Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study
Abstract
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Instruments
2.3. Preparation of Nano-Sized Activated Carbon
2.4. Adsorption Process
3. Results and Discussion
3.1. Characterization
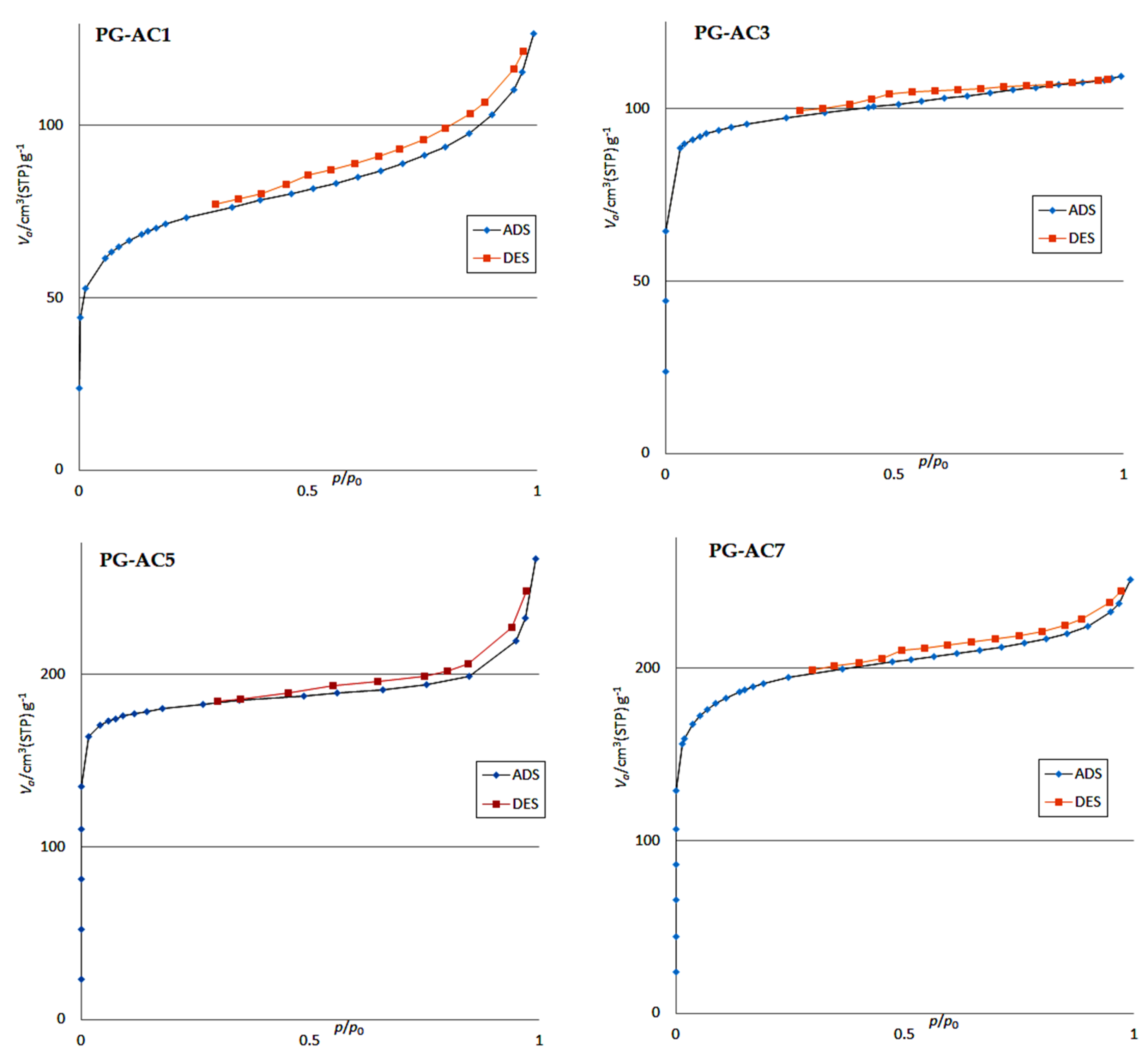
3.1.1. BET Analysis
3.1.2. FT-IR Spectroscopy
3.1.3. X-ray Diffractometry
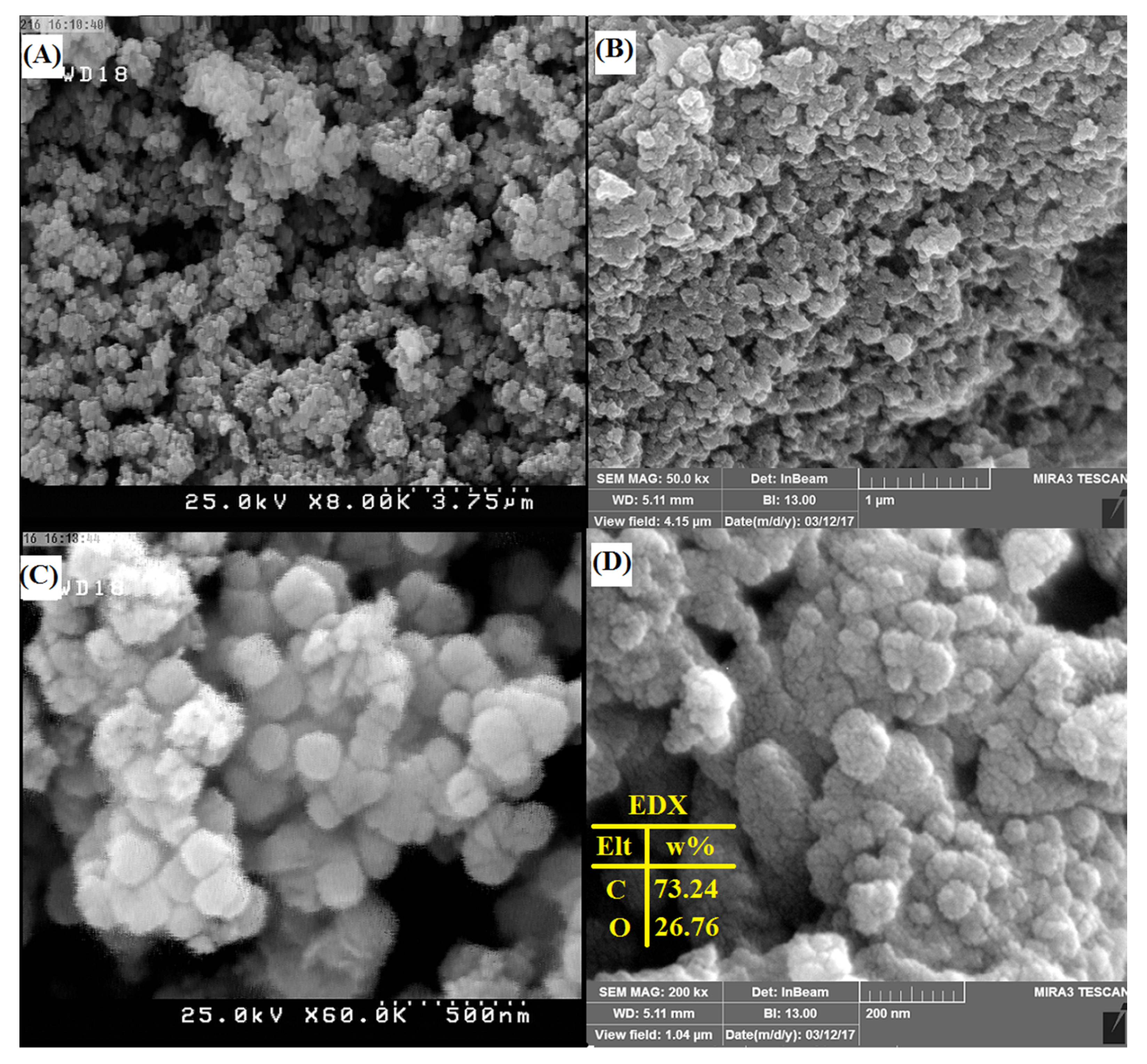
3.1.4. Field Emission Scanning Electron Microscopy
3.2. Effect of pH
3.3. Effect of Adsorbent Dosage
3.4. Effect of Salt Concentration
3.5. Adsorption Time
3.6. Adsorption Kinetics
3.7. Initial Concentration and Adsorption Isotherm
3.8. Effect of Temperature and Thermodynamics
3.9. Regeneration and Recovery
3.10. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M.N. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nodeh, M.K.; Radfard, M.; Zardari, L.A.; Rashidi Nodeh, H. Enhanced removal of naproxen from wastewater using silica magnetic nanoparticles decorated onto graphene oxide; parametric and equilibrium study. Sep. Sci. Technol. 2018, 15, 2476–2485. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Shokoohia, R.; Leilia, M.; Dargahib, A.; Vazirib, Y.; Khamutianb, R. Common antibiotics in wastewater of Sina and Besat Hospitals, Hamadan, Iran. Arch. Hyg. Sci. 2017, 6, 152–159. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Fakhri, A.; Adami, S. Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J. Taiwan Inst. Chem. Eng. 2014, 45, 1001–1006. [Google Scholar] [CrossRef]
- Zavareh, S.; Eghbalazar, T. Efficient and selective removal of cefixime form aqueous solution by a modified bionanocomposite. J. Environ. Chem. Eng. 2017, 5, 3337–3347. [Google Scholar] [CrossRef]
- Rouhbakhsh, Z.; Verdian, A.; Rajabzadeh, G. Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline. Talanta 2020, 206, 120246. [Google Scholar] [CrossRef]
