Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement Site
2.2. Meteorological Conditions
2.3. PTR-MS Sampling Methodology
2.4. Data Analysis
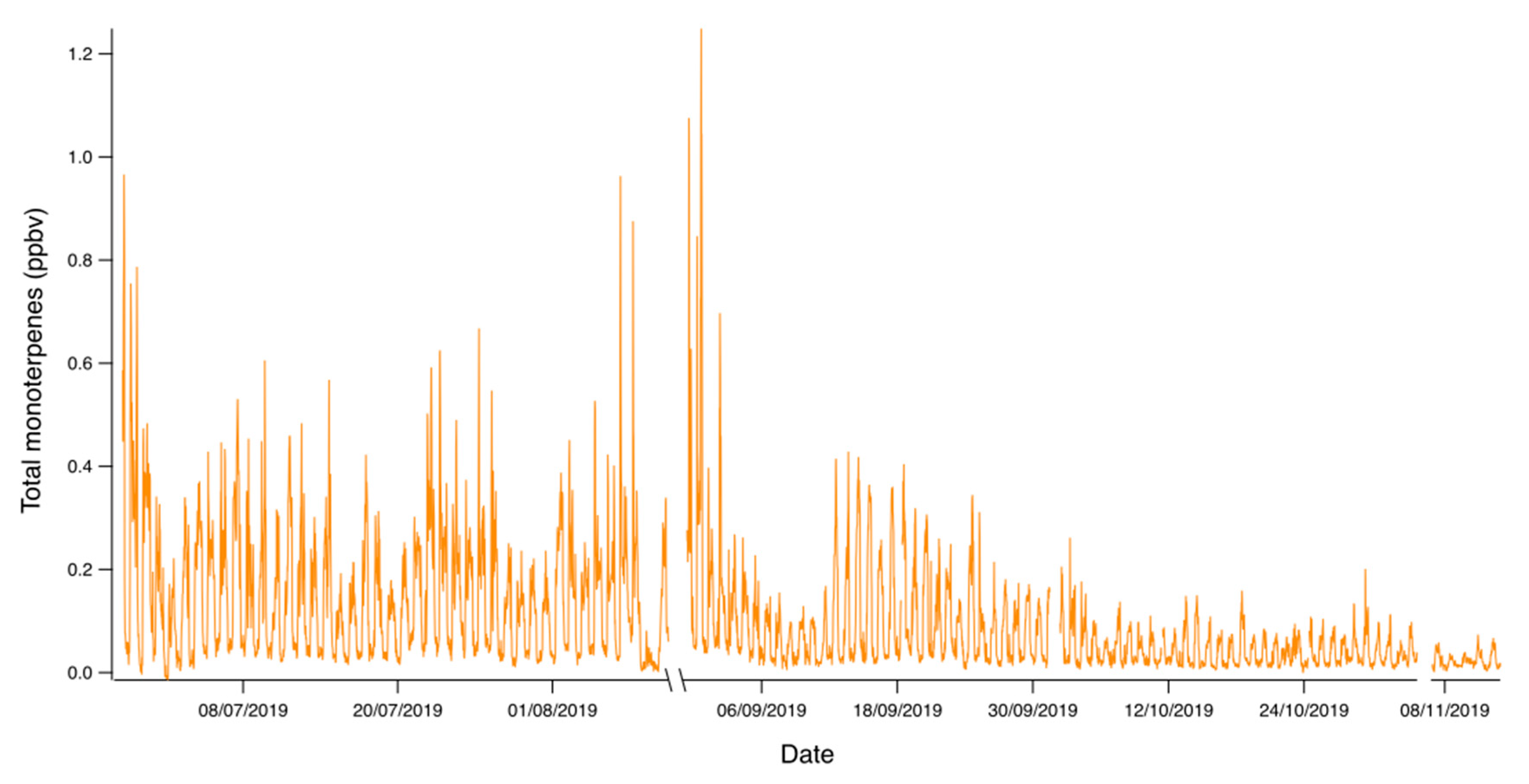
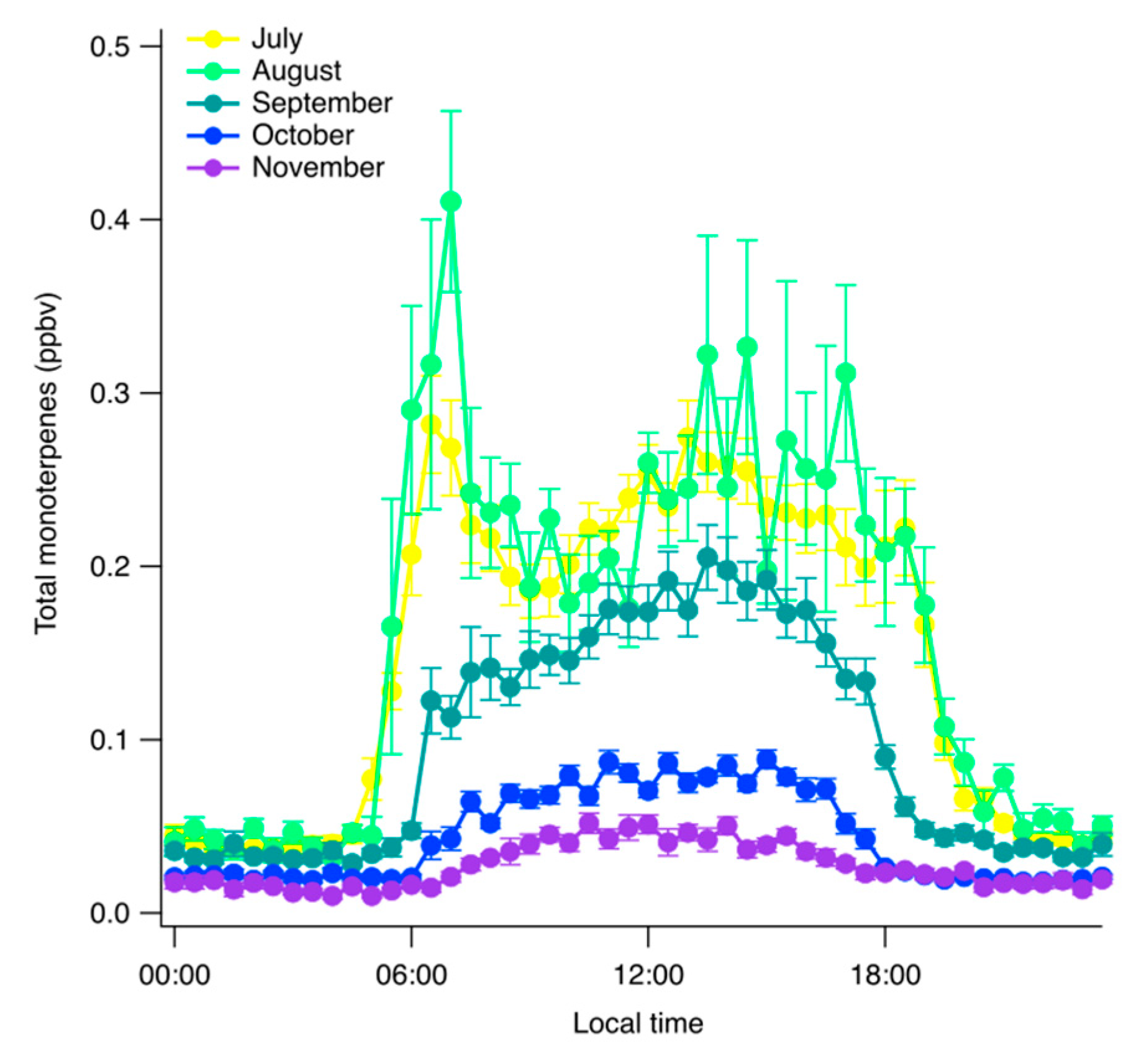
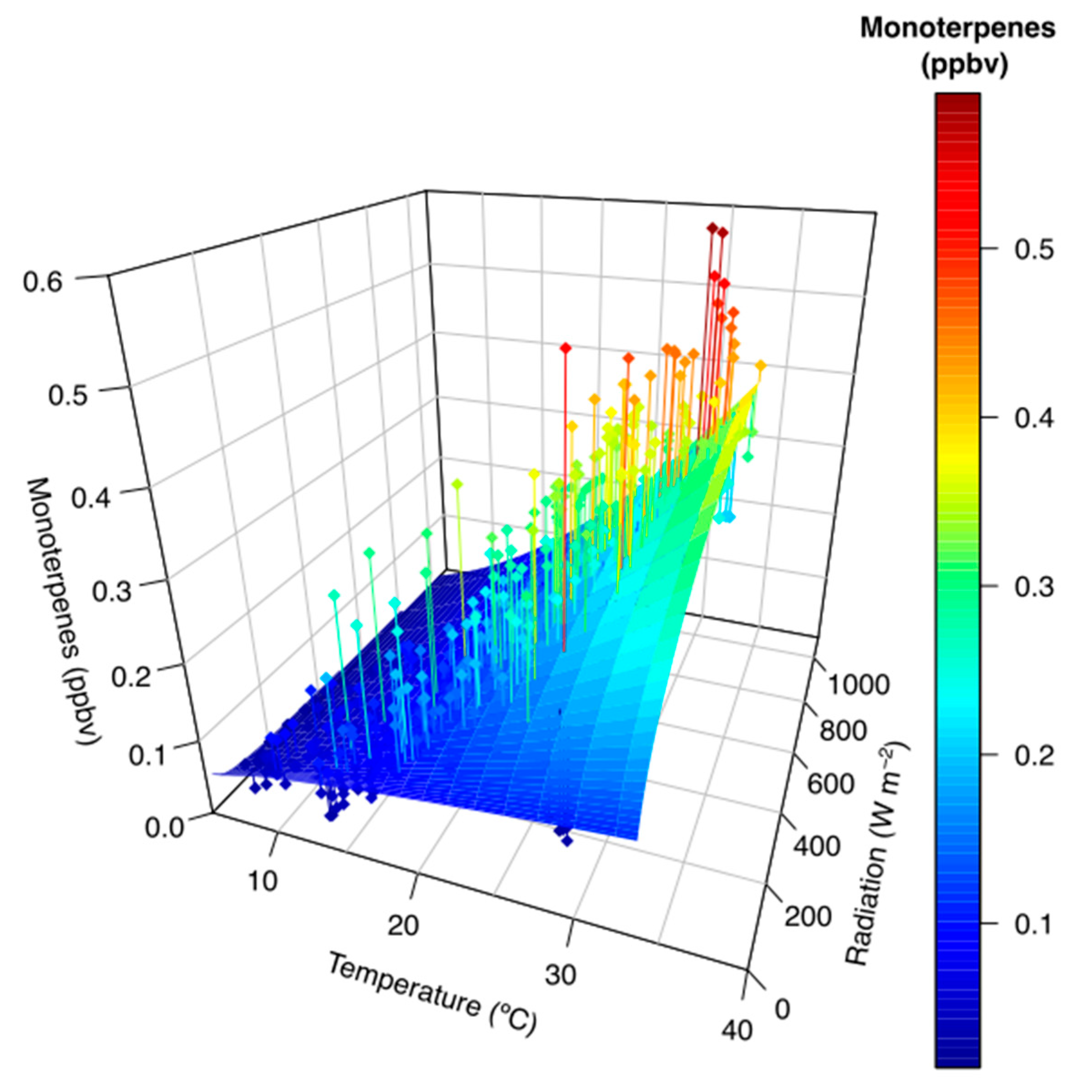
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of Shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Peñuelas, J.; Clarà, J.; Llusià, J.; Campillo, I.; López, F.; Maneja, R. How Should Forests Be Characterized in Regard to Human Health? Evidence from Existing Literature. Int. J. Environ. Res. Public Health 2020, 17, 1027. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef]
- Lee, K.J.; Hur, J.; Yang, K.S.; Lee, M.K.; Lee, S.J. Acute Biophysical Responses and Psychological Effects of Different Types of Forests in Patients With Metabolic Syndrome. Environ. Behav. 2018, 50, 298–323. [Google Scholar] [CrossRef]
- Li, Q.; Nakadai, A.; Matsushima, H.; Miyazaki, Y.; Krensky, A.; Kawada, T.; Morimoto, K. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunopharmacol. Immunotoxicol. 2006, 28, 319–333. [Google Scholar] [CrossRef]
- Laothawornkitkul, J.; Taylor, J.E.; Paul, N.D.; Hewitt, C.N. Biogenic volatile organic compounds in the Earth system. New Phytol. 2009, 183, 27–51. [Google Scholar] [CrossRef]
- Kulmala, M.; Nieminen, T.; Chellapermal, R.; Makkonen, R.; Bäck, J.; Kerminen, V.-M. Climate Feedbacks Linking the Increasing Atmospheric CO2 Concentration, BVOC Emissions, Aerosols and Clouds in Forest Ecosystems. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Springer: Dordrecht, The Netherlands, 2013; pp. 489–508. [Google Scholar]
