Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review
Abstract
1. Introduction
1.1. Burden of Wound Healing
1.2. Therapies for Skin Wound Healing
1.3. Nigella sativa and Its Bioactive Component in Skin Wound Healing
1.4. Wound Healing Cascade
1.5. Wound Healing Models
1.6. Wound Healing Parameters
2. Methods
2.1. Literature Review
2.2. Selection of Research Articles
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Management
3. Results
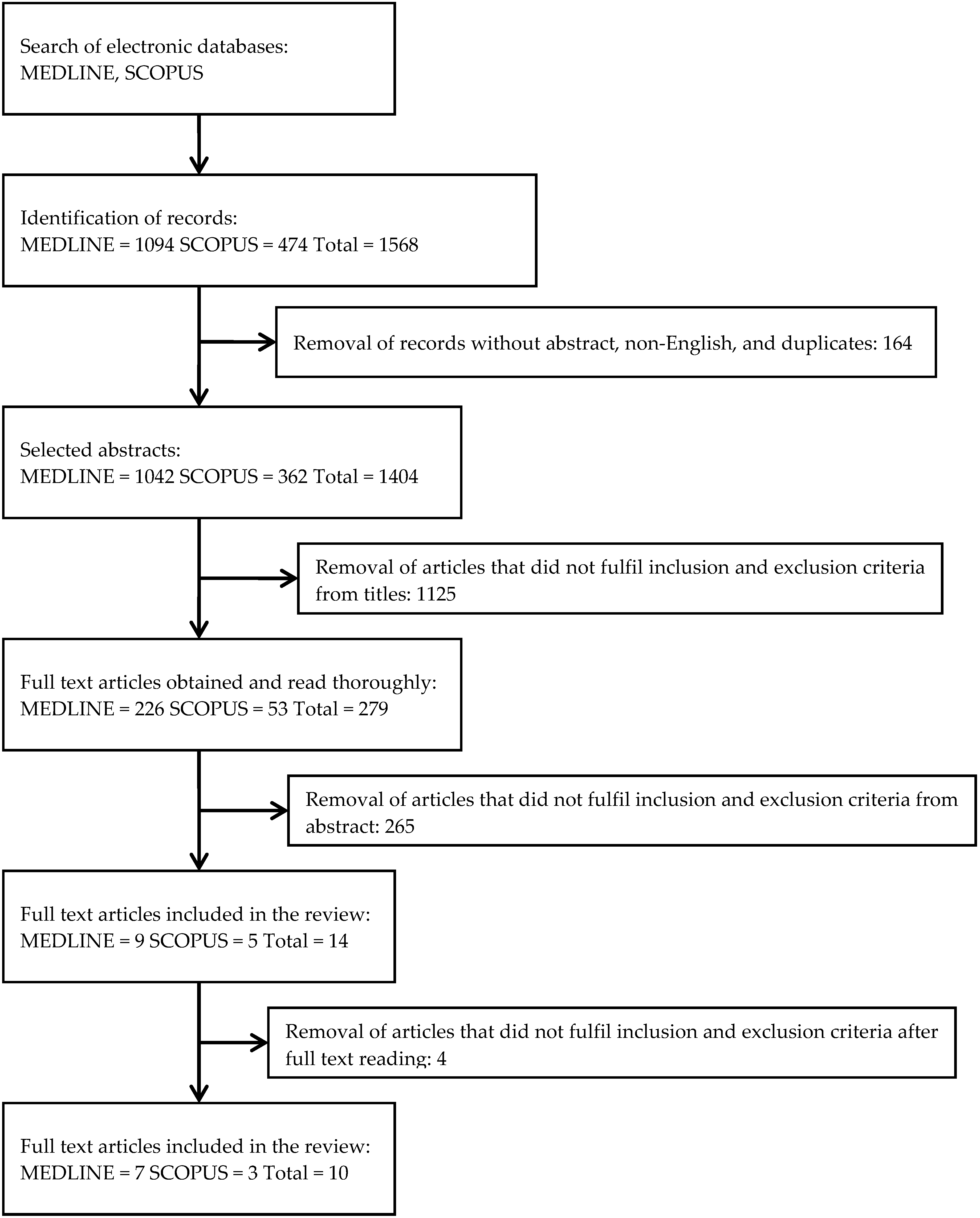
3.1. Search Results
3.2. Study Characteristics
3.3. NS in Skin Wound Healing
3.3.1. Gross Appearances
3.3.2. Microscopic Findings
3.3.3. Biochemical Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.S.; Bebell, L.M.; Meney, C.; Valeri, L.; White, M.C. Epidemiology of antibiotic-resistant wound infections from six countries in Africa. BMJ Glob. Health 2018, 2 (Suppl. 4), e000475. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Bartolo, P.J. Traditional therapies for skin wound healing. Adv. Wound Care 2016, 5, 208–229. [Google Scholar] [CrossRef] [PubMed]
- Turhan, Y.; Arıcan, M.; Karaduman, Z.O.; Turhal, O.; Gamsızkan, M.; Aydın, D.; Ozkan, K. Comparison of the Effects of Nigella sativa Oil and Nano-silver on Wound Healing in an Experimental Rat Model. Iran. Red Crescent Med. J. 2019, 21, e84650. [Google Scholar] [CrossRef]
- Al-Zamily, R.H.; Al-Temimi, S.M.A. Comparison of the effects of Nigella sativa oil and laser on treatments on experimental wound healing in rats. Asian J. Pharm. Clin. Res. 2019, 12, 295–299. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Qureshi, J.; Danish, Z.; Musayab, S.; Akhtar, M.F.; Saleem, A.; Khan, K.K.; Zaman, M.; Waheed, I.; et al. Nigella sativa (Prophetic Medicine): A Review. Pak. J. Pharm. Sci. 2017, 30, 229–234. [Google Scholar]
- Dafni, A.; Böck, B. Medicinal plants of the Bible—Revisited. J. Ethnobiol. Ethnomed. 2019, 15, 1–4. [Google Scholar] [CrossRef]
- Polat, R.; Satil, F.; Cakilcioglu, U. Medicinal plants and their use properties of sold in herbal market in Bingoel(Turkey) district. Biodivers Conserv. 2011, 4, 25–35. [Google Scholar]
- Rajsekhar, S.; Kuldeep, B. Pharmacognosy and pharmacology of Nigella sativa-A review. Int. Res. J. Pharm. 2011, 2, 36–39. [Google Scholar]
- Tiwari, P.; Jena, S.; Satpathy, S.; Sahu, P.K. Nigella sativa: Phytochemistry, Pharmacology and its Therapeutic Potential. Res. J. Pharm. Technol. 2019, 12, 3111–3116. [Google Scholar] [CrossRef]
- Cascella, M.; Palma, G.; Barbieri, A.; Bimonte, S.; Amruthraj, N.J.; Muzio, M.R.; Del Vecchio, V.; Rea, D.; Falco, M.; Luciano, A.; et al. Role of Nigella sativa and Its Constituent Thymoquinone on Chemotherapy-Induced Nephrotoxicity: Evidences from Experimental Animal Studies. Nutrients 2017, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del, V.V.; Muzio, M.R.; Vitale, A.; Benincasa, G.; Ferriello, A.B.; Azzariti, A.; Arra, C.; et al. Dissecting the Potential Roles of Nigella sativa and Its Constituent Thymoquinone on the Prevention and on the Progression of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Shahani, M.Y.; Memon, S.; Shahani, S.B.; Bano, U.; Arain, S.N. Effect of Nigella Sativa Extract Oil on Granulation Tissue in Cutaneous Wound: An Experimental Study in a Rabbit Model. Med. Forum Mon. 2013, 24, 72–77. [Google Scholar]
- Abu-Al-Basal, M.A. Influence of Nigella sativa fixed oil on some blood parameters and histopathology of skin in staphylococcal infected BALB/c mice. Pak. J. Biol. Sci. 2011, 14, 1038–1046. [Google Scholar]
