Comparisons of Dipstick Test, Urine Protein-to-Creatine Ratio, and Total Protein Measurement for the Diagnosis of Preeclampsia
Abstract
1. Introduction
2. Materials and Methods
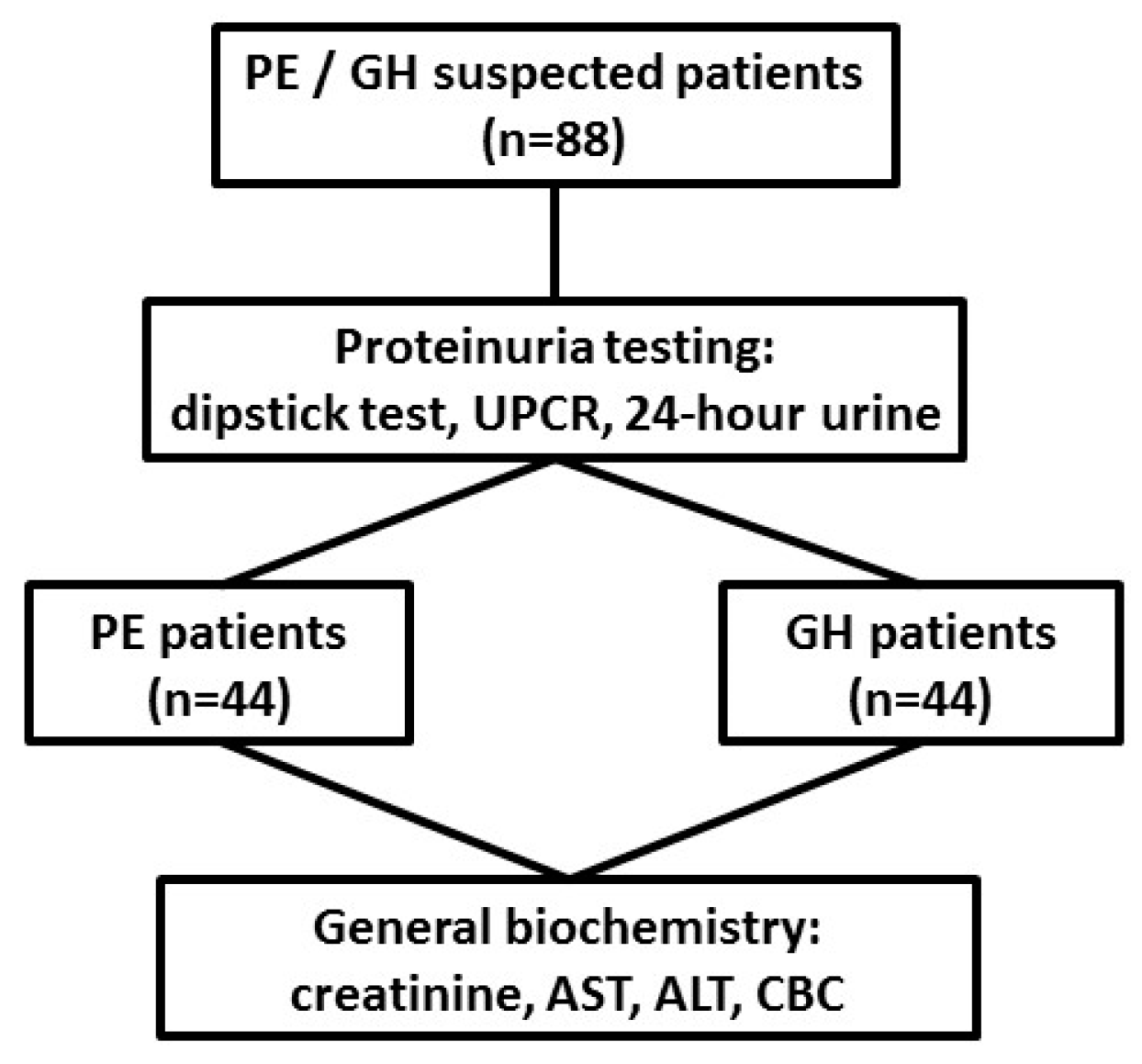
2.1. Study Design
2.2. Patients
2.3. Methods
2.4. Statistical Analysis
3. Results
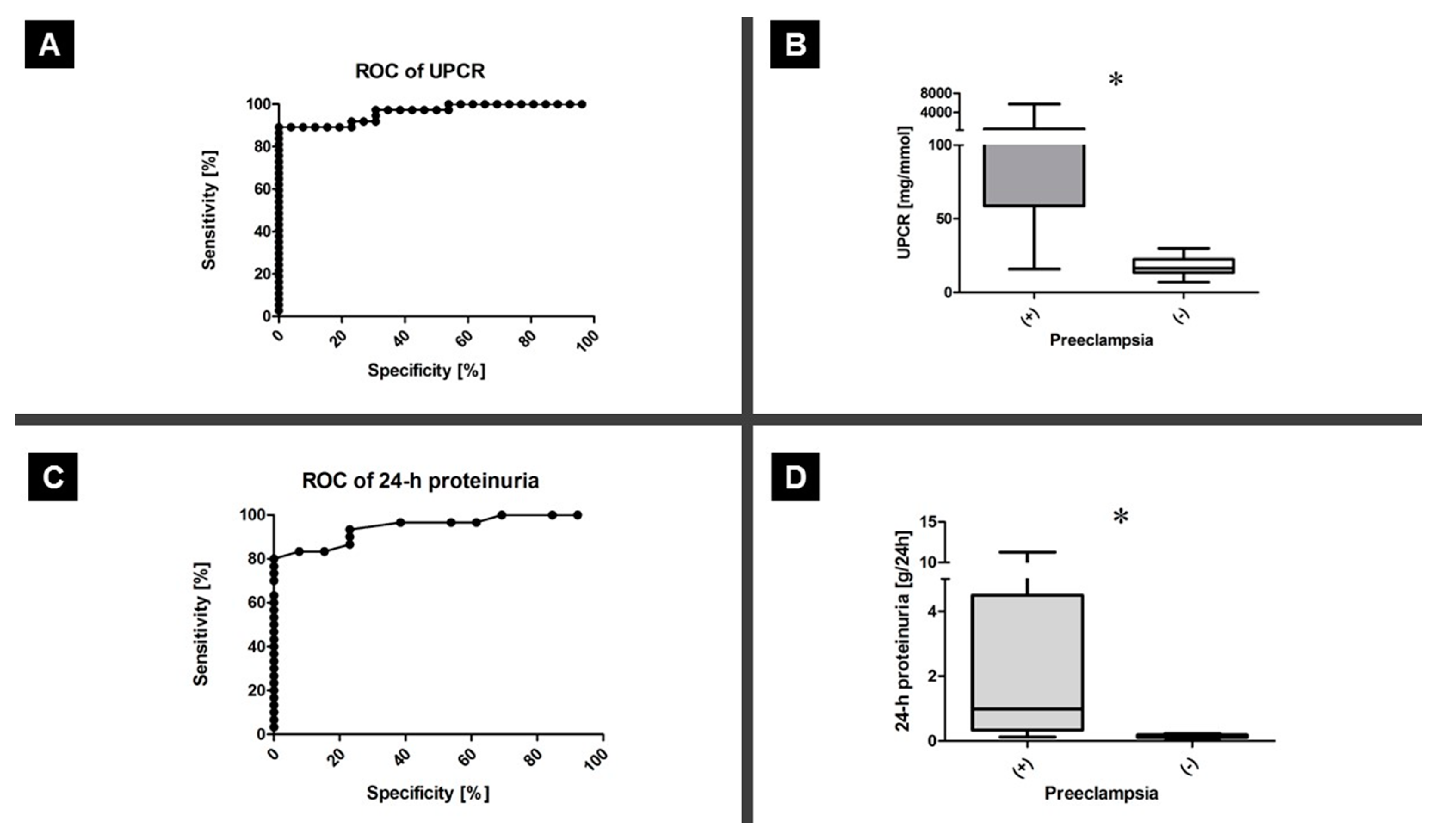
3.1. Proteinuria Testing
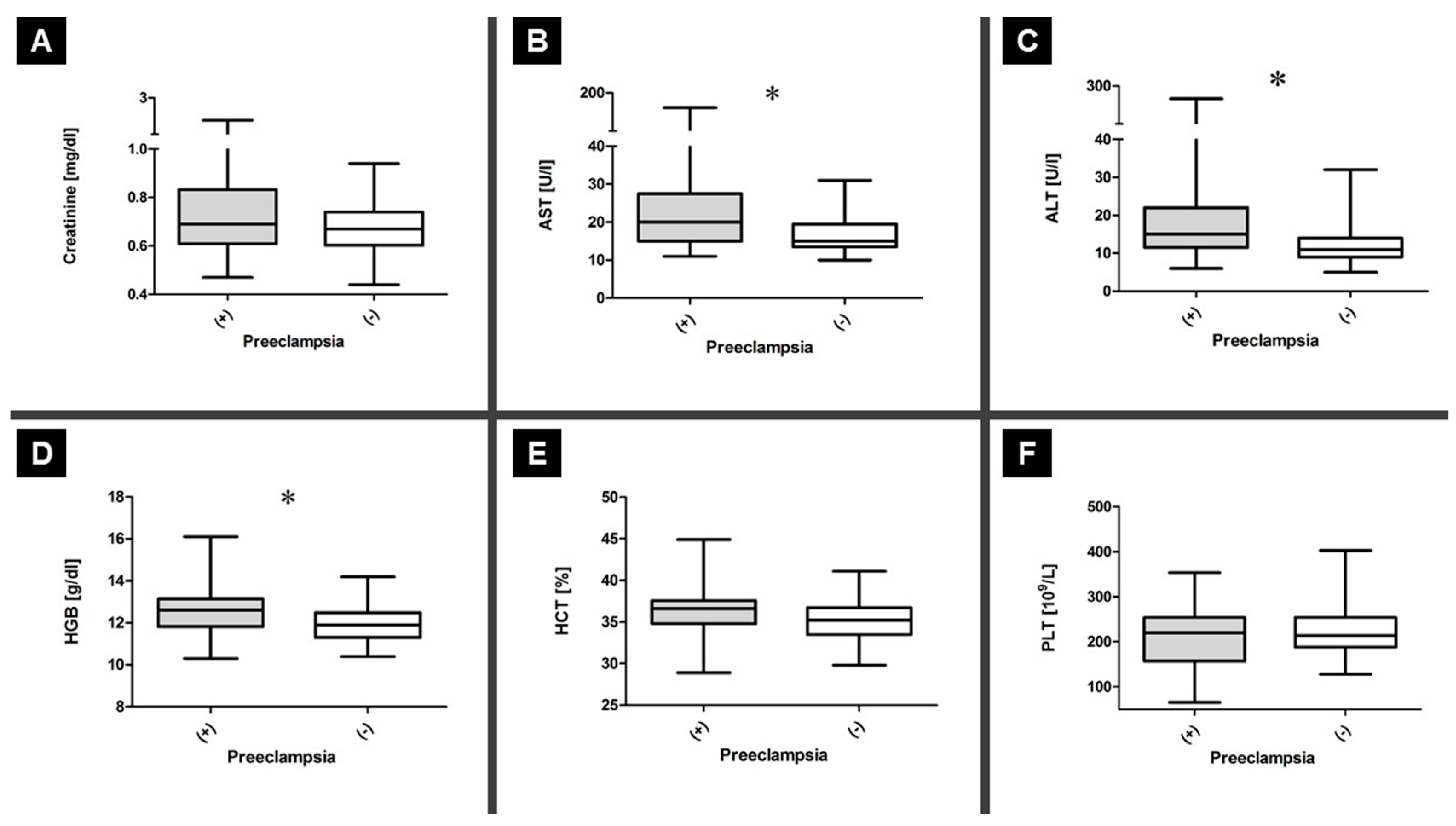
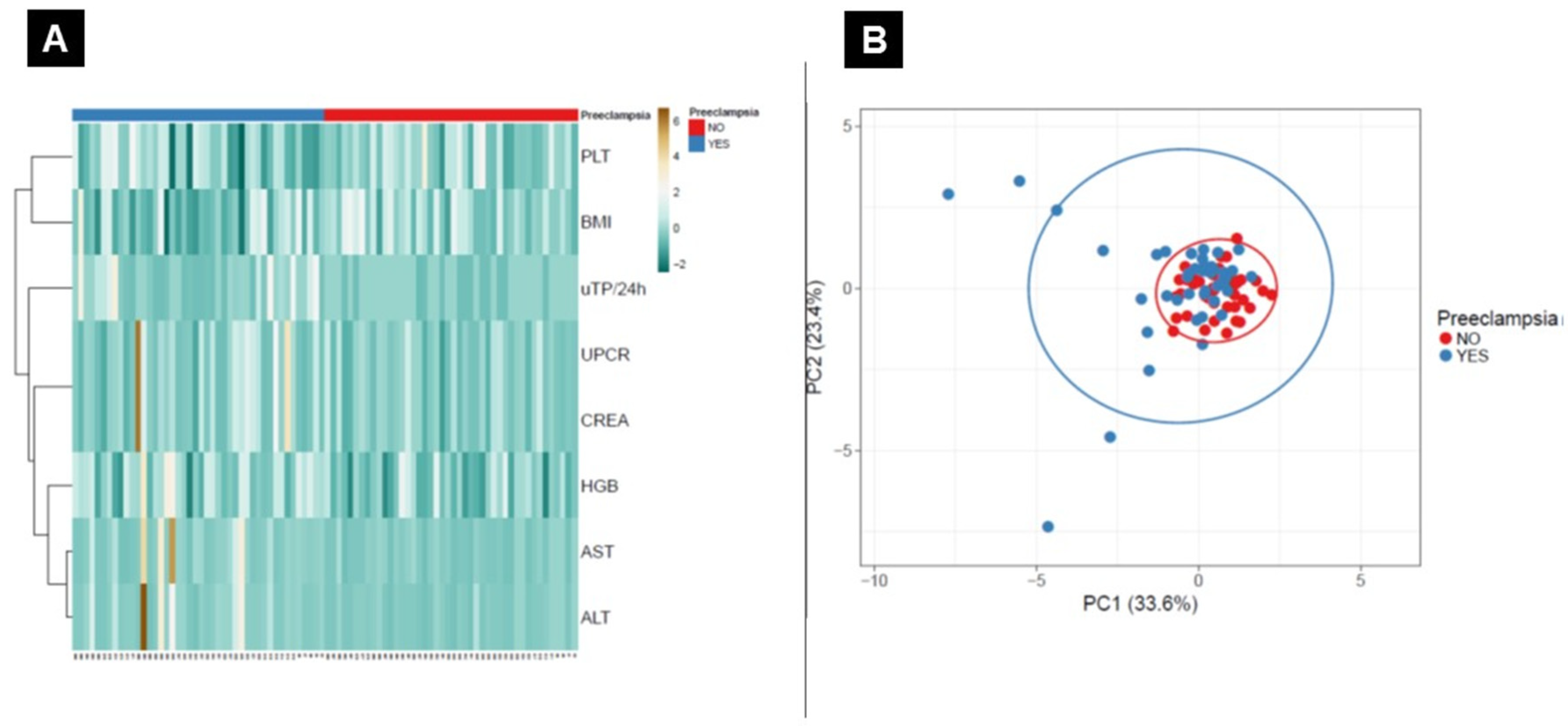
3.2. General Biochemistry Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145, 1–33. [Google Scholar] [CrossRef]
- Ronsmans, C.; Graham, W.J. Lancet Maternal Survival Series steering group. Maternal mortality: Who, when, where, and why. Lancet 2006, 368, 1189–1200. [Google Scholar] [CrossRef]
- Kuklina, E.V.; Ayala, C.; Callaghan, W.M. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol. 2009, 113, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P. Canadian Hypertensive Disorders of Pregnancy (HDP) Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens. 2014, 4, 105–145. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynecol. 2015, 55, e1–e29. [Google Scholar] [CrossRef]