- Pöschl, U.; Martin, S.T.; Sinha, B.; Chen, Q.; Gunthe, S.S.; Huffman, J.A.; Borrmann, S.; Farmer, D.K.; Garland, R.M.; Helas, G.; et al. Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Mewalal, R.; Rai, D.K.; Kainer, D.; Chen, F.; Külheim, C.; Peter, G.F.; Tuskan, G.A. Plant-Derived Terpenes: A Feedstock for Specialty Biofuels. Trends Biotechnol. 2017, 35, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.M.; Gershenzon, J.; Bohlmann, J. Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce. Plant Physiol. 2003, 132, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.F.; Kaminski, M.; Schlag, P.; Fuchs, H.; Acir, I.-H.; Bohn, B.; Häseler, R.; Kiendler-Scharr, A.; Rohrer, F.; Tillmann, R.; et al. Secondary organic aerosol formation from hydroxyl radical oxidation and ozonolysis of monoterpenes. Atmos. Chem. Phys. 2015, 15, 991–1012. [Google Scholar] [CrossRef]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. Environ. Pollut. 2000, 109, 175. [Google Scholar]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef]
- Harrison, S.P.; Morfopoulos, C.; Dani, K.G.S.; Prentice, I.C.; Arneth, A.; Atwell, B.J.; Barkley, M.P.; Leishman, M.R.; Loreto, F.; Medlyn, B.E.; et al. Volatile isoprenoid emissions from plastid to planet. New Phytol. 2013, 197, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. 2014, 14, 9317–9341. [Google Scholar] [CrossRef]
- Hantson, S.; Knorr, W.; Schurgers, G.; Pugh, T.A.M.; Arneth, A. Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use. Atmos. Environ. 2017, 155, 35–45. [Google Scholar] [CrossRef]
- Peñuelas, J.; Staudt, M. BVOCs and global change. Trends Plant Sci. 2010, 15, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Nam, E.-S.; Uhm, D.