Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018
Abstract
1. Introduction
2. Materials and Methods
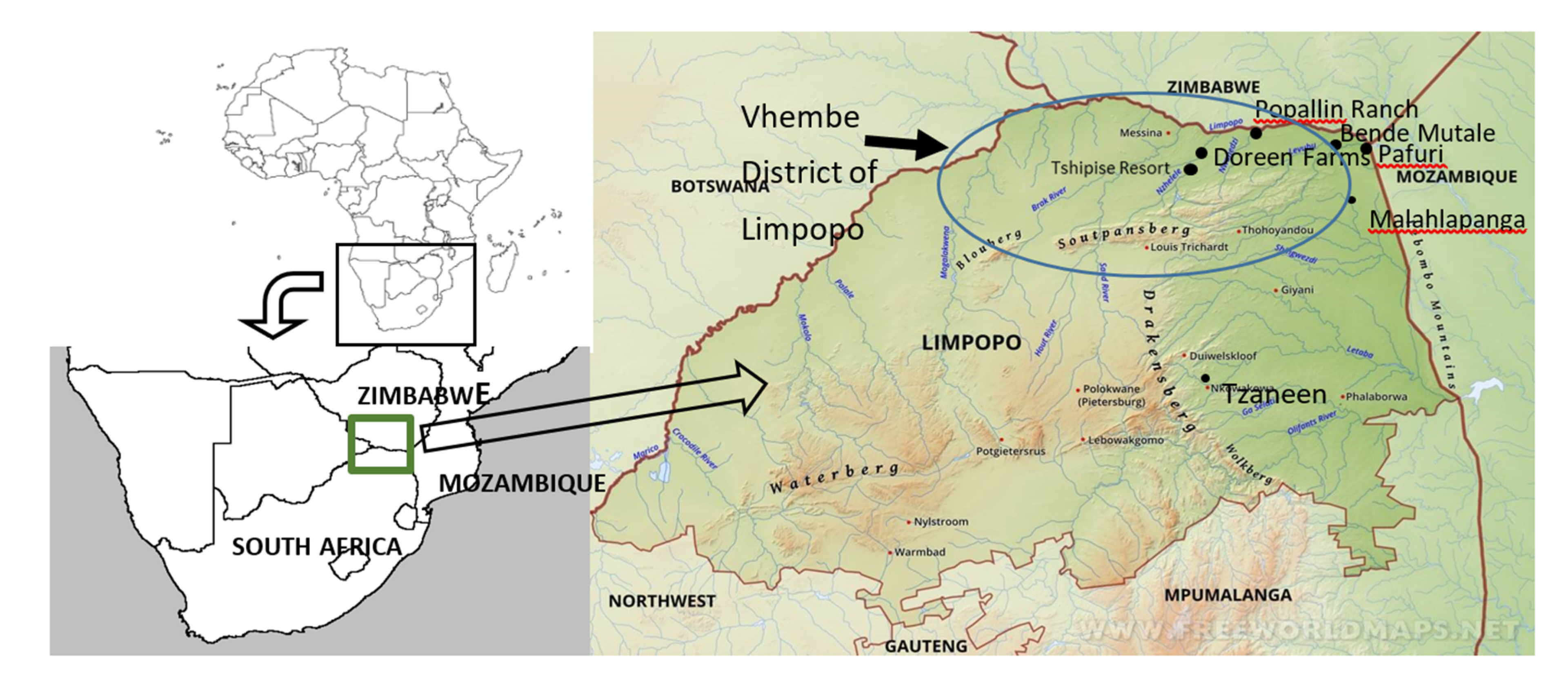
2.1. Study Area
2.2. Data Sources
2.3. Vector Surveillance and Methods Used for New Data Presented Here
3. Results
3.1. Historical Surveys in Limpopo Province
3.2. Surveys in Surrounding Areas
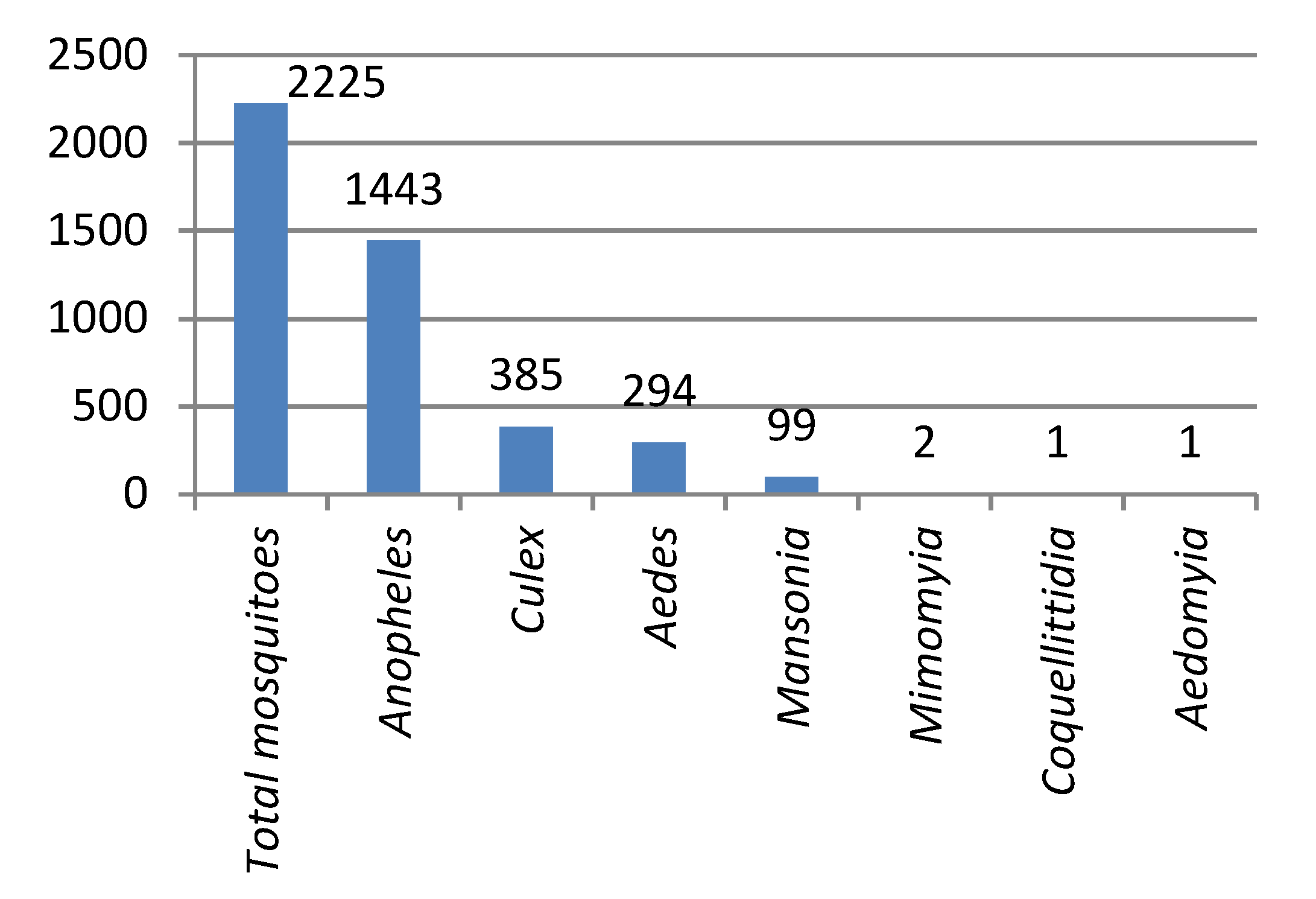
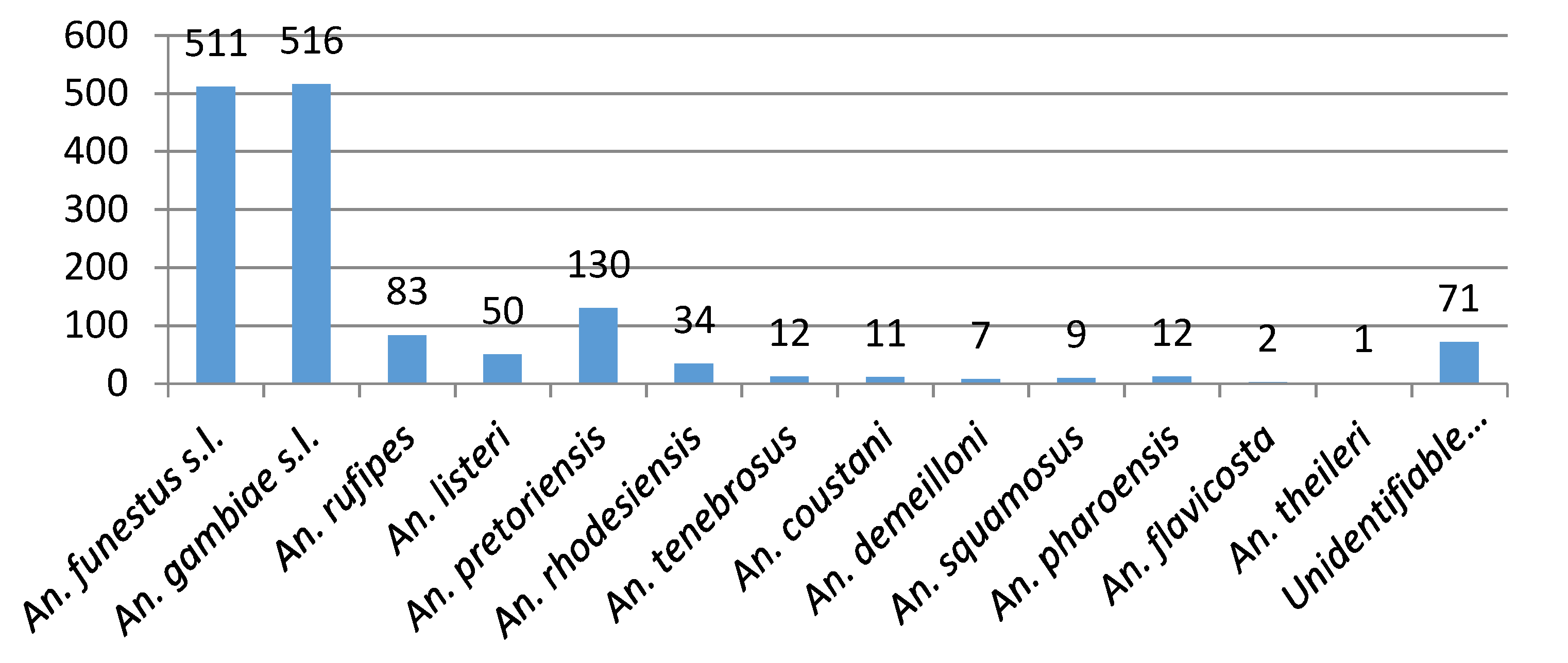
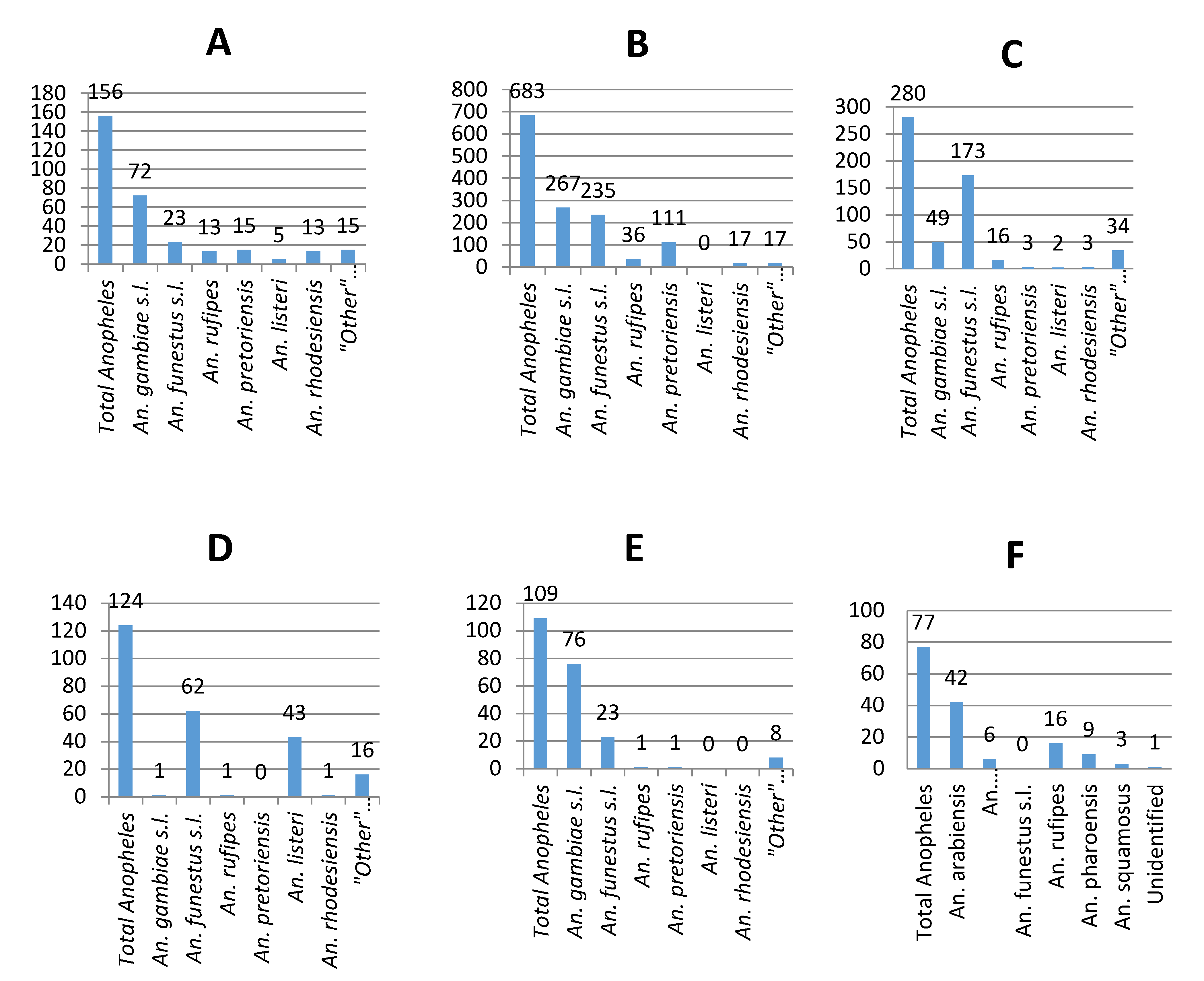
3.3. 2017/2018 Vector Surveys
3.4. Sporozoite Assays
4. Discussion
4.1. Vector Status of Members of the Anopheles Gambiae Complex and Their Role in the Limpopo River Valley
4.2. Vector Status of Members of the Anopheles Funestus Group and Their Role in the Limpopo River Valley
4.3. Other Anophelines of Potential Vector Importance
4.4. Importation of Infective Mosquitoes into South Africa
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
Abbreviations
| CDC | Centres for Disease Control and Prevention |
| DDT | Dichlorodiphenyltrichloroethane |
| IRS | Indoor Residual Spraying (of insecticides inside houses) |
| KNP | Kruger National Park |
| NDoH | National Department of Health |
| NICD | National Institute for Communicable Diseases |
| PCR | Polymerase Chain Reaction |
| s.l. | sensu lato (in the broad sense, i.e., the group or complex) |
| s.s. | sensu stricto (in the strict sense, i.e., the original species) |
| UP ISMC | University of Pretoria Institute for Sustainable Malaria Control |
References
- Bulpin, T.V. Lost Trails of the Transvaal; Books of Africa Party publisher: Cape Town, South Africa, 1983. [Google Scholar]
- Swellengrebel, N.H.; Annecke, S.; De Meillon, B. Malaria investigations in some parts of the Transvaal and Zululand. Publ S. Afr. Inst. Med. Res. 1931, 4, 245–274. [Google Scholar]
- Mabaso, M.L.; Sharp, B.; Lengeler, C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop. Med. Int. Health 2004, 9, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Kruger, P.; Hunt, R.; Durrheim, D.; Urbach, J.; Hansford, C. Malaria in South Africa: 110 years of learning to control the disease. S. Afr. Med. J. 2013, 103, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasit. Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Ingram, A.; De Meillon, B. A mosquito survey of certain parts of South Africa, with special reference to the carriers of malaria and their control. Part, I. Publ. S. Afr. Inst. Med. Res. 1927, 22, 1–81. [Google Scholar]
- Ingram, A.; De Meillon, B. A mosquito survey of certain parts of South Africa, with special reference to the carriers of malaria and their control. Part II. Publ. S. Afr. Inst. Med. Res. 1929, 4, 83–170. [Google Scholar]
- De Meillon, B. Entomological studies—Observations on Anopheles funestus and Anopheles gambiae in the Transvaal. Publ. S. Afr. Inst. Med. Res. 1934, 32, 195–248. [Google Scholar]
- Paterson, H.E. Direct evidence for the specific distinctness of forms A, B and C of the Anopheles gambiae complex. Riv. Malariol. 1964, 43, 191–196. [Google Scholar]
- Paterson, H.E.; Paterson, J.S.; Van Eeden, G.J. A new member of the Anopheles gambiae complex: A preliminary report. Med. Proc. 1963, 9, 414–418. [Google Scholar]