- Amin, S.V.; Illipilla, S.; Hebbar, S.; Rai, L.; Kumar, P.; Pai, M.V. Quantifying proteinuria in hypertensive disorders of pregnancy. Int. J. Hypertens. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Cheung, H.; Leung, K.; Choi, C. Diagnostic accuracy of spot urine protein-to-creatinine ratio for proteinuria and its association with adverse pregnancy outcomes in Chinese pregnant patients with pre-eclampsia. Hong Kong Med. J. 2016, 22, 249–255. [Google Scholar] [CrossRef]
- Gangaram, R.; Naicker, M.; Moodley, J. Accuracy of the spot urinary microalbumin:creatinine ratio and visual dipsticks in hypertensive pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 146–148. [Google Scholar] [CrossRef]
- Waugh, J.J.; Clark, T.J.; Divakaran, T.G.; Khan, K.S.; Kilby, M.D. Accuracy of urinalysis dipstick techniques in predicting significant proteinuria in pregnancy. Obstet. Gynecol. 2004, 103, 769–777. [Google Scholar] [CrossRef]
- Waugh, J.J.; Bell, S.C.; Kilby, M.D.; Blackwell, C.N.; Seed, P.; Shennan, A.H.; Halligan, A.W. Optimal bedside urinalysis for the detection of proteinuria in hypertensive pregnancy: A study of diagnostic accuracy. BJOG 2005, 112, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.