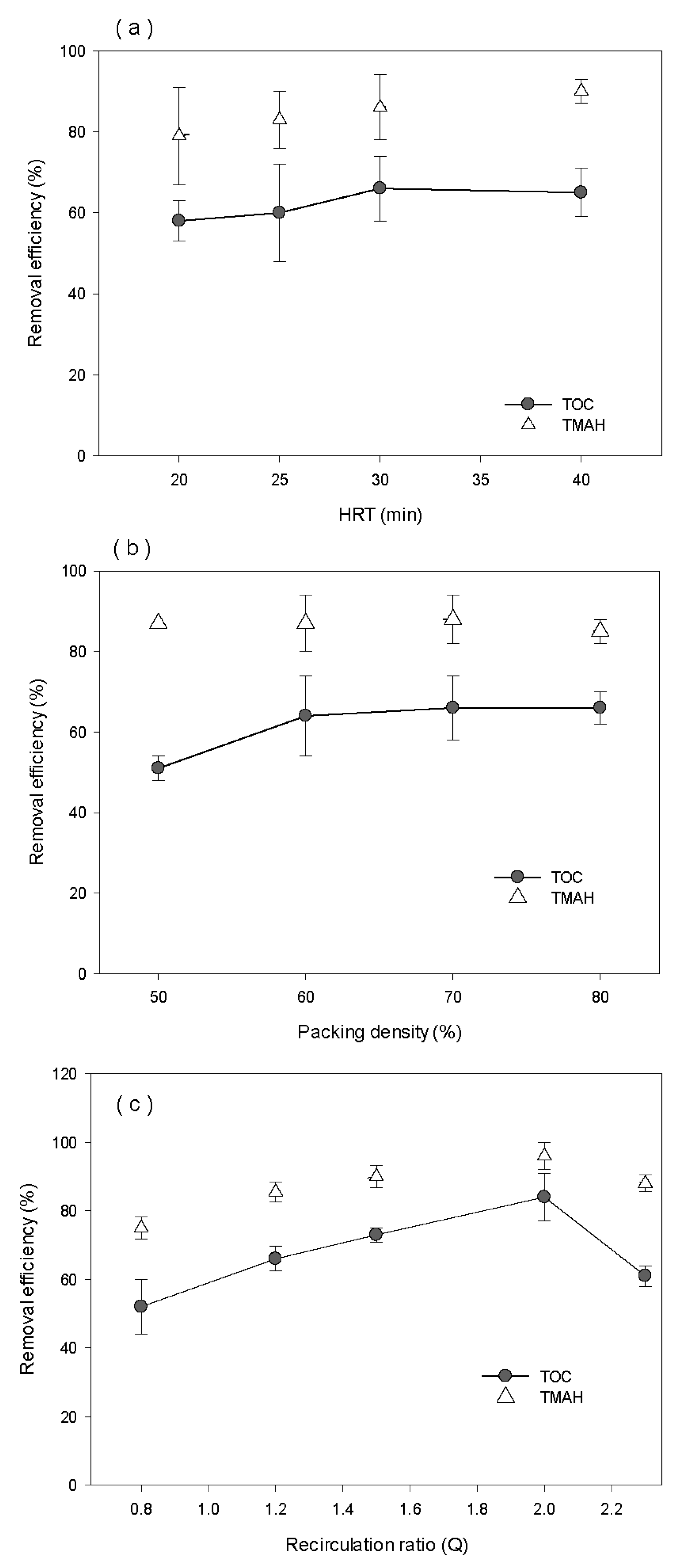
3.5. Optimization of Inoculation Method and Operating Conditions
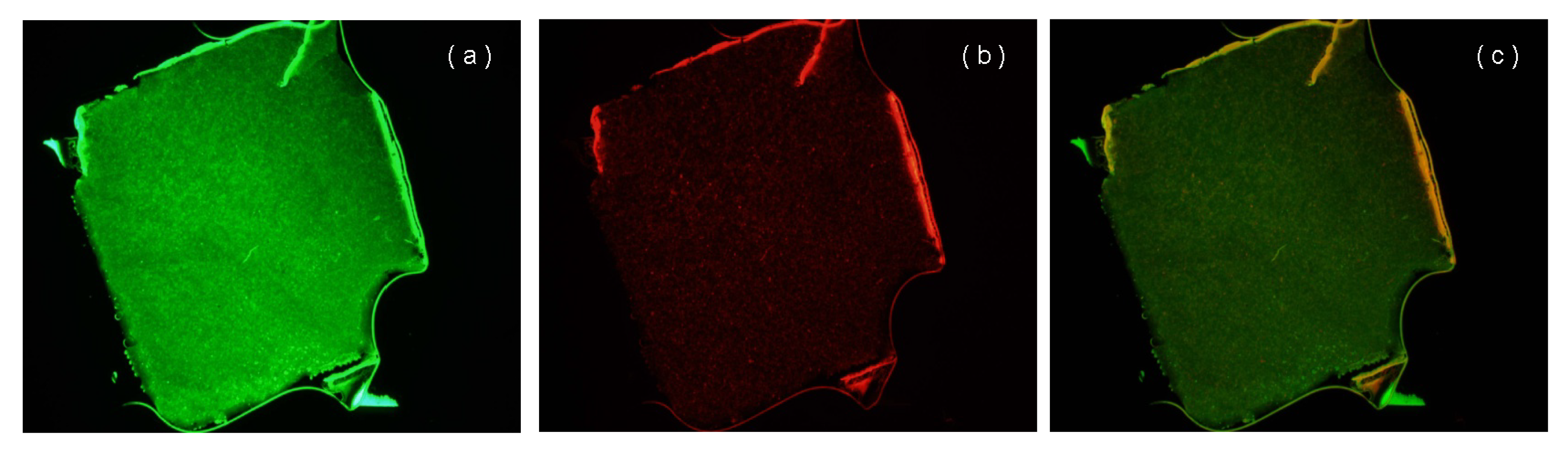
3.6. Viability of Inoculated Pellets
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, J.; Chung, J. Evaluation of potential for reuse of industrial wastewater using metal-immobilized catalysts and reverse osmosis. Chemosphere 2015, 125, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, J.; Chung, J. Removal of isopropyl alcohol and methanol in ultrapure water production system using a 185 nm ultraviolet and ion exchange system. Chemosphere 2016, 156, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Choi, W.Y.; Lee, Y.W. Removal of isopropyl alcohol (IPA) using anodized photocatalytic metal membrane reactor. Mater. Sci. Forum 2012, 724, 25–28. [Google Scholar] [CrossRef]
- Chang, K.F.; Yang, S.Y.; You, H.S.; Pan, J.R. Anaerobic treatment of tetra-methyl ammonium hydroxide (TMAH) containing wastewater, semiconductor manufacturing. IEEE Trans. Semicond. Manuf. 2008, 21, 486–491. [Google Scholar] [CrossRef]