-C. Effects of Phytoncides Inhalation on Serum Cortisol Level and Life Stress of College Students. Korean J. Adult Nurs. 2008, 20, 697–706. [Google Scholar]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Swatko-Ossor, M.; Augustynowicz-Kopec, E. The Effect of Combining Natural Terpenes and Antituberculous Agents against Reference and Clinical Mycobacterium tuberculosis Strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Fujiwara, R.; Tanida, M.; Nomura, J.; Yokoyama, M.M. Effects of citrus fragrance on immune function and depressive states. Neuroimmunomodulation 1995, 2, 174–180. [Google Scholar] [CrossRef]
- Lin, J.J.; Lu, K.W.; Ma, Y.S.; Tang, N.Y.; Wu, P.P.; Wu, C.C.; Lu, H.F.; Lin, J.G.; Chung, J.G. Alpha-phellandrene, a natural active monoterpene, influences a murine WEHI-3 leukemia model in vivo by enhancing macrophague phagocytosis and natural killer cell activity. In Vivo (Brooklyn) 2014, 28, 583–588. [Google Scholar]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest bathing enhances human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Li, Y.J.; Wakayama, T.; et al. A forest bathing trip increases human natural killer activity and expression of anti-cancer proteins in female subjects. J. Biol. Regul. Homeost. Agents 2008, 22, 45–55. [Google Scholar] [PubMed]
- Cho, K.S.; Lim, Y.R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.S. Terpenes from forests and human health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, K.; Akutsu, H.; Fukuyama, S.; Minoshima, A.; Kukita, S.; Yamamura, Y.; Sato, Y.; Hayasaka, T.; Osanai, S.; Funakoshi, H.; et al. Conifer-Derived Monoterpenes and Forest Walking. Mass Spectrom. (Tokyo, Japan) 2015, 4, A0042. [Google Scholar] [CrossRef]
- Terradas, J. Holm Oak and Holm Oak Forests: An Introduction. In Ecology of Mediterranean Evergreen oak Forests; Springer: Berlin/Heidelberg, Germany, 1999; pp. 3–14. [Google Scholar]