-M.; Firoz, T.; Mattman, A.; Lam, E.M.; von Dadelszen, P.; Magee, L.A. The 24-hour urine collection: Gold standard or historical practice? Am. J. Obstet. Gynecol. 2008, 199, 625.e1. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.; Bell, S.C.; Kilby, M.D.; Lambert, P.; Shennan, A.; Halligan, A. Urine protein estimation in hypertensive pregnancy: Which thresholds and laboratory assay best predict clinical outcome? Hypertens. Pregnancy 2005, 24, 291–302. [Google Scholar] [CrossRef]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, Y.; Yu, Y.; Wang, W.; Wang, S.; Zhong, M. Urinary spot albumin:creatinine ratio for documenting proteinuria in women with preeclampsia. Rev. Obstet. Gynecol. 2012, 5, 9–15. [Google Scholar]
- Sanchez-Ramos, L.; Gillen, G.; Zamora, J.; Stenyakina, A.; Kaunitz, A.M. The protein-to-creatinine ratio for the prediction of significant proteinuria in patients at risk for preeclampsia: A meta-analysis. Ann. Clin. Lab. Sci. 2013, 43, 211–220. [Google Scholar]
- Côté, A.M.; Brown, M.A.; Lam, E.; von Dadelszen, P.; Firoz, T.; Liston, R.M.; Magee, L.A. Diagnostic accuracy of urinary spot protein:creatinine ratio for proteinuria in hypertensive pregnant women: Systematic review. BMJ 2008, 336, 1003–1006. [Google Scholar] [CrossRef]
- Morris, R.K.; Riley, R.D.; Doug, M.; Deeks, J.J.; Kilby, M.D. Diagnostic accuracy of spot urinary protein and albumin to creatinine ratios for detection of significant proteinuria or adverse pregnancy outcome in patients with suspected pre-eclampsia: Systematic review and meta-analysis. BMJ 2012, 345, e4342. [Google Scholar] [CrossRef]
- Papanna, R.; Mann, L.K.; Kouides, R.W.; Glantz, J.C. Protein/creatinine ratio in preeclampsia: A systematic review. Obstet. Gynecol. 2008, 112, 135–144. [Google Scholar] [CrossRef]
- Price, C.P.; Newall, R.G.; Boyd, J.C. Use of protein: Creatinine ratio measurements on random urine samples for prediction of significant proteinuria: A systematic review. Clin. Chem. 2005, 51, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.; Hooper, R.; Lamb, E.; Robson, S.; Shennan, A.; Milne, F.; Price, C.; Thangaratinam, S.; Berdunov, V. Bingham Spot protein-creatinine ratio and spot albumin-creatinine ratio in the assessment of pre-eclampsia: A diagnostic accuracy study with decision-analytic model-based economic evaluation and acceptability analysis. Health Technol. Assess. 2017, 21, 1–90. [Google Scholar] [CrossRef] [PubMed]