- Davidson, G.; Paterson, H.E.; Coluzzi, M.; Mason, G.F.; Micks, D.W. The Anopheles gambiae complex. In Genetics of Insect Vectors of Disease; Wright, J.W., Pal, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1972; pp. 211–250. [Google Scholar]
- Coluzzi, M.; Sabatini, A.; Petrarca, V.; Di Deco, M.A. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 483–497. [Google Scholar] [CrossRef]
- Steyn, J.J.; Rose Innes, R.; Schulz, K.H. A culicine mosquito survey of the upper Limpopo River Valley. J. Entomol. Soc. S. Afr. 1955, 18, 238–246. [Google Scholar]
- La Grange, J.; Coetzee, M. A mosquito survey of Thomo village, Northern Province, South Africa, with special reference to the bionomics of exophilic members of the Anopheles funestus group (Diptera: Culicidae). Afr. Entomol. 1997, 5, 295–299. [Google Scholar]
- Govere, J.; Durrheim, D.N.; Coetzee, M.; Hunt, R.H.; La Grange, J.J. Captures of mosquitoes of the Anopheles gambiae complex (Diptera: Culicidae) in the Lowveld Region of Mpumalanga Province, South Africa. Afr. Entomol. 2000, 8, 91–99. [Google Scholar]
- Munhenga, G.; Brooke, B.D.; Spillings, B.; Essop, L.; Hunt, R.H.; Midzi, S.; Govender, D.; Braack, L.; Koekemoer, L.L. Field study site selection, species abundance and monthly distribution of anopheline mosquitoes in the northern Kruger National Park, South Africa. Malar. J. 2014, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.J.; Lee, Y.; Almeida, A.P.G.; Johnson, T.; Mouatcho, J.; Venter, M.; De Jager, C.; Braack, L. Mosquito community composition in South Africa and some neighboring countries. Parasit. Vectors 2018, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Brisco, K.K.; Cornel, A.J.; Lee, Y.; Mouatcho, J.; Braack, L. Comparing efficacy of a sweep net and a dip method for collection of mosquito larvae in large bodies of water in South Africa. F1000Research 2016, 5, 713. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; Coetzee, M. A supplement to the Anophelinae of Africa south of the Sahara (Afrotropical Region). Publ. S. Afr. Inst. Med. Res. 1987, 55, 1–143. [Google Scholar]
- Koekemoer, L.L.; Kamau, L.; Hunt, R.H.; Coetzee, M. A cocktail polymerase chain reaction (PCR) assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am. J. Trop. Med. Hyg. 2002, 66, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Padley, D.; Moody, A.H.; Chiodini, P.L.; Saldanha, J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 2003, 97, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mahon, R.J.; Green, C.A.; Hunt, R.H. Diagnostic allozymes for routine identification of adults of the Anopheles gambiae complex (Diptera, Culicidae). Bull. Entomol. Res. 1976, 66, 25–31. [Google Scholar] [CrossRef]
- Munhenga, G.; Brooke, B.D.; Chirwa, T.F.; Hunt, R.H.; Coetzee, M.; Govender, D.; Koekemoer, L.L. Evaluating the potential of the sterile insect technique for malaria control: Relative fitness and mating compatibility between laboratory colonized and a wild population of Anopheles arabiensis from the Kruger National Park, South Africa. Parasit. Vectors 2011, 4, 208. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, M.; Hunt, R.H.; Braack, L.; Davidson, G. Distribution of mosquitoes belonging to the Anopheles gambiae complex, including malaria vectors, south of latitude 15° S. S. Afr. J. Sci. 1993, 89, 227–231. [Google Scholar]