- Avila, A.; Rodrigo, A. Trace metal fluxes in bulk deposition, throughfall and stemflow at two evergreen oak stands in NE Spain subject to different exposure to the industrial environment. Atmos. Environ. 2004, 38, 171–180. [Google Scholar] [CrossRef]
- Lindinger, W.; Jordan, A. Proton-transfer-reaction mass spectrometry (PTR–MS): On-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 1998, 27, 347. [Google Scholar] [CrossRef]
- Peñuelas, J.; Guenther, A.; Rapparini, F.; Llusia, J.; Filella, I.; Seco, R.; Estiarte, M.; Mejia-Chang, M.; Ogaya, R.; Ibañez, J.; et al. Intensive measurements of gas, water, and energy exchange between vegetation and troposphere during the MONTES campaign in a vegetation gradient from short semi-desertic shrublands to tall wet temperate forests in the NW Mediterranean Basin. Atmos. Environ. 2013, 75, 348–364. [Google Scholar] [CrossRef]
- Seco, R.; Peñuelas, J.; Filella, I.; Llusià, J.; Molowny-Horas, R.; Schallhart, S.; Metzger, A.; Müller, M.; Hansel, A. Contrasting winter and summer VOC mixing ratios at a forest site in the Western Mediterranean Basin: The effect of local biogenic emissions. Atmos. Chem. Phys. 2011, 11, 13161–13179. [Google Scholar] [CrossRef]
- Ruuskanen, T.M.; Taipale, R.; Rinne, J.; Kajos, M.K.; Hakola, H.; Kulmala, M. Quantitative long-term measurements of VOC concentrations by PTR-MS: Annual cycle at a boreal forest site. Atmos. Chem. Phys. Discuss. 2009, 9, 81–134. [Google Scholar] [CrossRef]
- Jordan, C.; Fitz, E.; Hagan, T.; Sive, B.; Frinak, E.; Haase, K.; Cottrell, L.; Buckley, S.; Talbot, R. Long-term study of VOCs measured with PTR-MS at a rural site in New Hampshire with urban influences. Atmos. Chem. Phys. 2009, 9, 4677–4697. [Google Scholar] [CrossRef]
- Llusia, J.; Peñuelas, J.; Seco, R.; Filella, I. Seasonal changes in the daily emission rates of terpenes by Quercus ilex and the atmospheric concentrations of terpenes in the natural park of Montseny, NE Spain. J. Atmos. Chem. 2012, 69, 215–230. [Google Scholar] [CrossRef]