- Hu, T.H.; Whang, L.M.; Liu, P.W.G.; Hung, Y.C.; Chen, H.W.; Lin, L.B.; Chen, C.F.; Chen, S.K.; Hsu, S.F.; Shen, W.; et al. Biological treatment of TMAH (tetramethyl ammonium hydroxide) in a full-scale TFT-LCD wastewater treatment plant. Bioresour. Technol. 2012, 113, 303–310. [Google Scholar] [CrossRef]
- Sumino, T.; Nakamura, H.; Mori, N. Immobilization of activated sludge by the acrylamide method. J. Ferment. Technol. 1991, 72, 141–143. [Google Scholar] [CrossRef]
- Sumino, T.; Nakamura, H.; Mori, N.; Kawaguchi, Y. Immobilization of nitrifying bacteria by polyethylene glycol prepolymer. J. Ferment. Technol. 1992, 73, 37–42. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Tu, B. Immobilization of ammonia-oxidizing bacteria by polyvinyl alcohol and sodium alginate. Braz. J. Microbiol. 2017, 48, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, C.; Tramper, J.; Luyben, K.; Klapwijk, A. Characterization of Nitrosomonas europae immobilized in calcium alginate. Enzym. Microb. Technol. 1983, 5, 297–303. [Google Scholar] [CrossRef]