- Braack, L.E.O.; Coetzee, M.; Hunt, R.H.; Biggs, H.; Cornel, A.; Gericke, A. Biting pattern and host-seeking behavior of Anopheles arabiensis (Diptera: Culicidae) in northeastern South Africa. J. Med. Entomol. 1994, 31, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Mbokazi, F.; Coetzee, M.; Brooke, B.; Govere, J.; Reid, A.; Owiti, P.; Kosgei, R.; Zhou, S.; Magagula, R.; Kok, G.; et al. Changing distribution and abundance of the malaria vector Anopheles merus in Mpumalanga Province, South Africa. Public Health Action 2018, 8, S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Sande, S.; Zimba, M.; Chinwada, P.; Masendu, H.; Makuwaza, A. Malaria vector species composition and relative abundance in Mutare and Mutasa districts, Zimbabwe. J. Entomol. Acarol. Res. 2015, 47, 79–85. [Google Scholar] [CrossRef]
- Masendu, H.; Hunt, R.H.; Koekemoer, L.L.; Brooke, B.; Govere, J.; Coetzee, M. Spatial and temporal distributions and insecticide susceptibility of malaria vectors in Zimbabwe. Afr. Entomol. 2005, 13, 25–34. [Google Scholar]
- Mpofu, S.M. Seasonal vector density and disease incidence patterns of malaria in an area of Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 1985, 79, 169–175. [Google Scholar] [CrossRef]
- Taylor, P.; Mutambu, S. A review of the malaria situation in Zimbabwe with special reference to the period 1972–1981. Trans. R. Soc. Trop. Med. Hyg. 1986, 80, 12–19. [Google Scholar] [CrossRef]
- Zengenene, M.P.; Soko, W.; Brooke, B.D.; Koekemoer, L.L.; Govere, J.; Mazarire, T.T. Anopheles species composition and breeding habitat characterisation in Chiredzi District, Zimbabwe. Afr. Entomol. 2020, 28, 84–94. [Google Scholar] [CrossRef]
- Casimiro, S.; Coleman, M.; Hemingway, J.; Sharp, B. Insecticide resistance in Anopheles arabiensis and Anopheles gambiae from Mozambique. J. Med. Entomol. 2006, 43, 276–282. [Google Scholar] [CrossRef]
- Kyalo, D.; Amratia, P.; Mundia, C.W.; Mbogo, C.M.; Coetzee, M.; Snow, R.W. A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. Wellcome Open Res. 2017, 2, 57. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.D.; Kloke, G.; Hunt, R.H.; Koekemoer, L.L.; Temu, E.A.; Taylor, M.E.; Small, G.; Hemingway, J.; Coetzee, M. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bull. Entomol. Res. 2001, 91, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.; Coleman, M.; Mohloai, P.; Hemingway, J.; Sharp, B. Insecticide resistance in Anopheles funestus (Diptera: Culicidae) from Mozambique. J. Med. Entomol. 2006, 43, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, S.; Hemingway, J.; Sharp, B.; Coleman, M. Monitoring the operational impact of insecticide usage for malaria control on Anopheles funestus from Mozambique. Malar. J. 2007, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; De Meillon, B. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publ. S. Afr. Inst. Med. Res. 1968, 54, 1–343. [Google Scholar]