- Llusia, J.; Peñuelas, J.; Guenther, A.; Rapparini, F. Seasonal variations in terpene emission factors of dominant species in four ecosystems in NE Spain. Atmos. Environ. 2013, 70, 149–158. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J. The complexity of factors driving volatile organic compound emissions by plants. Biol. Plant. 2001, 44, 481–487. [Google Scholar] [CrossRef]
- Loreto, F.; Forster, A.; Durr, M.; Csiky, O.; Seufert, G. On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ. 1998, 21, 101–107. [Google Scholar] [CrossRef]
- Loreto, F.; Pinelli, P.; Manes, F.; Kollist, H. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol. 2004, 24, 361–367. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Pinus halepensis and Quercus ilex terpene emission as affected by temperature and humidity. Biol. Plant. 1999, 42, 317–320. [Google Scholar] [CrossRef]
- Staudt, M.; Rambal, S.; Joffre, R.; Kesselmeier, J. Impact of drought on seasonal monoterpene emissions from Quercus ilex in southern France. J. Geophys. Res. Atmos. 2002, 107, ACH 15-1–ACH 15-9. [Google Scholar] [CrossRef]
- Seco, R.; Filella, I.; Llusià, J.; Peñuelas, J. Methanol as a signal triggering isoprenoid emissions and photosynthetic performance in Quercus ilex. Acta Physiol. Plant. 2011, 33, 2413–2422. [Google Scholar] [CrossRef]
- Seco, R.; Peñuelas, J.; Filella, I.; Llusia, J.; Schallhart, S.; Metzger, A.; Müller, M.; Hansel, A. Volatile organic compounds in the western Mediterranean basin: Urban and rural winter measurements during the DAURE campaign. Atmos. Chem. Phys. 2013, 13, 4291–4306. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Nölscher, A.C.; Williams, J.; Wolff, S.; Alves, E.G.; Martins, G.A.; Bourtsoukidis, E.; Brito, J.; Jardine, K.; Artaxo, P.; et al. Diel and seasonal changes of biogenic volatile organic compounds within and above an Amazonian rainforest. Atmos. Chem. Phys. 2015, 15, 3359–3378. [Google Scholar] [CrossRef]