- Venkatachallam, S.K.; Pattekhan, H.; Divakar, S.; Kadimi, U.S. Chemical composition of Nigella sativa L. seed extracts obtained by supercritical carbon dioxide. Int. J. Food Sci. Tech. 2010, 47, 598–605. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar]
- Darakhshan, S.; Bidmeshki, P.A.; Hosseinzadeh, C.A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Singh, S.; Young, A.; McNaught, C.E. The physiology of wound healing. Surgery 2017, 35, 473–477. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Kumar, V.; Khan, A.A.; Nagarajan, K. Animal models for the evaluation of wound healing activity. Int. Bull. Drug Res. 2013, 3, 93–107. [Google Scholar]
- Percival, N.J. Classification of wounds and their management. Surgery (Oxford) 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Jockenhöfer, F.; Gollnick, H.; Herberger, K.; Isbary, G.; Renner, R.; Stücker, M.; Valesky, E.; Wollina, U.; Weichenthal, M.; Karrer, S.; et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: Retrospective evaluation of 1 000 patients from 10 specialised dermatological wound care centers in Germany. Int. Wound J. 2016, 13, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013. [Google Scholar] [CrossRef]
- Milne, K.; Penn-Barwell, J. Classification and management of acute wounds and open fractures. Surgery (Oxford) 2020, 38, 143–149. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A.; Wysocki, A.B. Rat models of skin wound healing. In Sourcebook of Models for Biomedical Research; Humana Press: Totowa, NJ, USA, 2008; pp. 631–638. [Google Scholar]
- Estevão, L.R.; Cassini-Vieira, P.; Leite, A.B.; Bulhões, A.A.; Barcelos, L.S.; Evêncio-Neto, J. Morphological Evaluation of Wound Healing Events in the Excisional Wound Healing Model in Rats. Bio-Protocol 2019, 9, e3285. [Google Scholar] [CrossRef]
- Takeo, M.; Lee, W.; Ito, M. Wound Healing and Skin Regeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a023267. [Google Scholar] [CrossRef]
- Mh Busra, F.; Rajab, N.F.; Tabata, Y.; Saim, A.B.; BH Idrus, R.; Chowdhury, S.R. Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef]
- Grada, A.; Mervis, J.; Falanga, V. Research techniques made simple: Animal models of wound healing. J. Investig. Dermatol. 2018, 138, 2095–2105. [Google Scholar] [CrossRef]
- Parnell, L.K.; Volk, S.W. The Evolution of Animal Models in Wound Healing Research: 1993–2017. Adv. Wound Care 2019, 8, 692–702. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets. 2018, 29, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-specific contribution of macrophages to wound healing. Semin. Cell Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Kopecki, Z.; Cowin, A.J. The Role of Actin Remodelling Proteins in Wound Healing and Tissue Regeneration. In Wound Healing-New insights into Ancient Challenges; IntechOpen: London, UK, 2016. [Google Scholar]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Prescott, C.; Bottle, S.E. Biological Relevance of Free Radicals and Nitroxides. Cell Biochem. Biophys. 2017, 75, 227. [Google Scholar] [CrossRef]
- Nordin, A.; Kamal, H.; Yazid, M.D.; Saim, A.; Idrus, R. Effect of Nigella sativa and its bioactive compound on type 2 epithelial to mesenchymal transition: A systematic review. BMC Complement. Altern. Med. 2019, 19, 290. [Google Scholar] [CrossRef]