- Kaya, Z.; Kürekçi, F.; Akkuzu, E.; Göral, S.; Kalkan, G. Autoimmune Hemolytic Anemia, Erythrophagocytosis and Liver Dysfunction After Cefixime Use for Urinary Tract Infection in a Child. Indian J. Hematol. Blood Transfus. 2019, 35, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.M.; Ciorba, P.; Döhla, M.; Exner, M.; Felder, C.; Lenz-Plet, F.; Sib, E.; Skutlarek, D.; Schmithausen, R.M.; Faerber, H.A. The investigation of antibiotic residues, antibiotic resistance genes and antibiotic-resistant organisms in a drinking water reservoir system in Germany. Int. J. Hyg. Environ. Health 2020, 224, 113449. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.M.; Zacharias, N.; Timm, C.; Wasser, F.; Sib, E.; Skutlarek, D.; Parcina, M.; Schmithausen, R.M.; Schwartz, T.; Hembach, N. Association between antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in anthropogenic wastewater—An evaluation of clinical influences. Chemosphere 2020, 241, 125032. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kumar, A.; Sharma, G.; Stadler, F.J.; Naushad, M.; Ghfar, A.A.; Ahamad, T. LaTiO2N/Bi2S3 Z-scheme nano heterostructures modified with rGO with high interfacial contact for rapid photocatalytic degradation of tetracycline. J. Mol. Liq. 2020, 311, 113300. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Naushad, M.; Veses, R.C.; Ghfar, A.A.; Stadler, F.J.; Khan, M.R. Solar-driven photodegradation of 17-β-estradiol and ciprofloxacin from waste water and CO 2 conversion using sustainable coal-char/polymeric-gC 3 N 4/RGO metal-free nano-hybrids. New J. Chem. 2017, 41, 10208–10224. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Bhogal, S.; Naushad, M.; Kumar, A.; Stadler, F.J. Fabrication and characterization of trimetallic nano-photocatalyst for remediation of ampicillin antibiotic. J. Mol. Liq. 2018, 260, 342–350. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Wan Ibrahim, W.A.; Rashidi Nodeh, H.; Sanagi, M.M. Graphene-Based Materials as Solid Phase Extraction Sorbent for Trace Metal Ions, Organic Compounds, and Biological Sample Preparation. Crit. Rev. Anal. Chem. 2016, 46, 267–283. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Sereshti, H.; Afsharian, E.Z.; Bidhendi, M.E.; Nodeh, H.R.; Kamboh, M.A.; Yilmaz, M. Removal of phosphate and nitrate ions aqueous using strontium magnetic graphene oxide nanocomposite: Isotherms, kinetics, and thermodynamics studies. Environ. Prog. Sustain. Energy 2020, 39, e13332. [Google Scholar] [CrossRef]
