Cross-Sectional Associations of Total Daily Volume and Activity Patterns across the Activity Spectrum with Cardiometabolic Risk Factors in Children and Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Accelerometry
2.2.2. Cardiometabolic Risk Factors
2.2.3. Participant Characteristics
2.3. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Accelerometer-Derived Variables
3.3. Associations between Total Daily Volumes and Cardiometabolic Risk Factors
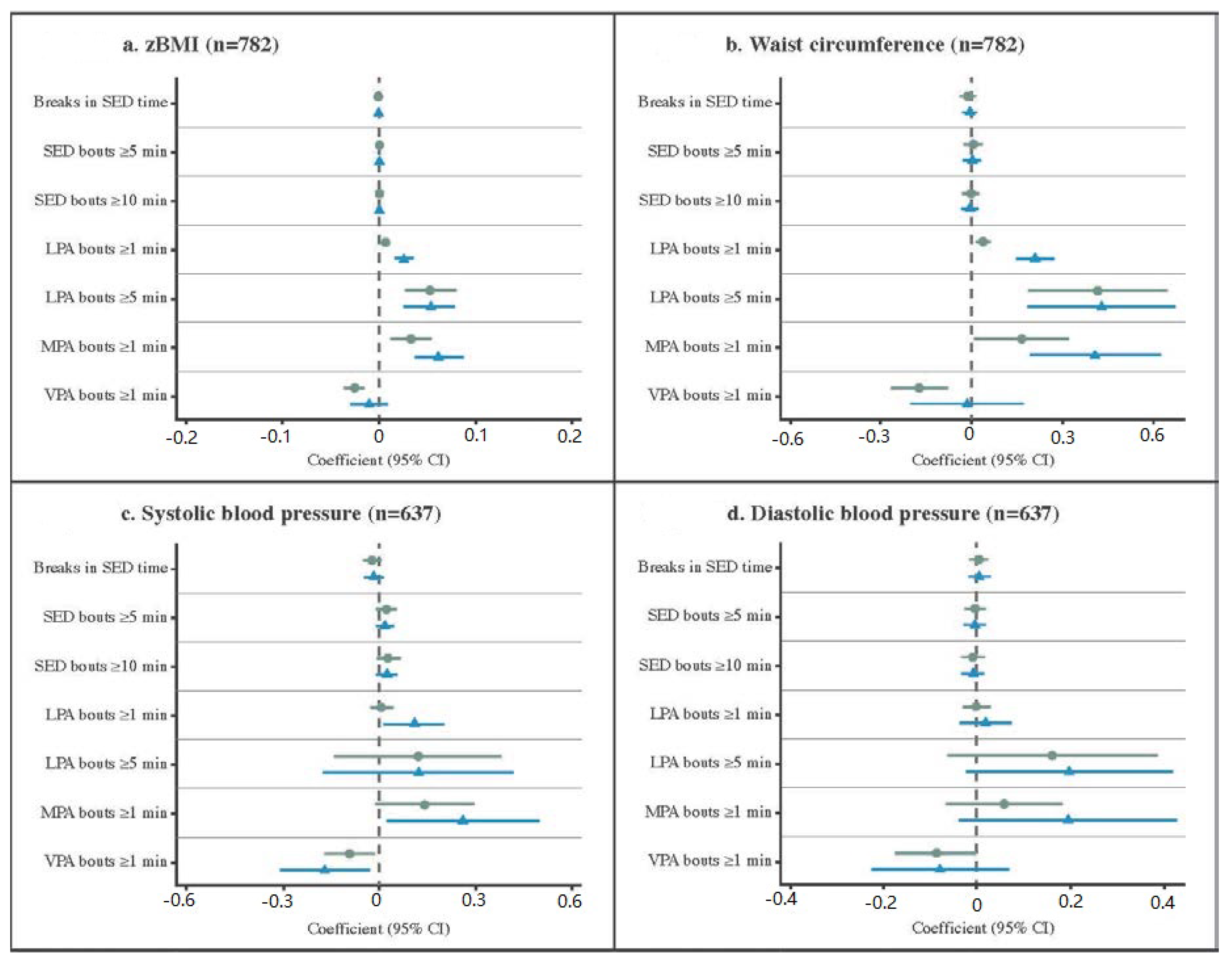
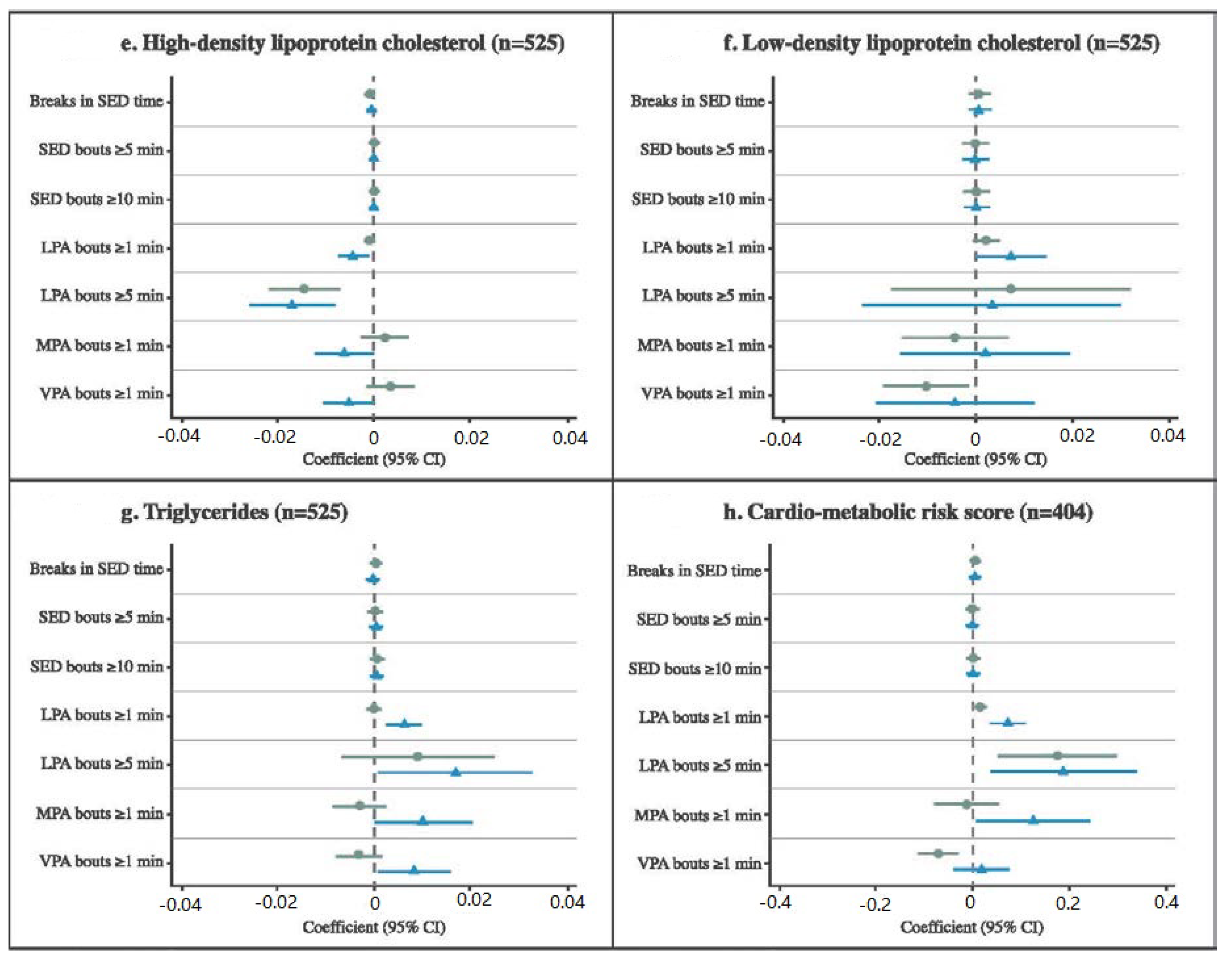
3.4. Associations between Activity Patterns and Cardiometabolic Risk Factors
4. Discussion
4.1. Defining Activity Patterns
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carson, V.; Hunter, S.; Kuzik, N.; Gray, C.E.; Poitras, V.J.; Chaput, J.P.; Saunders, T.J.; Katzmarzyk, P.T.; Okely, A.D.; Connor Gorber, S.; et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth: An update. Appl. Physiol. Nutr. Metab. 2016, 41, S240–S265. [Google Scholar] [CrossRef] [PubMed]
- Poitras, V.J.; Gray, C.; Borghese, M.M.; Carson, V.; Chaput, J.-P.; Janssen, I.; Katzmarzyk, P.T.; Pate, R.R.; Gorber, S.C.; Kho, M.E.; et al. Systematic review of the relationships between objectively measured physical activity and health indicators in school-aged children and youth. Appl. Physiol. Nutr. Metab. 2016, 41, S197–S239. [Google Scholar] [CrossRef] [PubMed]
- Verswijveren, S.; Lamb, K.E.; Bell, L.A.; Timperio, A.; Salmon, J.; Ridgers, N.D. Associations between activity patterns and cardio-metabolic risk factors in children and adolescents: A systematic review. PLoS ONE 2018, 13, e0201947. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.; Egerton, T.; Leask, C.; Stamatakis, E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity 2015, 23, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Telama, R. Tracking of physical activity from childhood to adulthood: A review. Obes. Facts 2009, 2, 187–195. [Google Scholar] [CrossRef]