- Javadi, S.M.; Hashemi, M.; Mohammadi, Y.; MamMohammadi, A.; Sharifi, A.; Makarchian, H.R. Synergistic effect of honey and Nigella sativa on wound healing in rats. Acta Cir. Bras. 2018, 33, 518–523. [Google Scholar] [CrossRef]
- Han, M.C.; Durmuş, A.S.; Sağliyan, A.; Günay, C.; Özkaraca, M.; Kandemir, F.M.; Comakli, S.; Öztopalan, D.F. Effects of Nigella sativa and Hypericum perforatum on wound healing. Turk. J. Vet. Anim. Sci. 2017, 41, 99–105. [Google Scholar] [CrossRef]
- Kumandaş, A.; Karslı, B.; Kürüm, A.; Çınar, M.; Elma, E. Comparison of the effects of zinc-silver cream and Nigella sativa oil on wound healing and oxidative stress in the wound model in rats. Vet. Fak. Derg. 2019, 67, 33–40. [Google Scholar] [CrossRef]
- Sari, Y.; Purnawan, I.; Kurniawan, D.W.; Sutrisna, E. A comparative study of the effects of Nigella sativa oil gel and aloe vera gel on wound healing in diabetic rats. J. Evid.-Based Integr. Med. 2018, 23, 2515690X18772804. [Google Scholar] [CrossRef] [PubMed]
- Yusmin, A.; Ahmad, N. Effect of Thymoquinone On Wound Healing in Alloxan-Induced Diabetic Rats. Asian J. Pharm. Clin. Res. 2017, 10, 242–245. [Google Scholar] [CrossRef]
- Nourbar, E.; Mirazi, N.; Yari, S.; Rafeian-Kopaei, M.; Nasri, H. Effect of Hydroethanolic Extract of Nigella sativa L. on Skin Wound Healing Process in Diabetic Male Rats. Int. J. Prev. Med. 2019, 1, 10–18. [Google Scholar]
- Selçuk, C.T.; Durgun, M.; Tekin, R.; Yolbas, L.; Bozkurt, M.; Akçay, C.; Alabalık, U.; Basarali, M.K. Evaluation of the effect of thymoquinone treatment on wound healing in a rat burn model. J. Burn. Care Res. 2013, 34, e274–e281. [Google Scholar] [CrossRef]
- Yaman, I.; Durmus, A.S.; Ceribasi, S.; Yaman, M. Effects of Nigella sativa and silver sulfadiazine on burn wound healing in rats. Vet. Med. 2010, 55, 619–624. [Google Scholar] [CrossRef]
- Elgohary, H.M.; Al Jaouni, S.K.; Selim, S.A. Effect of ultrasound-enhanced Nigella sativa seeds oil on wound healing: An animal model. J. Taibah Univ. Sci. 2018, 13, 438–443. [Google Scholar] [CrossRef]
- Sheehan, P.; Jones, P.; Caselli, A.; Giurini, J.M.; Veves, A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003, 26, 1879–1882. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care (New Rochelle) 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Theunissen, D.; Seymour, B.; Forder, M.; Cox, S.G.; Rode, H. Measurements in wound healing with observations on the effects of topical agents on full thickness dermal incised wounds. Burns 2016, 42, 556–563. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119. [Google Scholar] [CrossRef]
- Mahant, S.; Kumar, V.; Rao, R.; Nanda, S. Physical methods for enhancement of transdermal drug delivery in pain management. Int. J. Pharm. Sci. 2017, 8, 353. [Google Scholar]
- Belwal, T.; Devkota, H.P.; Singh, M.K.; Sharma, R.; Upadhayay, S.; Joshi, C.; Bisht, K.; Gour, J.K.; Bhatt, I.D.; Rawal, R.S.; et al. St. John’s Wort (Hypericum perforatum). In Nonvitamin and Nonmineral Nutritional Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 415–432. [Google Scholar]
- Hesselink, J.M. Phenytoin repositioned in wound healing: Clinical experience spanning 60 years. Drug Discov. Today 2018, 23, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. JAAOS Glob. Res. Rev. 2017, 1, e022. [Google Scholar] [CrossRef]
- Radekovic, M.; Stojanović, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol. Toxicol. Methods 2016, 78, 13–31. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthet Res. 2015, 2, 239–242. [Google Scholar] [CrossRef]