- Mohammadi Nodeh, M.K.; Soltani, S.; Shahabuddin, S.; Rashidi Nodeh, H.; Sereshti, H. Equilibrium, Kinetic and Thermodynamic Study of Magnetic Polyaniline/Graphene Oxide Based Nanocomposites for Ciprofloxacin Removal from Water. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1226–1234. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, Z.; Wei, Y.; Zhou, Y.; Yang, X.; Yang, Y.; Yang, J.; Zhang, J.; Luo, L.; Zhou, Z. Carbon-based materials as adsorbent for antibiotics removal: Mechanisms and influencing factors. J. Environ. Manag. 2019, 237, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, J.; Xiong, W.; Yang, Z.; Sun, S.; Jia, M.; Xu, Z. In-situ growing of metal-organic frameworks on three-dimensional iron network as an efficient adsorbent for antibiotics removal. Chem. Eng. J. 2020, 392, 124844. [Google Scholar] [CrossRef]
- Balasubramani, K.; Sivarajasekar, N.; Naushad, M. Effective adsorption of antidiabetic pharmaceutical (metformin) from aqueous medium using graphene oxide nanoparticles: Equilibrium and statistical modelling. J. Mol. Liq. 2020, 301, 112426. [Google Scholar] [CrossRef]
- Teixeira, S.; Delerue-Matos, C.; Santos, L. Application of experimental design methodology to optimize antibiotics removal by walnut shell based activated carbon. Sci. Total Environ. 2019, 646, 168–176. [Google Scholar] [CrossRef]
- Berrios, M.; Martín, M.Á.; Martín, A. Treatment of pollutants in wastewater: Adsorption of methylene blue onto olive-based activated carbon. J. Ind. Eng. Chem. 2012, 18, 780–784. [Google Scholar] [CrossRef]
- Açıkyıldız, M.; Gürses, A.; Karaca, S. Preparation and characterization of activated carbon from plant wastes with chemical activation. Microporous Mesoporous Mater. 2014, 198, 45–49. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H. Advanced porous hierarchical activated carbon derived from agricultural wastes toward high performance supercapacitors. J. Alloys Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Ververi, M.; Goula, A.M. Pomegranate peel and orange juice by-product as new biosorbents of phenolic compounds from olive mill wastewaters. Chem. Eng. Process. Intensif. 2019, 138, 86–96. [Google Scholar] [CrossRef]