- Smith, A.; Hansford, C.F.; Thomson, J.F. Malaria along the southernmost fringe of its distribution in Africa: Epidemiology and control. Bull. World Health Org. 1977, 55, 95–103. [Google Scholar] [PubMed]
- Miles, S.J. Enzyme variation in the Anopheles gambiae Giles group of species (Diptera: Culicidae). Bull. Entomol. Res. 1978, 68, 85–96. [Google Scholar] [CrossRef]
- De Meillon, B. The control of malaria in South Africa by measures directed against the adult mosquitoes in habitations. Quart Bull. Health Org. L.o N. 1936, 5, 134–137. [Google Scholar]
- Park Ross, G.A. Insecticide as a major measure in control of malaria, being an account of the methods and organisation put in force in Natal and Zululand during the past six years. Quart Bull. Health Org. L.o N. 1936, 5, 114–133. [Google Scholar]
- Brooke, B.; Koekemoer, L.; Kruger, P.; Urbach, J.; Misiani, E.; Coetzee, M. Malaria vector control in South Africa. S. Afr. Med. J. 2013, 103, 784–788. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharp, B.L.; Ngxongo, S.; Botha, M.J.; Ridl, F.; Le Sueur, D. An analysis of 10 years of retrospective malaria data from the KwaZulu areas of Natal. S. Afr. J. Sci. 1988, 84, 102–106. [Google Scholar]
- Hargreaves, K.; Koekemoer, L.L.; Brooke, B.D.; Hunt, R.H.; Mthembu, J.; Coetzee, M. Anopheles funestus is resistant to pyrethroid insecticides in South Africa. Med. Vet. Entomol. 2000, 14, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Hansford, C.F. Recent trends in the control and treatment of malaria. S. Afr. Med. J. 1972, 46, 635–637. [Google Scholar]
- Sharp, B.L.; Le Sueur, D.; Bekker, P. Effect of DDT on survival and blood feeding success of Anopheles arabiensis in northern KwaZulu, Republic of South Africa. J. Am. Mosq. Control Assoc. 1990, 6, 197–202. [Google Scholar] [PubMed]
- Burke, A.; Dahan-Moss, Y.; Duncan, F.; Qwabe, B.; Coetzee, M.; Koekemoer, L.; Brooke, B. Anopheles parensis contributes to residual malaria transmission in South Africa. Malar. J. 2019, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Dandalo, L.C.; Brooke, B.D.; Munhenga, G.; Lobb, L.N.; Zikhali, J.; Ngxongo, S.P.; Zikhali, P.M.; Msimang, S.; Wood, O.R.; Mofokeng, M.; et al. Population dynamics and Plasmodium falciparum (Haemosporida: Plasmodiidae) infectivity rates for the malaria vector Anopheles arabiensis (Diptera: Culicidae) at Mamfene, KwaZulu-Natal, South Africa. J. Med. Entomol. 2017, 54, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Eling, W.; Hooghof, J.; Dekker, T.; Hunt, R.H.; Coetzee, M. Susceptibility of Anopheles quadriannulatus Theobald (Diptera: Culicidae) to Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 1999, 93, 578–580. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasit. Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Irish, S.R.; Kyalo, D.; Snow, R.W.; Coetzee, M. Updated list of Anopheles species (Diptera: Culicidae) by country in the Afrotropical Region and associated islands. Zootaxa 2020, 4747, 401–449. [Google Scholar]
- Temu, E.; Minjas, J.; Coetzee, M.; Hunt, R.; Shiff, C. The role of four anopheline species (Diptera: Culicidae) in malaria transmission in coastal Tanzania. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 152–158. [Google Scholar] [CrossRef]