- Hashimoto, N.; Sumino, T. Wastewater treatment using activated sludge entrapped in polyethylene glycol prepolymer. J. Ferment. Technol. 1998, 86, 424–426. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, Y.W.; Chung, J.; Lee, J.K.; Lee, J.Y.; Yu, Y.; Jahng, D.; Cha, Y. Reuse of low concentrated electronic wastewater using selected microbe immobilised cell system. Water Sci. Technol. 2008, 57, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, A.K.; Bhanu, S. Immobilization of α–amylase in vinyl-polymer-based interpenertrating polymer networks. Colloid Polym. Sci. 2003, 282, 76–83. [Google Scholar] [CrossRef]
- Zhou, L.; Li, G.; An, T.; Fu, J.; Sheng, G. recent patents on immobilized microorganism technology and its engineering application in wastewater treatment. Recent Pat. Eng. 2008, 2, 28–35. [Google Scholar]
- Kim, S.K.; Kong, I.; Lee, B.H.; Kang, L.; Lee, M.G.; Suh, K.H. Removal of ammonium-N from a recirculation aquacultural system using an immobilized nitrifier. Aquacult. Eng. 2000, 21, 139–150. [Google Scholar] [CrossRef]
- Singj, L.; Siddiqui, M.F.; Ahmad, A.; Rahim, M.H.A.; Sakinah, M.; Wahid, Z.A. Application of polyethylene glycol immobilized Clostridium sp. LS2 for continuous hydrogen production from palm oil mill effluent in upflow anaerobic sludge blanket reactor. Biochem. Eng. J. 2013, 70, 158–165. [Google Scholar] [CrossRef]
- Yang, B.; Oh, H.; Lee, Y.; Jung, J.; Jahng, D. Low-strength electronic wastewater treatment using immobilized cells of TMAH-degrading bacterium followed by activated carbon adsorption. Desalin. Water Treat. 2015, 54, 3639–3645. [Google Scholar] [CrossRef]
- Chung, J.; Choi, J.; Chung, S. Pilot study of specific microbe immobilization cells (SMICs) technology in removing tetramethyl ammonium hydroxide for reuse of low-strength electronics wastewater. J. Hazard. Mater. 2020, 384, 120829. [Google Scholar] [CrossRef]