- Camhi, S.M.; Katzmarzyk, P.T. Tracking of cardiometabolic risk factor clustering from childhood to adulthood. Pediatr. Obes. 2010, 5, 122–129. [Google Scholar] [CrossRef]
- Rodrigues, A.N.; Abreu, G.R.; Resende, R.S.; Goncalves, W.L.; Gouvea, S.A. Cardiovascular risk factor investigation: A pediatric issue. Int. J. Gen. Med. 2013, 6, 57–66. [Google Scholar] [CrossRef]
- Telford, R.D.; Basse, S.L.; Budge, M.M.; Byrne, D.G.; Carlson, J.S.; Coles, D.; Cunningham, R.B.; Daly, R.M.; Dunstan, D.W.; English, R.; et al. The lifestyle of our kids (LOOK) project: Outline of methods. J. Sci. Med. Sport 2009, 12, 156–163. [Google Scholar] [CrossRef]
- Salmon, J.; Arundell, L.; Hume, C.; Brown, H.; Hesketh, K.D.; Dunstan, D.W.; Daly, R.; Pearson, N.; Cerin, E.; Moodie, M.; et al. A cluster-randomized controlled trial to reduce sedentary behavior and promote physical activity and health of 8-9 year olds: The Transform-Us! Study. BMC Public Health 2011, 11, 759. [Google Scholar] [CrossRef]
- Telford, R.M.; Telford, R.D.; Cunningham, R.B.; Cochrane, T.; Davey, R.; Waddington, G. Longitudinal patterns of physical activity in children aged 8 to 12 years: The LOOK study. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Robusto, K.M.; Trost, S.G. Comparison of three generations of ActiGraph activity monitors in children and adolescents. J. Sports Sci. 2012, 30, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Cain, K.L.; Sallis, J.F.; Conway, T.L.; Van Dyck, D.; Calhoon, L. Using accelerometers in youth physical activity studies: A review of methods. J. Phys. Act. Health 2013, 10, 437–450. [Google Scholar] [CrossRef]
- Carson, V.; Ridgers, N.D.; Howard, B.J.; Winkler, E.A.; Healy, G.N.; Owen, N.; Dunstan, D.W.; Salmon, J. Light-intensity physical activity and cardiometabolic biomarkers in US adolescents. PLoS ONE 2013, 8, e71417. [Google Scholar] [CrossRef] [PubMed]