- Sühnholz, S.; Kopinke, F.-D.; Weiner, B. Hydrothermal treatment for regeneration of activated carbon loaded with organic micropollutants. Sci. Total Environ. 2018, 644, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Spessato, L.; Bedin, K.C.; Cazetta, A.L.; Souza, I.P.A.F.; Duarte, V.A.; Crespo, L.H.S.; Silva, M.C.; Pontes, R.M.; Almeida, V.C. KOH-super activated carbon from biomass waste: Insights into the paracetamol adsorption mechanism and thermal regeneration cycles. J. Hazard. Mater. 2019, 371, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Xu, X.; Gao, B.; Yue, Q.; Yanan, S.; Khan, R.; Inam, M.A. Adsorptive removal of phosphate by the bimetallic hydroxide nanocomposites embedded in pomegranate peel. J. Environ. Sci. 2020, 91, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.K. Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Puad, N.A.A.; Bello, O.S. Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave-induced KOH activation. Water Resour. Ind. 2014, 6, 18–35. [Google Scholar] [CrossRef]
- Gündüz, F.; Bayrak, B. Biosorption of malachite green from an aqueous solution using pomegranate peel: Equilibrium modelling, kinetic and thermodynamic studies. J. Mol. Liq. 2017, 243, 790–798. [Google Scholar] [CrossRef]
- Ali, M.E.M.; Abdelsalam, H.; Ammar, N.S.; Ibrahim, H.S. Response surface methodology for optimization of the adsorption capability of ball-milled pomegranate peel for different pollutants. J. Mol. Liq. 2018, 250, 433–445. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Lillo-Rodenas, M.A.; Marco Lozar, J.P.; Kunowsky, M.; Romero Anaya, A.J. NaOH and KOH for preparing activated carbons used in energy and environmental applications. Int. J. Energy Environ. Econ. 2012, 20, 59–91. [Google Scholar]