- Cuamba, N.; Mendis, C. The role of Anopheles merus in malaria transmission in an area of southern Mozambique. J. Vector-borne Dis. 2009, 46, 157–159. [Google Scholar] [PubMed]
- Burke, A.; Dandalo, L.; Munhenga, G.; Dahan-Moss, Y.; Mbokazi, F.; Ngxongo, S.; Coetzee, M.; Koekemoer, L.; Brooke, B. A new malaria vector mosquito in South Africa. Sci. Rep. 2017, 7, 43779. [Google Scholar] [CrossRef] [PubMed]
- Mouatcho, J.; Cornel, A.J.; Dahan-Moss, Y.; Koekemoer, L.L.; Coetzee, M.; Braack, L. Detection of Anopheles rivulorum-like, a member of the Anopheles funestus group, in South Africa. Malar. J. 2018, 17, 195. [Google Scholar] [CrossRef] [PubMed]
- Temu, E.A.; Minjas, J.N.; Tuno, N.; Kawada, H.; Takagi, M. Identification of four members of the Anopheles funestus (Diptera: Culicidae) group and their role in Plasmodium falciparum transmission in Bagamoyo coastal Tanzania. Acta Trop. 2007, 102, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.; Matola, Y.; Charlwood, J. Anopheles rivulorum, a vector of human malaria in Africa. Med. Vet. Entomol. 1996, 10, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Dida, G.O.; Sonye, G.; Njenga, S.M.; Mwandawiro, C.; Minakawa, N. Reconsideration of Anopheles rivulorum as a vector of Plasmodium falciparum in western Kenya: Some evidence from biting time, blood preference, sporozoite positive rate, and pyrethroid resistance. Parasit. Vectors. 2012, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Frean, J.; Brooke, B.; Thomas, J.; Blumberg, L. Odyssean malaria outbreaks in Gauteng Province, South Africa, 2007–2013. S. Afr. Med. J. 2014, 104, 335–338. [Google Scholar] [CrossRef] [PubMed][Green Version]

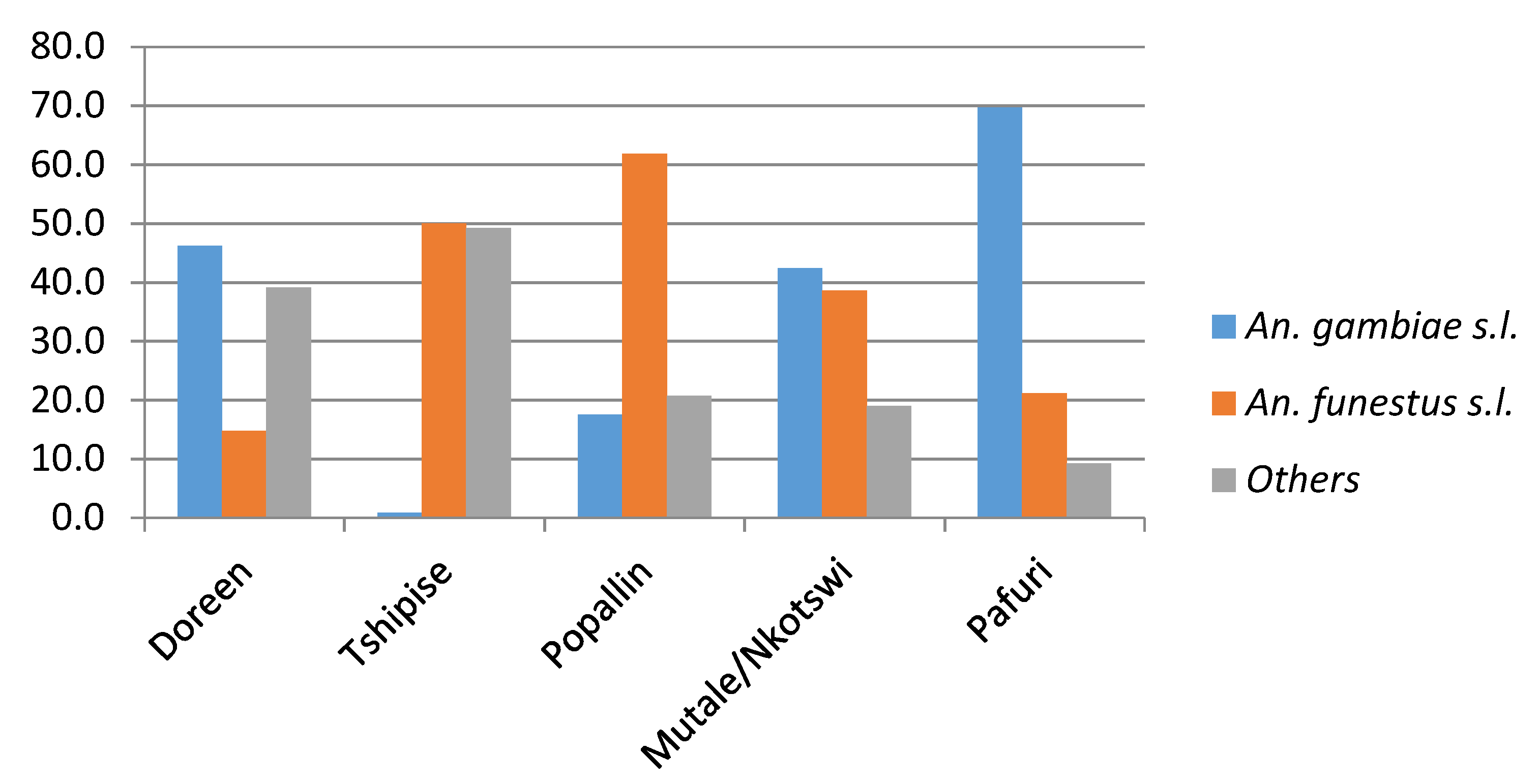
| Author(s) [Reference] | Date | Outline of Publication Content | Key Findings |
|---|---|---|---|
| Ingram & De Meillon [6] | 1927 | Mosquito survey in 1926, results covering northern Transvaal (the current Limpopo Province) and coastal Zululand (current KwaZulu-Natal), indicating distribution and breeding sites (larval collections having been the primary survey tool), with discussion around malaria vector species and control options. | Thirteen anopheline species/species groups found, speculating that An. funestus and An. gambiae are the main vectors despite strongly fluctuating population numbers and apparent extended absence even during malaria transmission periods. |
| Ingram & De Meillon [7] | 1929 | Mosquito survey in 1928, results covering northern and eastern Transvaal (Limpopo and Mpumalanga Provinces respectively) with discussion around malaria vector species and control options. | Thirteen anopheline species found, mostly through larval collections. |
| Swellengrebel et al. [2] | 1931 | Survey of anophelines in different habitat settings in “Transvaal” and “Zululand” to detect parasite positivity rates. | Six Anopheles species found indoors, and malaria parasites found in An. funestus, An. gambiae and also An. pretoriensis. |
| Steyn et al. [13] | 1955 | Two-week survey in March 1953 of mainly culicine mosquitoes by way of mostly larval collections, in the general area from Vaalwater to Musina in current Limpopo Province | 538 mosquito specimens making up 21 species in three genera (Anopheles 6 species; Aedes 9 spp.; Culex 6 spp.) |