- Freedson, P.; Pober, D.; Janz, K.F. Calibration of accelerometer output for children. Med. Sci. Sports Exerc. 2005, 37, S523–S530. [Google Scholar] [CrossRef] [PubMed]
- Baquet, G.; Stratton, G.; van Praagh, E.; Berthoin, S. Improving physical activity assessment in prepubertal children with high-frequency accelerometry monitoring: A methodological issue. Prev. Med. 2007, 44, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, T.M.; de Niet, M.; Verloigne, M.; De Bourdeaudhuij, I.; Androutsos, O.; Manios, Y.; Kovacs, E.; Bringolf-Isler, B.; Brug, J.; Chinapaw, M.J. Occurrence and duration of various operational definitions of sedentary bouts and cross-sectional associations with cardiometabolic health indicators: The ENERGY-project. Prev. Med. 2015, 71, 101–106. [Google Scholar] [CrossRef]
- Gabel, L.; Ridgers, N.D.; Della Gatta, P.A.; Arundell, L.; Cerin, E.; Robinson, S.; Daly, R.M.; Dunstan, D.W.; Salmon, J. Associations of sedentary time patterns and TV viewing time with inflammatory and endothelial function biomarkers in children. Pediatr. Obes. 2016, 11, 194–201. [Google Scholar] [CrossRef]
- Fletcher, E.A.; Carson, V.; McNaughton, S.A.; Dunstan, D.W.; Healy, G.N.; Salmon, J. Does diet mediate associations of volume and bouts of sedentary time with cardiometabolic health indicators in adolescents? Obesity 2017, 25, 591–599. [Google Scholar] [CrossRef]
- Garnett, S.P.; Baur, L.A.; Cowell, C.T. Waist-to-height ratio: A simple option for determining excess central adiposity in young people. Int. J. Obes. 2008, 32, 1028–1030. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; WHO, Ed.; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia—Data Only. 2006; Cat: 2033.0.55.001. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.003Chapter12011-2012 (accessed on 20 December 2017).