- Cui, X.; Gong, J.; Han, H.; He, L.; Teng, Y.; Tetley, T.; Sinharay, R.; Chung, K.F.; Islam, T.; Gilliland, F.; et al. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J. Thorac. Dis. 2018, 10, 3088. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns: Pathophysiology of systemic complications and current management. J. Burn. Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef]
- Vigani, A.; Culler, C.A. Systemic and local management of burn wounds. Vet. Clin. North AM. Small Anim. Pract. 2017, 47, 1149–1163. [Google Scholar] [CrossRef]
- Masood, R.A.; Wain, Z.N.; Tariq, R.; Bashir, I. Burn cases, their management and complications: A review. Int. Curr. Pharm. 2016, 5, 103–105. [Google Scholar] [CrossRef][Green Version]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Arici, M.; Sagdic, O.; Gecgel, U. Antibacterial effect of Turkish black cumin (Nigella sativa L.) oils. Grasas Aceites 2005, 56, 259–262. [Google Scholar] [CrossRef]
- Hassan, S.; Ahmed, W.; Galeb, F.M.; El-Taweel, M. In Vitro Challenge using Thymoquinone on Hepatocellular Carcinoma (HepG2) Cell Line. Iran. J. Pharm. Res. 2010, 7, 283–290. [Google Scholar]
- Basheer, I.; Qureshi, I.Z. Short and long term modulation of tissue minerals concentrations following oral administration of black cumin (Nigella sativa L.) seed oil to laboratory rats. Phytomedicine 2018, 39, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Suboh, S.M.; Bilto, Y.Y.; Aburjai, T.A. Protective effects of selected medicinal plants against protein degradation, lipid peroxidation and deformability loss of oxidatively stressed human erythrocytes. Phytother. Res. 2004, 18, 280–284. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Ng, P.Y.; Ng, S.F.; Ariffin, H.; Amin, M.C.I.M. Cellular and Molecular Interaction of Human Dermal Fibroblasts with Bacterial Nanocellulose Composite Hydrogel for Tissue Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 39532–39543. [Google Scholar] [CrossRef]
- Busra, M.F.; Chowdhury, S.R.; bin Ismail, F.; bin Saim, A.; Idrus, R.B. Tissue-Engineered Skin Substitute Enhances Wound Healing after Radiation Therapy. Adv. Skin Wound Care 2016, 29, 120–129. [Google Scholar] [CrossRef]
- Yan, H.; Peng, X.; Huang, Y.; Zhao, M.; Li, F.; Wang, P. Effects of early enteral arginine supplementation on resuscitation of severe burn patients. Burns 2007, 33, 179–184. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 2007, 14, 621–627. [Google Scholar] [CrossRef]
- Vorauer-Uhl, K.; Fürnschlief, E.; Wagner, A.; Ferko, B.; Katinger, H. Reepithelialization of experimental scalds effected by topically applied superoxide dismutase: Controlled animal studies. Wound Repair Regen. 2002, 10, 366–371. [Google Scholar] [CrossRef]
- Liu, P.; Chen, D.; Shi, J. Chemical constituents, biological activity and agricultural cultivation of aloe vera-a review. Asian J. Chem. 2013, 25, 6477. [Google Scholar] [CrossRef]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J. Ethnopharmacol. 1998, 59, 195–201. [Google Scholar] [CrossRef]
| References | Experimental Model | Form of NS | Methods | Results | Conclusion |
|---|---|---|---|---|---|
| Kumandaş et al., 2019 [42] | Excision wound in male Wistar-albino rats | NS oil cream | Treatment groups:
| Outcomes:
| NS oil caused better epithelialization and granulation tissue while reducing vascularization and inflammation during wound healing. |