- Le Van, K.; Luong Thi Thu, T. Preparation of Pore-Size Controllable Activated Carbon from Rice Husk Using Dual Activating Agent and Its Application in Supercapacitor. J. Chem. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Ji, Y.; Li, T.; Zhu, L.; Wang, X.; Lin, Q. Preparation of activated carbons by microwave heating KOH activation. Appl. Surf. Sci. 2007, 254, 506–512. [Google Scholar] [CrossRef]
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Comparisons of porous, surface chemistry and adsorption properties of carbon derived from Enteromorpha prolifera activated by H4P2O7 and KOH. Chem. Eng. J. 2013, 232, 582–590. [Google Scholar] [CrossRef]
- Rashidi Nodeh, H.; Sereshti, H. Synthesis of magnetic graphene oxide doped with strontium titanium trioxide nanoparticles as a nanocomposite for the removal of antibiotics from aqueous media. RSC Adv. 2016, 6, 89953–89965. [Google Scholar] [CrossRef]
- Shivashankar, M.; Mandal, B.K. Design and Evaluation of Chitosan-Based Novel pH-Sensitive Drug Carrier for Sustained Release of Cefixime. Trop. J. Pharm. Res. 2013, 12, 155–161. [Google Scholar]
- Güzel, F.; Sayğılı, H.; Sayğılı, G.A.; Koyuncu, F.; Yılmaz, C. Optimal oxidation with nitric acid of biochar derived from pyrolysis of weeds and its application in removal of hazardous dye methylene blue from aqueous solution. J. Clean. Prod. 2017, 144, 260–265. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of using green adsorbent of heavy metal removal from aqueous solutions: Adsorption kinetics, isotherm, thermodynamic, mechanism and economic analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Ghodbane, I.; Hamdaoui, O. Removal of mercury (II) from aqueous media using eucalyptus bark: Kinetic and equilibrium studies. J. Hazard. Mater. 2008, 160, 301–309. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (IUPAC recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kalantary, R.R.; Azari, A.; Esrafili, A.; Yaghmaeian, K.; Moradi, M.; Sharafi, K. The survey of Malathion removal using magnetic graphene oxide nanocomposite as a novel adsorbent: Thermodynamics, isotherms, and kinetic study. Desalin. Water Treat. 2016, 57, 1–14. [Google Scholar] [CrossRef]