| La Grange & Coetzee [14] | 1997 | Anopheline survey 1987–1989 in Thomo Village, Limpopo Province, using human landing catches, outdoor resting catches, and larval rearing. | Exophilic members of Anopheles funestus group most abundant, comprising 85.8% of total Anopheles catch (n = 23,252). Of Anopheles landing on humans, An coustani was most abundant at 70.1% (n = 2994), followed by members of the An. funestus group at 28.1%. Of An. gambiae complex captured (n = 245) or reared (n = 225), 155 and 164 respectively were An. quadriannulatus. No An. arabiensis were found. |
| Govere et al. [15] | 2000 | Monthly collections of Anopheles at 7 sites in the Lowveld region of Mpumalanga Province, August 1997–May 1998, using human landing catches, window exit traps, and indoor knockdown spraying. | A total of 5084 Anopheles were collected, of which 2837 (55.8%) were An. coustani, 1418 (27.9%) An. funestus group, 435 (8.6%) An. gambiae complex, 264 (5.2%) An. pretoriensis and 130 (2.6%) a mix of other anopheline species. Of the An. gambiae complex, An. merus (56%) and An. quadriannulatus (30.4%) dominated, with An. arabiensis making up 13.6%. |
| Munhenga et al. [16] | 2014 | Anopheline species collected from five sites over two years in the northern Kruger National Park as part of an assessment of sites for possible sterile male release for malaria vector control. | A total of 3311 anophelines comprising nine species, showing clear and consistent differences in Anopheles community composition between sites even relatively close to each other. |
| Cornel et al. [17] | 2018 | Description of mosquito diversity and abundance at multiple sites across southern Africa, including Shingwedzi and Lapalala Nature Reserve in Limpopo Province. | Eight species of Anopheles comprising 63.1% of the total catch of 168 mosquitoes at Shingwedzi. |
| Species | Zoutpans-Berg 1926 | Zoutpans-Berg 1928 | Waterberg 1926 | Waterberg 1928 | Skukuza 1928 | Tzaneen 1928 | Musina 1928 |
|---|---|---|---|---|---|---|---|
| An. cinereus | P | P | P | ||||
| An. coustani | P | P | P | P | P | P | |
| An. demeilloni | P | P | P | ||||
| An. funestus group | P | P | P | P | P | ||
| An. gambiae complex | P | P | P | P | P | P | |
| An. longipalpis | P | P | P | P | |||
| An. maculipalpis | P | ||||||
| An. marshallii | P | P | |||||
| An. natalensis | P | P | |||||
| An. nili | P | P | |||||
| An. pretoriensis | P | P | P | P | P | P | |
| An. quadriannulatus | P | ||||||
| An. rhodesiensis | P | P | P | P | P | ||
| An. rufipes | P | P | P | P | P | P | P |
| An. squamosus | P | P | P | P | |||
| An. theileri | P | P |
| Species | Letaba Foothills | Ofcolaco | ||
|---|---|---|---|---|
| Inside Rural Huts in Foothills Number of Mosquitoes (Number Parasite-Infected) | Inside Rural Farmhouses Number of Mosquitoes (Number Parasite-Infected) | Outside Rural Farmhouses Number of Mosquitoes (Number Parasite-Infected) | Inside Rural Huts in Lowland Area Number of Mosquitoes (Number Parasite-Infected) | |
| An. funestus group | 240 (44) | 44 (6) | 7 (0) | 53 (0) |
| An. gambiae complex | 6 (0) | - | 1 (0) | 161 (27) |