- Matsubara, H.; Yoshida, A.; Ohtani, H.; Tsuge, S. Compositional analysis of UV-cured acrylic ester resins by pyrolysis-gas chromatography in the presence of organic alkali. J. Anal. Appl. Pyrolysis 2002, 64, 159–175. [Google Scholar] [CrossRef]
- Elshereksi, N.W.; Mohamed, S.H.; Arifin, A.; Ishak, Z.A.M. Thermal characterisation of poly(methyl methacrylate) filled with barium titanate as denture base material. J. Phys. Sci. 2014, 25, 15–27. [Google Scholar]
- Qiao, X.; Liu, Z.; Liu, Z.; Zeng, Y.; Zhang, Z. Optimized immobilization of activated carbon sludge in poly(ethylene glycol) gels by UV technology. Process Biochem. 2010, 45, 1342–1347. [Google Scholar] [CrossRef]
- Virta, M.; Lineri, S.; Kankaanpää, P.; Karp, M.; Peltonen, K.; Nuutila, J.; Lilius, E.M. Determination of complement-mediated killing of bacteria by viability staining and bioluminescence. Appl. Environ. Microbiol. 1998, 64, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Jepras, T.I.; Carter, J.; Pearson, S.C.; Paul, F.E.; Wilkinson, M.J. Development of a robust flow cytometry assay for determining numbers of viable bacteria. Appl. Environ. Microbiol. 1995, 61, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.J.; Allman, R.; Sark, J.M.; Lloyd, D. Rapid estimation of antibiotic susceptibility with flow cytometry. J. Microsc. 1994, 176, 8–16. [Google Scholar] [CrossRef] [PubMed]
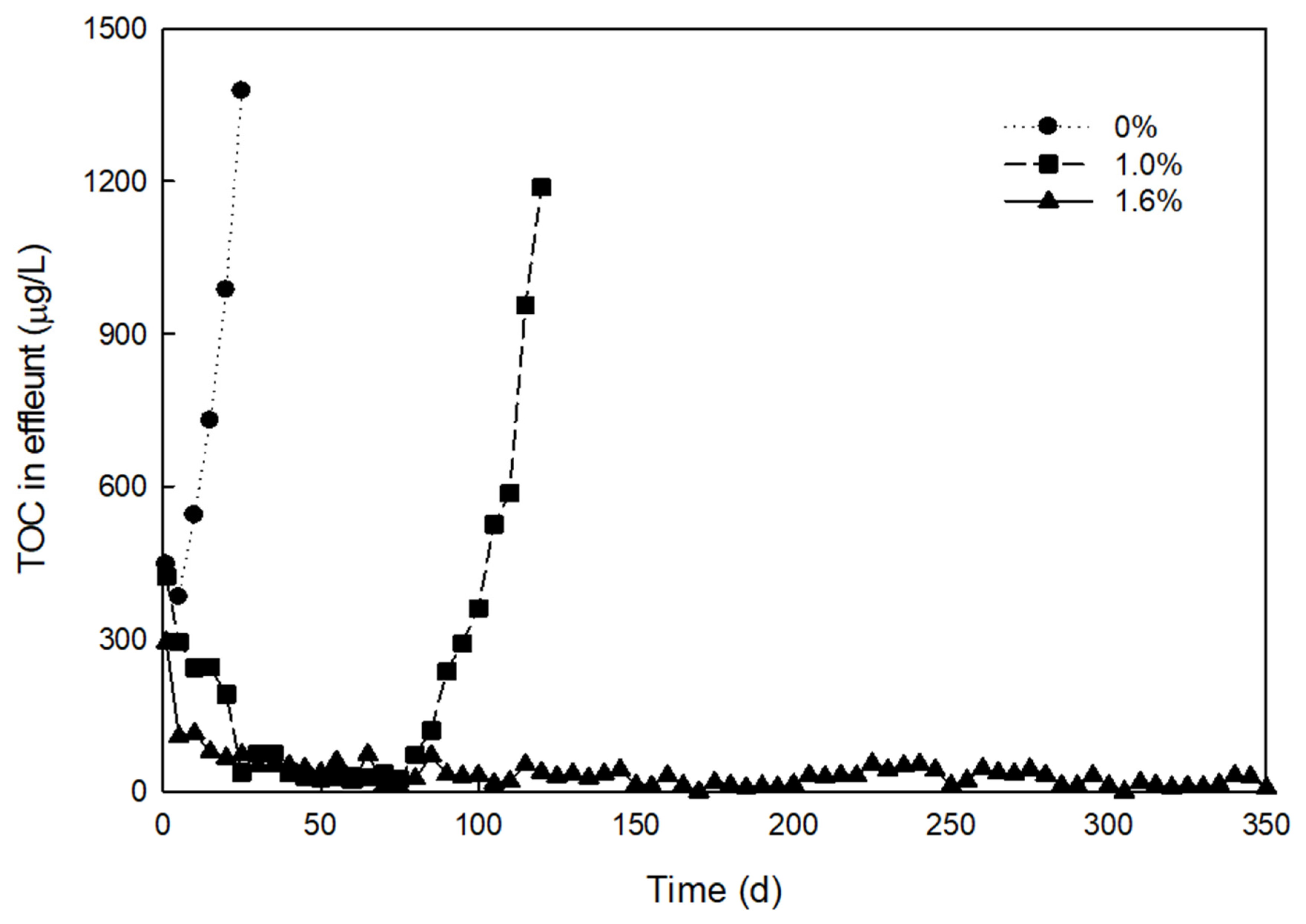
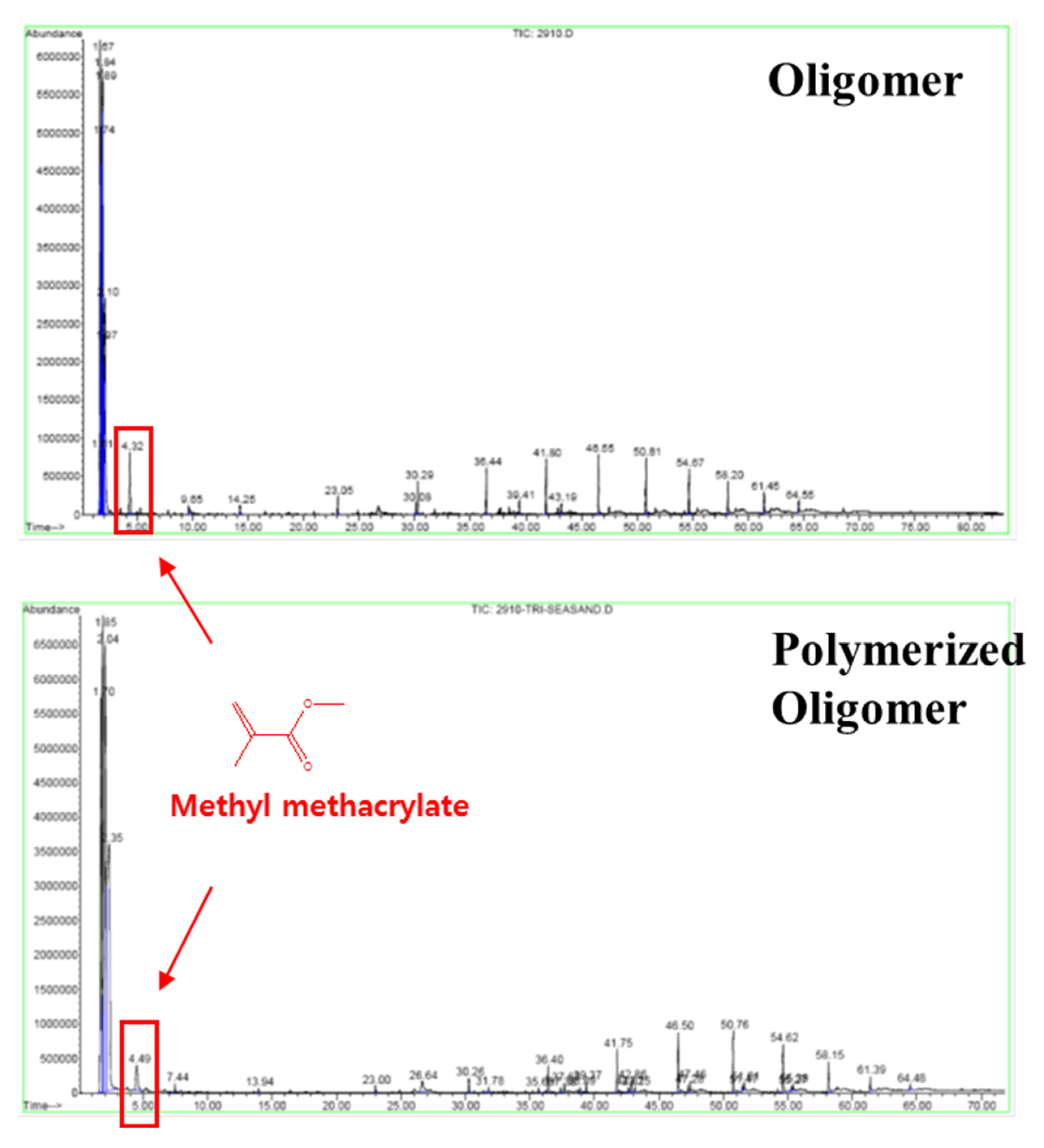
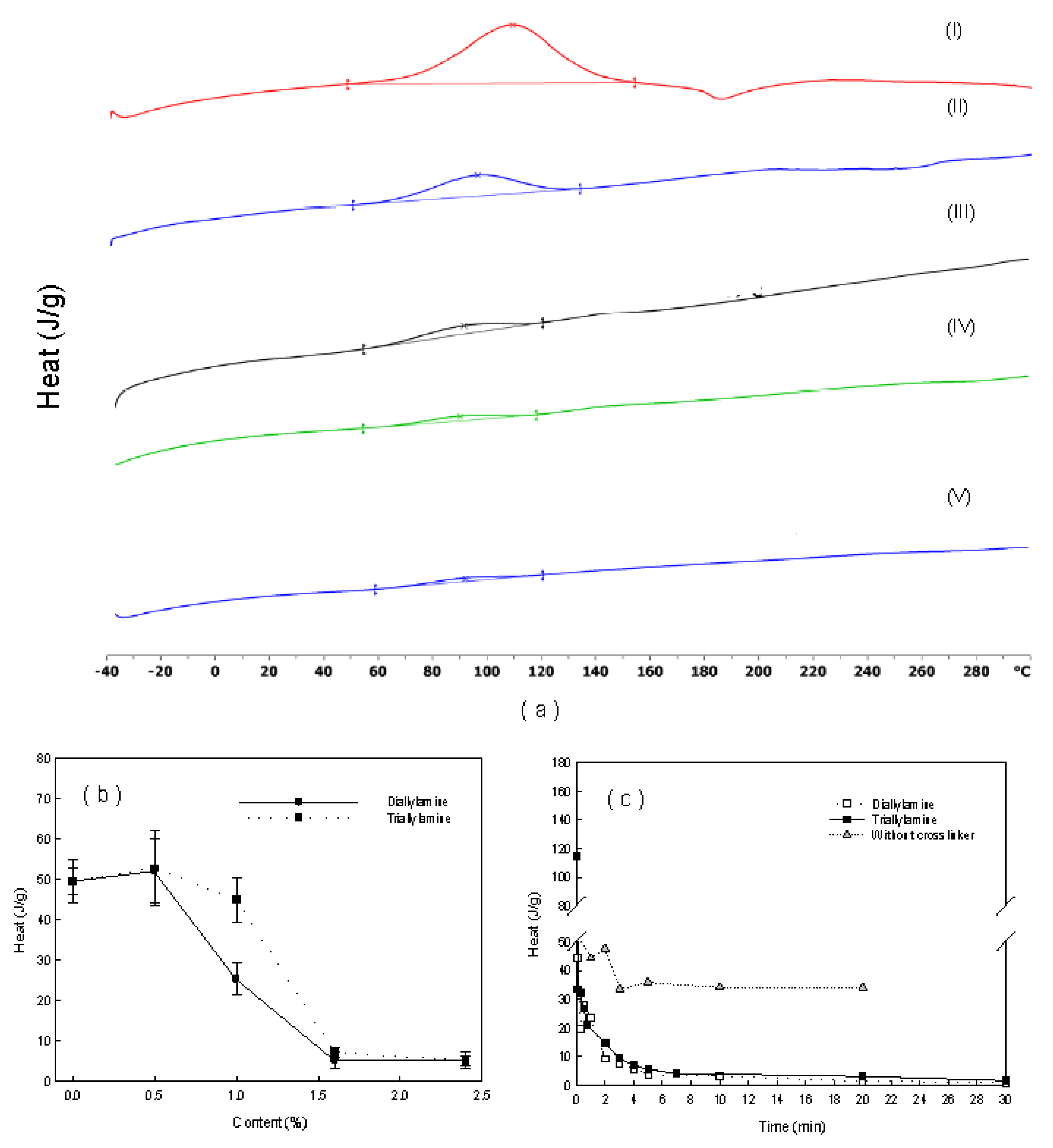
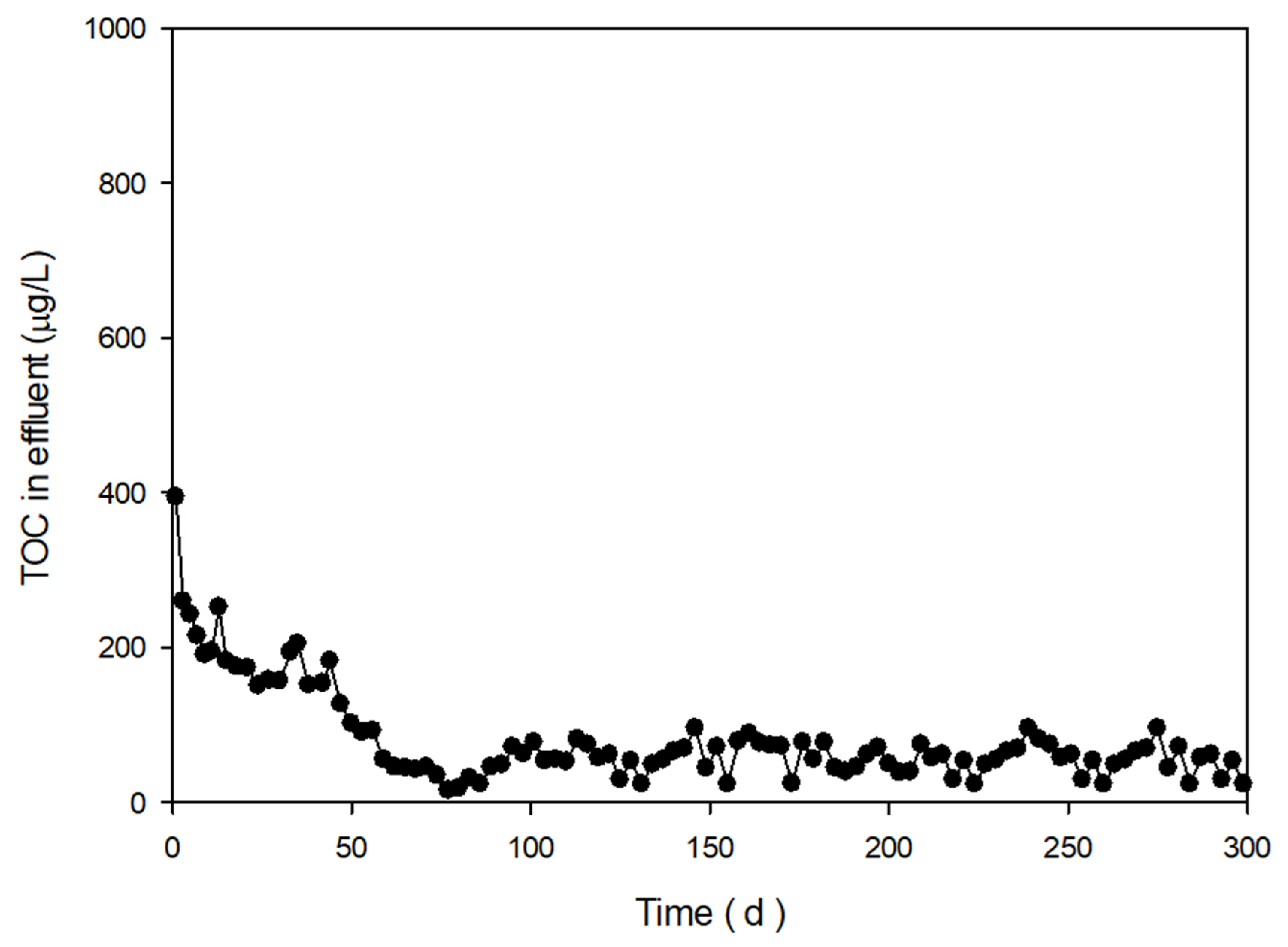
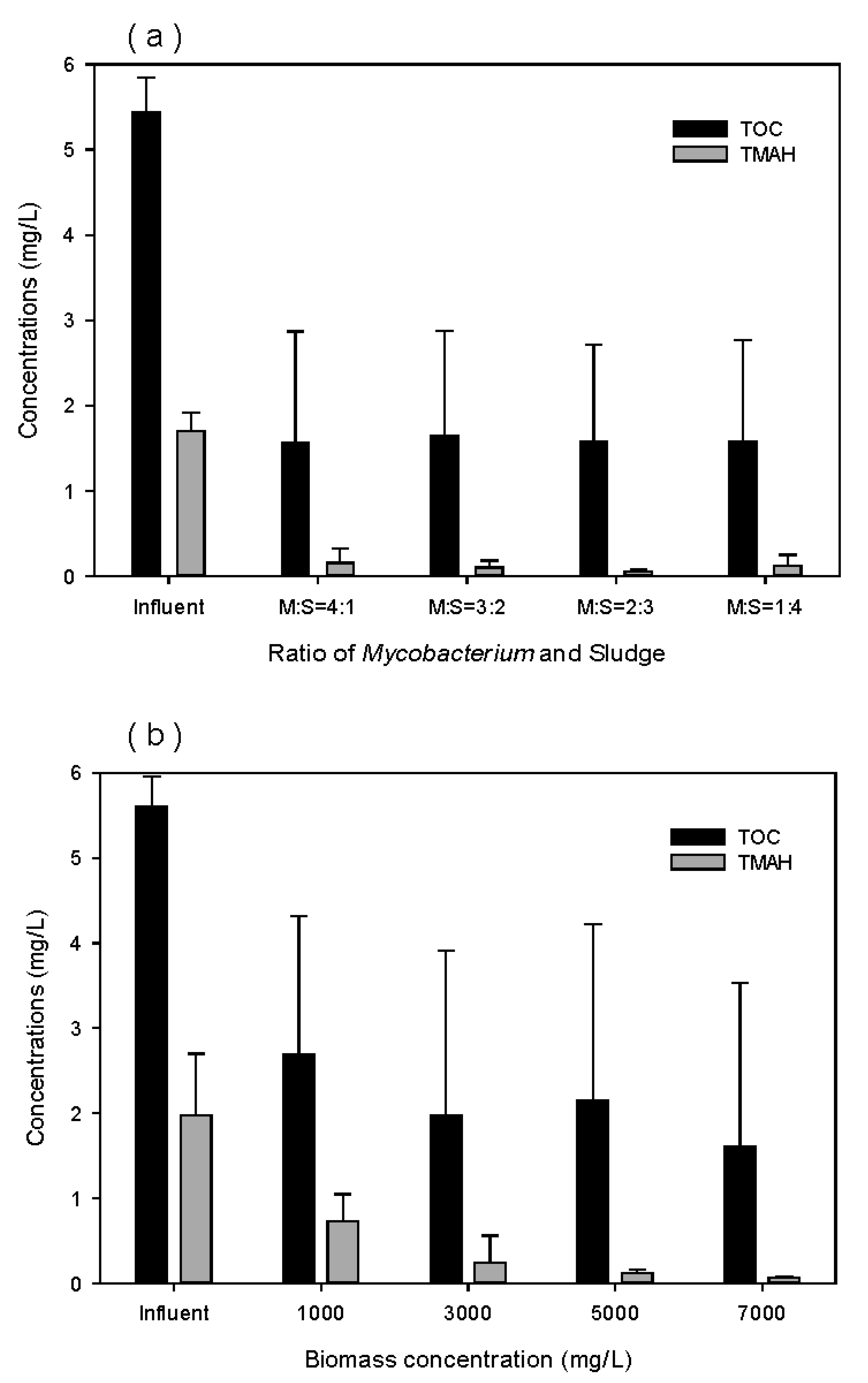
| No. | Type of Prepolymer | Type of Crosslinker | Dose of Crosslinker (wt%) | Dose of Initiator (mg/L) | a Breakthrough Time (days) |
|---|---|---|---|---|---|
| 1 | Polyethylene glycol (PEG) monomer | b DAA | 1.0 | 10 | 7 |
| 2 | PEG monomer | DAA | 1.6 | 10 | 13 |
| 3 | PEG monomer | DAA | 2.4 | 10 | 28 |
| 4 | PEG monomer | c TAA | 1.0 | 10 | 9 |