- Schisterman, E.F.; Cole, S.R.; Platt, R.W. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology 2009, 20, 488–495. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef] [PubMed]
- Tarp, J.; Child, A.; White, T.; Westgate, K.; Bugge, A.; Grontved, A.; Wedderkopp, N.; Andersen, L.B.; Cardon, G.; Davey, R.; et al. Physical activity intensity, bout-duration, and cardiometabolic risk markers in children and adolescents. Int. J. Obes. 2018, 42, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Aadland, E.; Andersen, L.B.; Anderssen, S.A.; Resaland, G.K.; Kvalheim, O.M. Associations of volumes and patterns of physical activity with metabolic health in children: A multivariate pattern analysis approach. Prev. Med. 2018, 115, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ottevaere, C.; Huybrechts, I.; Benser, J.; De Bourdeaudhuij, I.; Cuenca-Garcia, M.; Dallongeville, J.; Zaccaria, M.; Gottrand, F.; Kersting, M.; Rey-López, J.P.; et al. Clustering patterns of physical activity, sedentary and dietary behavior among European adolescents: The HELENA study. BMC Public Health 2011, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Blaes, A.; Baquet, G.; Fabre, C.; Van Praagh, E.; Berthoin, S. Is there any relationship between physical activity level and patterns, and physical performance in children? Int. J. Behav. Nutr. Phys. Act. 2011, 8, 1–8. [Google Scholar] [CrossRef]
- Smith, P.; Ma, H.; Glazier, R.H.; Gilbert-Ouimet, M.; Mustard, C. The relationship between occupational standing and sitting and incident heart disease over a 12-year period in Ontario, Canada. Am. J. Epidemiol. 2018, 187, 27–33. [Google Scholar] [CrossRef]
- Chastin, S.F.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: A novel compositional data analysis approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef]
| Minimally Adjusted Model 1 | ||||
| Cardiometabolic risk factor | SED A β (95% CI) | LPA A β (95% CI) | MPA A β (95% CI) | VPA A β (95% CI) |
| zBMI | −0.0003 (−0.0023, 0.0018) | 0.0023 (−0.0004, 0.0049) | 0.0015 (−0.0060, 0.0089) | −0.0168 ** (−0.0252, −0.0084) |
| WC | 0.0067 (−0.0061, 0.0195) | 0.0070 (−0.0110, 0.0249) | −0.0218 (−0.0667, 0.0231) | −0.1371 ** (−0.1898, −0.0843) |
| SBP B | 0.0061 (−0.0126, 0.0248) | −0.0067 (−0.0324, 0.0189) | 0.0031 (−0.0517, 0.0578) | −0.0103 (−0.0895, 0.0690) |
| DBP B | 0.0081 (−0.0063, 0.0224) | −0.0037 (−0.0236, 0.0163) | −0.0213 (−0.0670, 0.0243) | −0.0501 (−0.1249, 0.0248) |
| HDL-C C | −0.0004 (−0.0011, 0.0003) | −0.0003 (−0.0011, 0.0006) | 0.0031 ** (0.0009, 0.0052) | 0.0065 ** (0.0029, 0.0101) |
| LDL-C C | 0.0002 (−0.0011, 0.0014) | 0.0010 (−0.0010, 0.0029) | −0.0029 (−0.0070, 0.0012) | −0.0079 * (−0.0145, −0.0014) |
| TG C | 0.0009 * (0.0001, 0.0017) | −0.0004 (−0.0015, 0.0006) | −0.0042 ** (−0.0063, −0.0021) | −0.0073 ** (−0.0105, −0.0041) |
| CMR-score D | 0.0056 (−0.0035, 0.0147) | 0.0038 (−0.0075, 0.0151) | −0.0370 ** (−0.0609, −0.0131) | −0.0762 ** (−0.1144, −0.0380) |