| An. maculipalpis | - | 1 (0) | - | - |
| An. marshallii | - | - | - | 1 (0) |
| An. pretoriensis | 9 (0) | 1 (1) | 110 (1) | - |
| An. rufipes | 4 (0) | - | 14 (0) | 1 (0) |
| Species | Larvae | Adults | Total |
|---|---|---|---|
| Anopheles coustani | 7 | 2 | 9 |
| An. gambiae complex | 23 | 3 | 26 |
| An. listeri | 19 | - | 19 |
| An. rufipes | 2 | 1 | 3 |
| An. pretoriensis | 12 | 1 | 13 |
| An. squamosus | - | 1 | 1 |
| Aedes spp. (scatophagoides, fulgens, aegypti, metallicus, calceatus, vittatus, marshalli, dentatus, hirsutus) | 257 | 71 | 328 |
| Culex spp. (tigripes, nebulosus var. pseudocinereus, theileri, univittatus, simpsoni, decens) | 88 | 51 | 139 |
| Totals | 408 | 130 | 538 |
| Species | HLC | Pit F | Pit M | Natural F | Natural M | Cattle Enclosures | Total |
|---|---|---|---|---|---|---|---|
| An. funestus group | 842 | 9234 | 6807 | 1819 | 1099 | 157 | 19,958 |
| An. gambiae complex | 6 | 45 | 20 | 115 | 51 | 8 | 245 |
| An. coustani | 2100 | 3 | 3 | 9 | 1 | 21 | 2137 |
| An. rufipes | 6 | 41 | 22 | 343 | 361 | 24 | 797 |
| An. squamosus | 25 | - | - | 1 | - | 6 | 32 |
| An. pretoriensis | - | - | 1 | 17 | 11 | - | 29 |
| An. marshallii | 2 | 2 | - | 5 | - | 1 | 10 |
| An. pharoensis | 4 | - | - | - | - | 3 | 7 |
| An. longipalpis | 5 | 4 | - | 3 | - | 1 | 13 |
| An. demeilloni | 1 | 2 | - | 2 | - | 3 | 8 |
| An. maculipalpis | 2 | - | - | 3 | - | 9 | 14 |
| An. theileri | 1 | - | - | - | - | 1 | 2 |
| Totals | 2994 | 9331 | 6853 | 2317 | 1523 | 234 | 23,252 |
| Species | Total Collected | Percentage Composition (Aggregate of Specimens Caught at All Sites) | Number of Sites Collected from |
|---|---|---|---|
| An. arabiensis | 1352 ** | 44.3 | 3 |
| An. quadriannulatus | 870 | 28.5 | 4 |
| An. merus | 349 *** | 11.4 | 2 |
| An. coustani | 395 | 12.9 | 3 |
| An. pretoriensis | 35 | 1.1 | 2 |
| An. maculipalpis | 28 | 0.9 | 3 |
| An. rivulorum | 19 | 0.6 | 2 |
| An. squamosus | 3 | 0.1 | 1 |
| An. rufipes | 2 | 0.1 | 1 |
| Locality | Anopheles (n) | No. Culex | No. Other Genera | Summary |
|---|---|---|---|---|
| Shingwedzi River, Kruger National Park, eastern Limpopo Province | An. leesoni (2) An. parensis (1) An. pharoensis (3) An. quadriannulatus (66) An. rivulorum (6) An. rivulorum-like (26) An. squamosus (4) An. theileri (1) Total: 109 | 25 | 34 | 109 Anopheles out of 168 mosquitoes = 65% |
| Lapalala Nature Reserve, western Limpopo Province | An. coustani (41) An. longipalpis (1) An. marshallii (54) An. squamosus (6) An. theileri (42) Total: 144 | 43 | 109 | 144 Anopheles out of 296 = 49% |
| Month | Sampling Days (%) | An. coustani | An. funestus Group | An. gambiae Complex | An. pretoriensis | Other Anopheles |
|---|---|---|---|---|---|---|
| August 1997 | 15 (12.0) | 809 | 579 | 6 | 20 | 9 |
| September | 17 (13.6) | 650 | 321 | 8 | 9 | 1 |
| October | 20 (16.0) | 518 | 227 | 63 | 5 | 18 |
| November | 17 (13.6) | 291 | 212 | 95 | 14 | 7 |
| December | 8 (6.4) | 142 | 0 | 53 | 35 | 15 |
| January 1998 | 14 (11.2) | 105 | 12 | 117 | 17 | 6 |
| February | 15 (12.0) | 251 | 23 | 77 | 60 | 13 |
| March | 7 (5.6) | 55 | 6 | 3 | 44 | 7 |
| April | 4 (3.2) | 14 | 6 | 4 | 39 | 14 |
| May | 8 (6.4) | 2 | 32 | 9 | 21 | 40 |