- Bhomick, P.C.; Supong, A.; Baruah, M.; Pongener, C.; Sinha, D. Pine Cone biomass as an efficient precursor for the synthesis of activated biocarbon for adsorption of anionic dye from aqueous solution: Isotherm, kinetic, thermodynamic and regeneration studies. Sustain. Chem. Pharm. 2018, 10, 41–49. [Google Scholar] [CrossRef]
- Ge, X.; Wu, Z.; Cravotto, G.; Manzoli, M.; Cintas, P.; Wu, Z. Cork wastewater purification in a cooperative flocculation/adsorption process with microwave-regenerated activated carbon. J. Hazard. Mater. 2018, 360, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rasoulifard, M.H.; Khanmohammadi, S.; Heidari, A. Adsorption of cefixime from aqueous solutions using modified hardened paste of Portland cement by perlite; optimization by Taguchi method. Water Sci. Technol. 2016, 74, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Pouretedal, H.R.; Sadegh, N. Effective removal of Amoxicillin, Cephalexin, Tetracycline and Penicillin G from aqueous solutions using activated carbon nanoparticles prepared from vine wood. J. Water Process Eng. 2014, 1, 64–73. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Fluoroquinolones antibiotics adsorption onto microporous activated carbon from lignocellulosic biomass by microwave pyrolysis. J. Taiwan Inst. Chem. Eng. 2014, 45, 219–226. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, S.; Li, J. Fast and highly efficient tetracyclines removal from environmental waters by graphene oxide functionalized magnetic particles. Chem. Eng. J. 2013, 225, 679–685. [Google Scholar] [CrossRef]



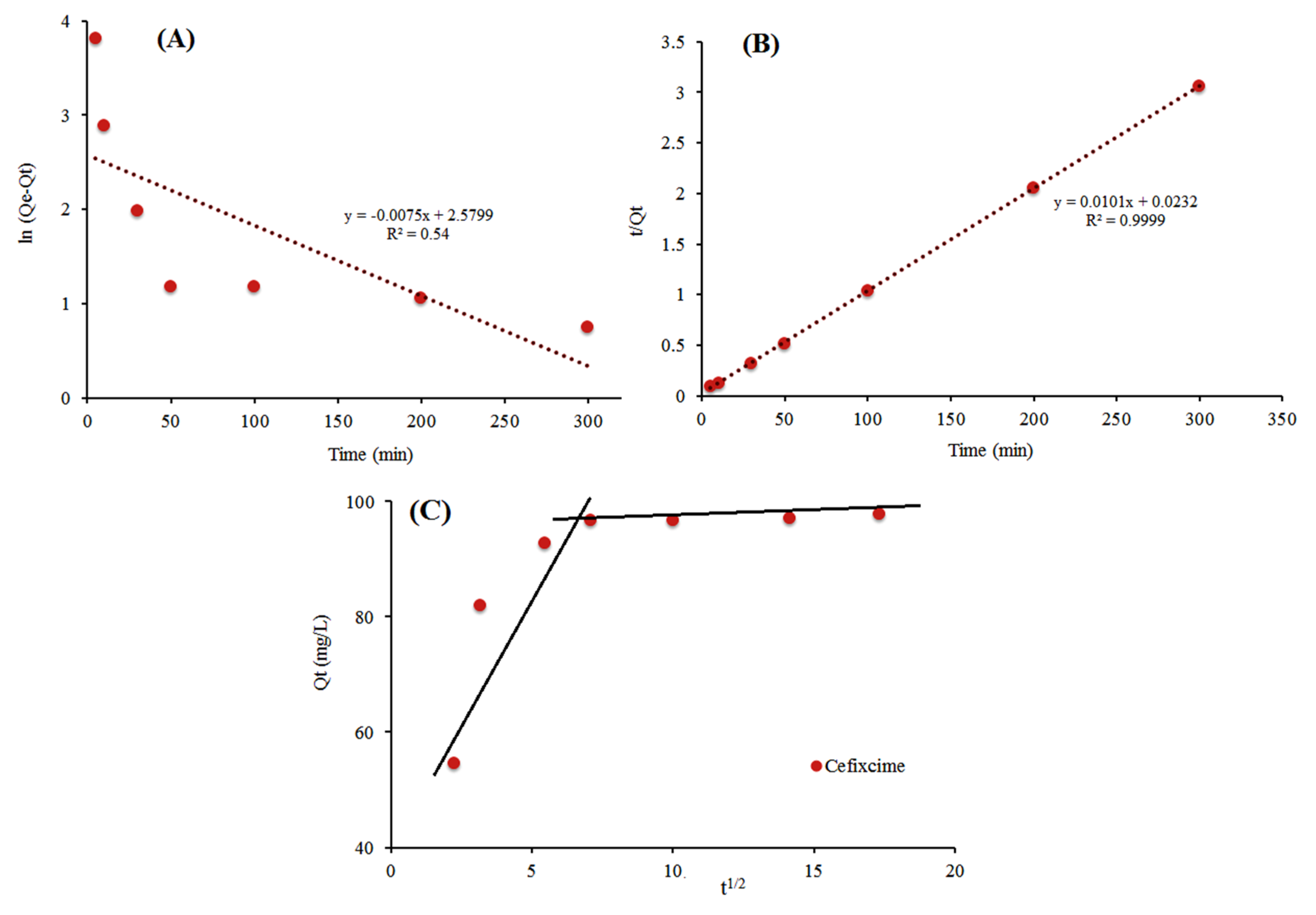

| Model | Parameters | Cefixime |
|---|---|---|
| Pseudo first order | qe (mg/g) | 12.84 |
| k1 (1/min) | 0.0075 | |
| R2 | 0.5418 | |
| Pseudo second order | qe (mg/g) | 99.09 |
| k2 (g/(mg·min)) | 0.0001 | |
| R2 | 0.9999 | |
| Intra-particle diffusion | Kid,1 | 0.7622 |
| Ci1 | 63.405 | |
| R12 | 0.6837 | |
| Kid,2 | 0.0047 | |
| Ci2 | 96.356 | |
| R22 | 0.9153 |
| Isotherms | Parameters | Cefixime |
|---|---|---|