| Fully Adjusted Model 3 | ||||
| SEDA β (95% CI) | LPAA β (95% CI) | MPAA β (95% CI) | VPAA β (95% CI) | |
| zBMI | 0.0004 (−0.0017, 0.0025) | 0.0020 (−0.0006, 0.0047) | −0.0028 (−0.0108, 0.0053) | −0.0245 ** (−0.0328, −0.0162) |
| WC | 0.0088 (−0.0045, 0.0221) | 0.0082 (−0.0100, 0.0264) | −0.0438 (−0.0918, 0.0042) | −0.1877 ** (−0.2395, −0.1359) |
| SBP B | 0.0046 (−0.0144, 0.0236) | −0.0017 (−0.0285, 0.0250) | −0.0010 (−0.0597, 0.0577) | −0.0349 (−0.1271, 0.0573) |
| DBP B | 0.0061 (−0.0081, 0.0202) | −0.0017 (−0.0221, 0.0186) | −0.0145 (−0.0650, 0.0360) | −0.0514 (−0.1390, 0.0362) |
| HDL-C C | −0.0002 (−0.0009, 0.0005) | −0.0003 (−0.0011, 0.0005) | 0.0023 * (0.0001, 0.0044) | 0.0056 ** (0.0018, 0.0094) |
| LDL-C C | 0.0001 (−0.0010, 0.0013) | 0.0010 (−0.0009, 0.0029) | −0.0031 (−0.0070, 0.0008) | −0.0088 * (−0.0164, −0.0013) |
| TG C | 0.0009 * (0.0002, 0.0016) | −0.0006 (−0.0016, 0.0004) | −0.0040 ** (−0.0064, −0.0016) | −0.0064 ** (−0.0103, −0.0025) |
| CMR-score D | 0.0056 (−0.0034, 0.0145) | 0.0034 (−0.0075, 0.0143) | −0.0369 ** (−0.0610, −0.0128) | −0.0798 ** (−0.1205, −0.0391) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verswijveren, S.J.J.M.; Lamb, K.E.; Timperio, A.; Salmon, J.; Telford, R.M.; Daly, R.M.; Cerin, E.; Hume, C.; Olive, L.S.; Mackintosh, K.A.; et al. Cross-Sectional Associations of Total Daily Volume and Activity Patterns across the Activity Spectrum with Cardiometabolic Risk Factors in Children and Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 4286. https://doi.org/10.3390/ijerph17124286
Verswijveren SJJM, Lamb KE, Timperio A, Salmon J, Telford RM, Daly RM, Cerin E, Hume C, Olive LS, Mackintosh KA, et al. Cross-Sectional Associations of Total Daily Volume and Activity Patterns across the Activity Spectrum with Cardiometabolic Risk Factors in Children and Adolescents. International Journal of Environmental Research and Public Health. 2020; 17(12):4286. https://doi.org/10.3390/ijerph17124286
Chicago/Turabian StyleVerswijveren, Simone J. J. M., Karen E. Lamb, Anna Timperio, Jo Salmon, Rohan M. Telford, Robin M. Daly, Ester Cerin, Clare Hume, Lisa S. Olive, Kelly A. Mackintosh, and et al. 2020. "Cross-Sectional Associations of Total Daily Volume and Activity Patterns across the Activity Spectrum with Cardiometabolic Risk Factors in Children and Adolescents" International Journal of Environmental Research and Public Health 17, no. 12: 4286. https://doi.org/10.3390/ijerph17124286
APA StyleVerswijveren, S. J. J. M., Lamb, K. E., Timperio, A., Salmon, J., Telford, R. M., Daly, R. M., Cerin, E., Hume, C., Olive, L. S., Mackintosh, K. A., McNarry, M. A., & Ridgers, N. D. (2020). Cross-Sectional Associations of Total Daily Volume and Activity Patterns across the Activity Spectrum with Cardiometabolic Risk Factors in Children and Adolescents. International Journal of Environmental Research and Public Health, 17(12), 4286. https://doi.org/10.3390/ijerph17124286