| Patients Status | PE n = 44 | GH n = 44 | p a |
|---|---|---|---|
| Age (years, mean ± SD) | 28 ± 4.05 | 30 ± 4.60 | 0.124 |
| Period of gestation | 35 | 39 | 2.469 × 10−6 |
| Body mass index (kg/m2) (median, min/max) | 30 (21/46) | 33 (26/42) | 7.013 × 10−8 |
| Parity | |||
| 0 | 36 | 34 | NT |
| 1 | 7 | 7 | NT |
| >1 | 1 | 3 | NT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefańska, K.; Zieliński, M.; Zamkowska, D.; Adamski, P.; Jassem-Bobowicz, J.; Piekarska, K.; Jankowiak, M.; Leszczyńska, K.; Świątkowska-Stodulska, R.; Preis, K.; et al. Comparisons of Dipstick Test, Urine Protein-to-Creatine Ratio, and Total Protein Measurement for the Diagnosis of Preeclampsia. Int. J. Environ. Res. Public Health 2020, 17, 4195. https://doi.org/10.3390/ijerph17124195
Stefańska K, Zieliński M, Zamkowska D, Adamski P, Jassem-Bobowicz J, Piekarska K, Jankowiak M, Leszczyńska K, Świątkowska-Stodulska R, Preis K, et al. Comparisons of Dipstick Test, Urine Protein-to-Creatine Ratio, and Total Protein Measurement for the Diagnosis of Preeclampsia. International Journal of Environmental Research and Public Health. 2020; 17(12):4195. https://doi.org/10.3390/ijerph17124195
Chicago/Turabian StyleStefańska, Katarzyna, Maciej Zieliński, Dorota Zamkowska, Przemysław Adamski, Joanna Jassem-Bobowicz, Karolina Piekarska, Martyna Jankowiak, Katarzyna Leszczyńska, Renata Świątkowska-Stodulska, Krzysztof Preis, and et al. 2020. "Comparisons of Dipstick Test, Urine Protein-to-Creatine Ratio, and Total Protein Measurement for the Diagnosis of Preeclampsia" International Journal of Environmental Research and Public Health 17, no. 12: 4195. https://doi.org/10.3390/ijerph17124195
APA StyleStefańska, K., Zieliński, M., Zamkowska, D., Adamski, P., Jassem-Bobowicz, J., Piekarska, K., Jankowiak, M., Leszczyńska, K., Świątkowska-Stodulska, R., Preis, K., Trzonkowski, P., & Marek-Trzonkowska, N. (2020). Comparisons of Dipstick Test, Urine Protein-to-Creatine Ratio, and Total Protein Measurement for the Diagnosis of Preeclampsia. International Journal of Environmental Research and Public Health, 17(12), 4195. https://doi.org/10.3390/ijerph17124195