| Total (%) | 125 (100.0) | 2837 (55.8%) | 1418 (27.9%) | 435 (8.6%) | 264 (5.2%) | 130 (2.6%) |
| Sampling Method | Sampling Region | Total Anopheles | An. funestus Group | An. gambiae Complex | An. pretoriensis |
|---|---|---|---|---|---|
| Pyrethrum spray catch | Burma Valley Ward, Mutare District | 795 | 96.6% | 3.3% | 0.1% |
| Zindi Ward, Mutasa District | 140 | 96.4% | 3.6% | 0% | |
| Reared from larvae | Burma Valley Ward, Mutare District | 3141 | 1.4% | 0.2% | 98.4% |
| Zindi Ward, Mutasa District | 1549 | 2.9% | 0.2% | 96.9% | |
| Totals | 5625 | 17.5% | 0.8% | 81.7% |
| Locality | Sampling Month | An. funestus | An. leesoni | An. parensis | An. rivulorum | An. rivulorum-Like | An. vaneedeni | An. gambiae s.s. | An. arabiensis | An. quadriannulatus | An. merus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bende Mutale | Feb 2018 | - | 3 | - | 165 | 3 | - | - | - | - | - |
| Bende Mutale | Apr 2018 | - | 2 | - | 2 | 9 | - | - | 1 | 59 | 6 |
| Bende Mutale | Oct 2018 | - | 7 | 2 | 7 | - | - | - | - | 35 | 1 |
| Popallin Ranch | Oct 2017 | - | 2 | - | 47 | - | - | - | - | - | - |
| Popallin Ranch | Feb 2018 | 1 | 1 | 2 | 29 | - | 1 | 1 | - | - | - |
| Popallin Ranch | Apr 2018 | - | 2 | - | 54 | 7 | - | - | - | 4 | 1 |
| Doreen Farms | Feb 2018 | - | - | - | 5 | - | 2 | - | - | - | - |
| Doreen Farms | Apr 2018 | - | 4 | - | 2 | - | 1 | - | 1 | 10 | - |
| Tshipise Resort | Apr 2018 | - | 4 | - | 1 | 1 | 42 | - | 1 | - | - |
| Nkotswi | Apr 2018 | - | - | - | - | - | - | - | - | 7 | - |
| Tshikuyu | Apr 2018 | - | - | - | - | - | - | - | - | 1 | - |
| Pafuri | Apr 2018 | - | - | - | - | - | - | - | 3 | 27 | - |
| Malahla-panga | Oct 2018 | - | - | - | - | - | - | - | 42 | 7 | - |
| Total | 1 | 25 | 4 | 312 | 20 | 46 | 1 | 48 | 150 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braack, L.; Bornman, R.; Kruger, T.; Dahan-Moss, Y.; Gilbert, A.; Kaiser, M.; Oliver, S.V.; Cornel, A.J.; Lee, Y.; Norris, D.E.; et al. Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018. Int. J. Environ. Res. Public Health 2020, 17, 4125. https://doi.org/10.3390/ijerph17114125
Braack L, Bornman R, Kruger T, Dahan-Moss Y, Gilbert A, Kaiser M, Oliver SV, Cornel AJ, Lee Y, Norris DE, et al. Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018. International Journal of Environmental Research and Public Health. 2020; 17(11):4125. https://doi.org/10.3390/ijerph17114125
Chicago/Turabian StyleBraack, Leo, Riana Bornman, Taneshka Kruger, Yael Dahan-Moss, Allison Gilbert, Maria Kaiser, Shüné V. Oliver, Anthony J. Cornel, Yoosook Lee, Douglas E. Norris, and et al. 2020. "Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018" International Journal of Environmental Research and Public Health 17, no. 11: 4125. https://doi.org/10.3390/ijerph17114125
APA StyleBraack, L., Bornman, R., Kruger, T., Dahan-Moss, Y., Gilbert, A., Kaiser, M., Oliver, S. V., Cornel, A. J., Lee, Y., Norris, D. E., Coetzee, M., Brooke, B., & de Jager, C. (2020). Malaria Vectors and Vector Surveillance in Limpopo Province (South Africa): 1927 to 2018. International Journal of Environmental Research and Public Health, 17(11), 4125. https://doi.org/10.3390/ijerph17114125