| Langmuir | qm (mg/g) | 181.81 |
| kL (L/mg) | 0.024 | |
| R2 | 0.787 | |
| Freundlich | KF [(mg/g) (L/mg)1/n] | 6.201 |
| n | 1.52 | |
| R2 | 0.992 |
| Temp °C | Removal% | qe (mg/g) | ∆G (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/mol K) |
|---|---|---|---|---|---|
| 25 | 89 | 69.97 | −2.09 | ||
| 35 | 87 | 65.42 | −1.63 | −13.89 | −0.038 |
| 55 | 85 | 58.13 | −0.89 |
| Adsorbent | Antibiotics | Time (min) | qe (mg/g) | Ref |
|---|---|---|---|---|
| Nano-activated carbon | Cefixime | 60 | 181.81 | This study |
| Portland cement | Cefixime | 180 | 6.99 | [52] |
| GO/MNPs–SrTiO3 | Cefotaxime | 30 | 18.21 | [43] |
| Vine wood AC | Cephalexin | 8 h | 7.2 | [53] |
| Biomass-AC | Norfloxacin | 40 | 166.99 | [54] |
| MGO | Tetracycline | 20 | 39.1 | [55] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaeili Bidhendi, M.; Poursorkh, Z.; Sereshti, H.; Rashidi Nodeh, H.; Rezania, S.; Afzal Kamboh, M. Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study. Int. J. Environ. Res. Public Health 2020, 17, 4223. https://doi.org/10.3390/ijerph17124223
Esmaeili Bidhendi M, Poursorkh Z, Sereshti H, Rashidi Nodeh H, Rezania S, Afzal Kamboh M. Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study. International Journal of Environmental Research and Public Health. 2020; 17(12):4223. https://doi.org/10.3390/ijerph17124223
Chicago/Turabian StyleEsmaeili Bidhendi, Mehdi, Zahra Poursorkh, Hassan Sereshti, Hamid Rashidi Nodeh, Shahabaldin Rezania, and Muhammad Afzal Kamboh. 2020. "Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study" International Journal of Environmental Research and Public Health 17, no. 12: 4223. https://doi.org/10.3390/ijerph17124223
APA StyleEsmaeili Bidhendi, M., Poursorkh, Z., Sereshti, H., Rashidi Nodeh, H., Rezania, S., & Afzal Kamboh, M. (2020). Nano-Size Biomass Derived from Pomegranate Peel for Enhanced Removal of Cefixime Antibiotic from Aqueous Media: Kinetic, Equilibrium and Thermodynamic Study. International Journal of Environmental Research and Public Health, 17(12), 4223. https://doi.org/10.3390/ijerph17124223