| Nourbar et al., 2019 [45] | Chronic delayed wound in streptozotocin-induced diabetic rats | NS extract | Treatment groups:
| Outcome:
| NS extract could accelerate wound healing in streptozotocin-induced diabetic rats. |
| Elgohary et al., 2018 [48] | Chemical burn using concentrated HCl (38%) in albino rabbits | NS oil | Treatment groups:
| Outcomes:
| Pulsed phonophoresis using NS oil can be used as an adjunct treatment with limited side effects to promote wound contraction and inhibit inflammation; thus, accelerating wound healing. |
| Javadi et al., 2018 [40] | Excision wound in male Wistar-albino rats | Cold pressed NS seed oil | Treatment groups:
| Outcomes:
| NS seed oil can accelerate wound healing and the effect is greater in combination with honey. |
| Sari et al., 2018 [43] | Chronic delayed wound in alloxan-induced diabetic male Wistar rats | NS oil gel | Treatment groups:
| Outcomes:
| NS oil gel has negligible effect on wound healing in diabetic rats. |
| Han et al., 2017 [41] | Full thickness wound in female Wistar-albino rats | NS oil cream | Treatment groups:
| Outcomes:
| NS exerts a wound healing effect through its antioxidant property, in contrast to HP that enhances wound healing via epithelialization and granulation-encouraging effects. |
| Shahani et al., 2013 [14] | Cutaneous wound in Wistar rabbits | NS extract oil | Treatment groups:
| Outcomes:
| NS extract oil induces angiogenesis, fibroblasts proliferation, and collagen synthesis during wound healing in rabbit. |
| Yaman et al., 2010 [47] | Burn wound model in male Wistar-albino rats | NS oil | Treatment groups:
| Outcomes:
| NS was found to accelerate healing process via its antimicrobial, antioxidant, anti-inflammatory, and immunomodulatory effects. |
| References | Experimental Model | Form of NS | Methods | Results | Conclusion |
|---|---|---|---|---|---|
| Yusmin and Ahmad 2017 [44] | Chronic delayed wound in alloxan-induced diabetic rats | TQ in petroleum jelly | Treatment groups:
| Outcomes:
| TQ accelerated wound healing during the inflammatory phase but decelerated wound healing during the granulation phase in diabetic rats. |
| Selçuk et al., 2013 [46] | Deep second degree burn in Sprague–Dawley rats | Topical and intraperitoneal delivery of TQ | Treatment groups:
| Outcomes:
| TQ appears to accelerate the rate of wound closure both in topical and systemic administrations, and this effect is stronger for the topical administration. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. Int. J. Environ. Res. Public Health 2020, 17, 4160. https://doi.org/10.3390/ijerph17114160
Sallehuddin N, Nordin A, Bt Hj Idrus R, Fauzi MB. Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. International Journal of Environmental Research and Public Health. 2020; 17(11):4160. https://doi.org/10.3390/ijerph17114160
Chicago/Turabian StyleSallehuddin, Nusaibah, Abid Nordin, Ruszymah Bt Hj Idrus, and Mh Busra Fauzi. 2020. "Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review" International Journal of Environmental Research and Public Health 17, no. 11: 4160. https://doi.org/10.3390/ijerph17114160
APA StyleSallehuddin, N., Nordin, A., Bt Hj Idrus, R., & Fauzi, M. B. (2020). Nigella sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. International Journal of Environmental Research and Public Health, 17(11), 4160. https://doi.org/10.3390/ijerph17114160