- Jardine, A.B.; Jardine, K.J.; Fuentes, J.D.; Martin, S.T.; Martins, G.; Durgante, F.; Carneiro, V.; Higuchi, N.; Manzi, A.O.; Chambers, J.Q. Highly reactive light-dependent monoterpenes in the Amazon. Geophys. Res. Lett. 2015, 42, 1576–1583. [Google Scholar] [CrossRef]
- Davison, B.; Taipale, R.; Langford, B.; Misztal, P.; Fares, S.; Matteucci, G.; Loreto, F.; Cape, J.N.; Rinne, J.; Hewitt, C.N. Concentrations and fluxes of biogenic volatile organic compounds above a Mediterranean macchia ecosystem in western Italy. Biogeosciences 2009, 6, 1655–1670. [Google Scholar] [CrossRef]
- Yassaa, N.; Song, W.; Lelieveld, J.; Vanhatalo, A.; Bäck, J.; Williams, J. Diel cycles of isoprenoids in the emissions of Norway spruce, four Scots pine chemotypes, and in Boreal forest ambient air during HUMPPA-COPEC-2010. Atmos. Chem. Phys. 2012, 12, 7215–7229. [Google Scholar] [CrossRef]
- Staudt, M.; Bertin, N. Light and temperature dependence of the emission of cyclic and acyclic monoterpenes from holm oak (Quercus ilex L.) leaves. Plant Cell Environ. 1998, 21, 385–395. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Bartolini, G.; Zabini, F. Temporal and spatial variability of volatile organic compounds in the forest atmosphere. Int. J. Environ. Res. Public Health 2019, 16, 4915. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Morimoto, K.I.; Kobayashi, M.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Suzuki, H.; Li, Y.; Wakayama, Y.; et al. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008, 21, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Park, B.J.; Yeon, P.S.; Lee, S.; Joung, D.; Park, C.; Koga, S. Case Study on the Changes in the Physical Environment in Forest Healing Spaces. J. Fac. Agr. Kyushu Univ. 2016, 61, 375–381. [Google Scholar]