| 5 | PEG monomer | TAA | 1.6 | 10 | 19 |
| 6 | PEG monomer | TAA | 2.4 | 10 | 37 |
| 7 | PEG oligomer | DAA | 1.0 | 10 | 50 |
| 8 | PEG oligomer | DAA | 1.6 | 10 | >360 |
| 9 | PEG oligomer | DAA | 2.4 | 10 | >360 |
| 10 | PEG oligomer | TAA | 1.0 | 10 | 70 |
| 11 | PEG oligomer | TAA | 1.6 | 10 | >360 |
| 12 | PEG oligomer | TAA | 2.4 | 10 | >360 |
| pH | 4.84 | TOC | 4.392 mg/L |
| Turbidity | 0.76 NTU | TMAH | 1.998 mg/L |
| T-N | 0.82 mg/L | IPA | 1.620 mg/L |
| T-P | 0.85 mg/L | Ethanol | 0.026 mg/L |
| NH4+-N | 0.05 mg/L | Methanol | 0.590 mg/L |
| COD | 10.58 mg/L | Acetone | 0.022 mg/L |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.; Choi, J.; Chung, J. Development of Novel Method for Immobilizing TMAH-Degrading Microbe into Pellet and Characterization Tool, for Verifying Its Robustness in Electronics Wastewater Treatment. Int. J. Environ. Res. Public Health 2020, 17, 4411. https://doi.org/10.3390/ijerph17124411
Chung S, Choi J, Chung J. Development of Novel Method for Immobilizing TMAH-Degrading Microbe into Pellet and Characterization Tool, for Verifying Its Robustness in Electronics Wastewater Treatment. International Journal of Environmental Research and Public Health. 2020; 17(12):4411. https://doi.org/10.3390/ijerph17124411
Chicago/Turabian StyleChung, Seungjoon, Jeongyun Choi, and Jinwook Chung. 2020. "Development of Novel Method for Immobilizing TMAH-Degrading Microbe into Pellet and Characterization Tool, for Verifying Its Robustness in Electronics Wastewater Treatment" International Journal of Environmental Research and Public Health 17, no. 12: 4411. https://doi.org/10.3390/ijerph17124411
APA StyleChung, S., Choi, J., & Chung, J. (2020). Development of Novel Method for Immobilizing TMAH-Degrading Microbe into Pellet and Characterization Tool, for Verifying Its Robustness in Electronics Wastewater Treatment. International Journal of Environmental Research and Public Health, 17(12), 4411. https://doi.org/10.3390/ijerph17124411