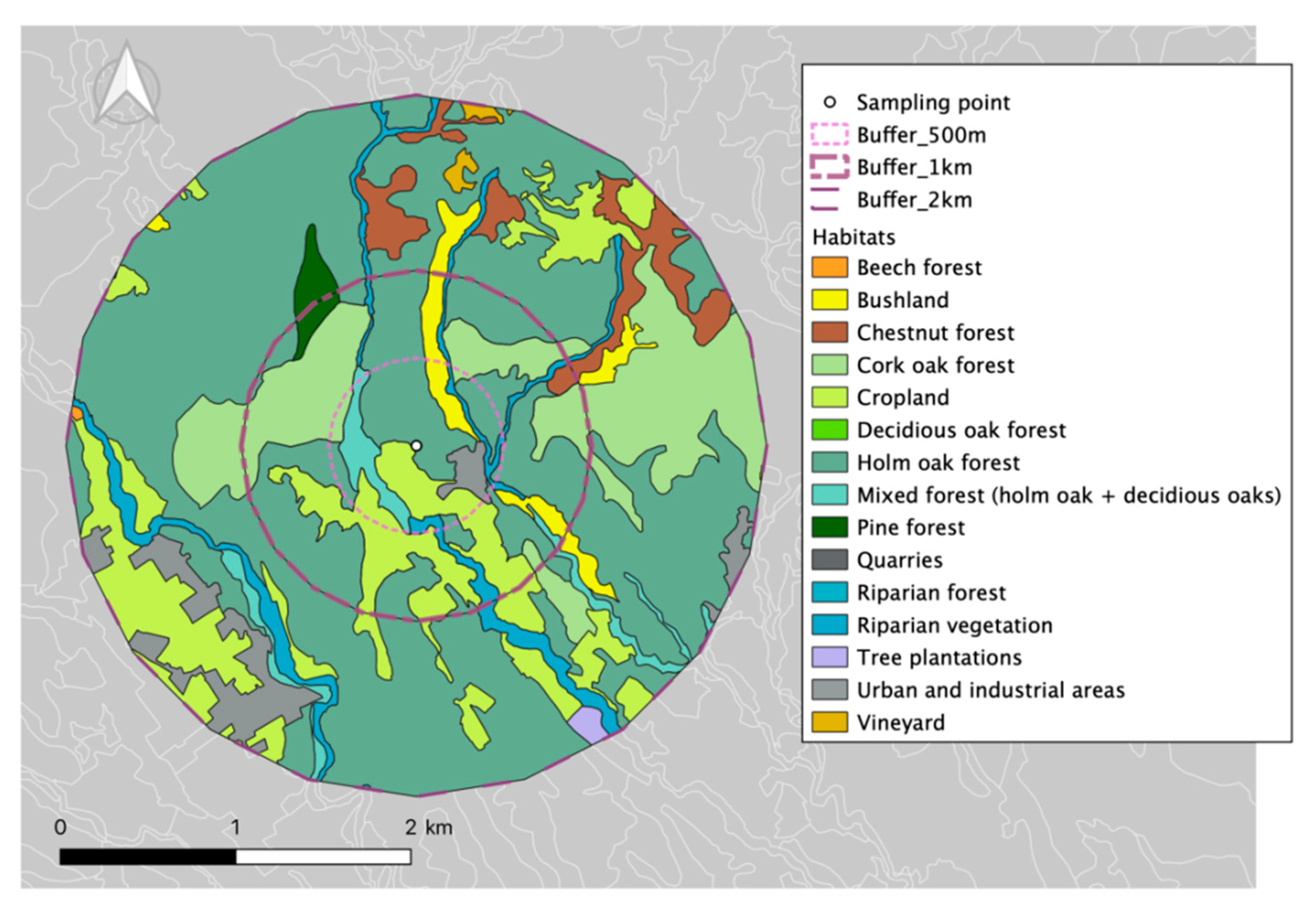
| Habitat | Surface (ha) | Percentage (%) |
|---|---|---|
| Holm Oak Forest | 33.63 | 43.50 |
| Cropland | 16.83 | 21.77 |
| Mixed forest (holm oak + deciduous oaks) | 9.97 | 12.90 |
| Urban and industrial areas | 5.41 | 6.99 |
| Bushland | 4.94 | 6.39 |
| Riparian vegetation | 4.19 | 5.42 |
| Cork oak forest | 2.34 | 3.03 |
| TOTAL | 77.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bach, A.; Yáñez-Serrano, A.M.; Llusià, J.; Filella, I.; Maneja, R.; Penuelas, J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. Int. J. Environ. Res. Public Health 2020, 17, 4391. https://doi.org/10.3390/ijerph17124391
Bach A, Yáñez-Serrano AM, Llusià J, Filella I, Maneja R, Penuelas J. Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. International Journal of Environmental Research and Public Health. 2020; 17(12):4391. https://doi.org/10.3390/ijerph17124391
Chicago/Turabian StyleBach, Albert, Ana Maria Yáñez-Serrano, Joan Llusià, Iolanda Filella, Roser Maneja, and Josep Penuelas. 2020. "Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy" International Journal of Environmental Research and Public Health 17, no. 12: 4391. https://doi.org/10.3390/ijerph17124391
APA StyleBach, A., Yáñez-Serrano, A. M., Llusià, J., Filella, I., Maneja, R., & Penuelas, J. (2020). Human Breathable Air in a Mediterranean Forest: Characterization of Monoterpene Concentrations under the Canopy. International Journal of Environmental Research and Public Health, 17(12), 4391. https://doi.org/10.3390/ijerph17124391






