Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes
Abstract
1. Introduction
2. Materials and Methods
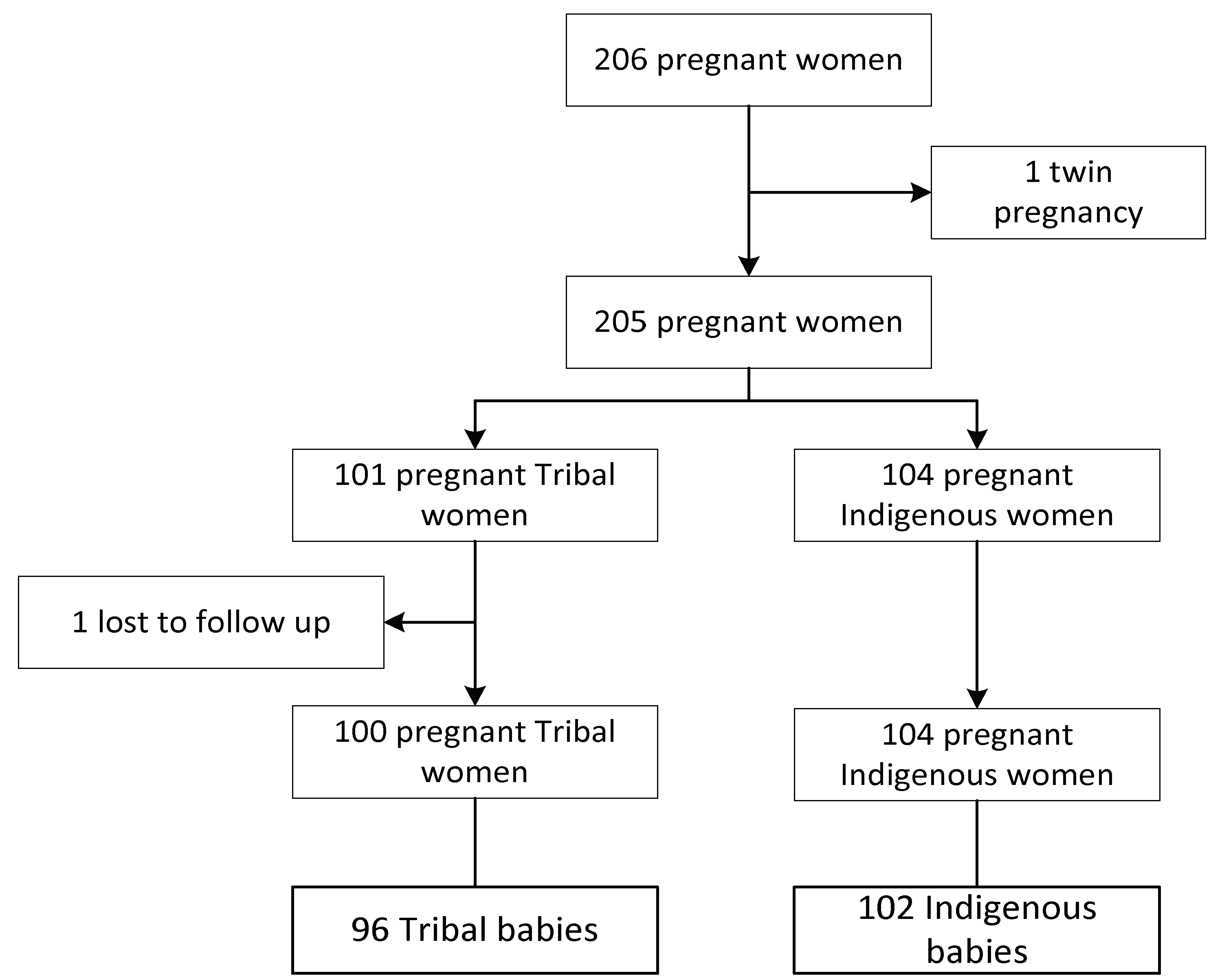
2.1. Data Sources
2.2. Protocols
2.3. Statistical Analysis
2.3.1. Mercury and Birth Outcomes
Contingency Table Analyses
Regression Analyses
2.3.2. Maternal Age and Birth Outcomes
Contingency Table Analyses
Regression Analyses
2.3.3. Gender Comparison among Newborns
3. Results
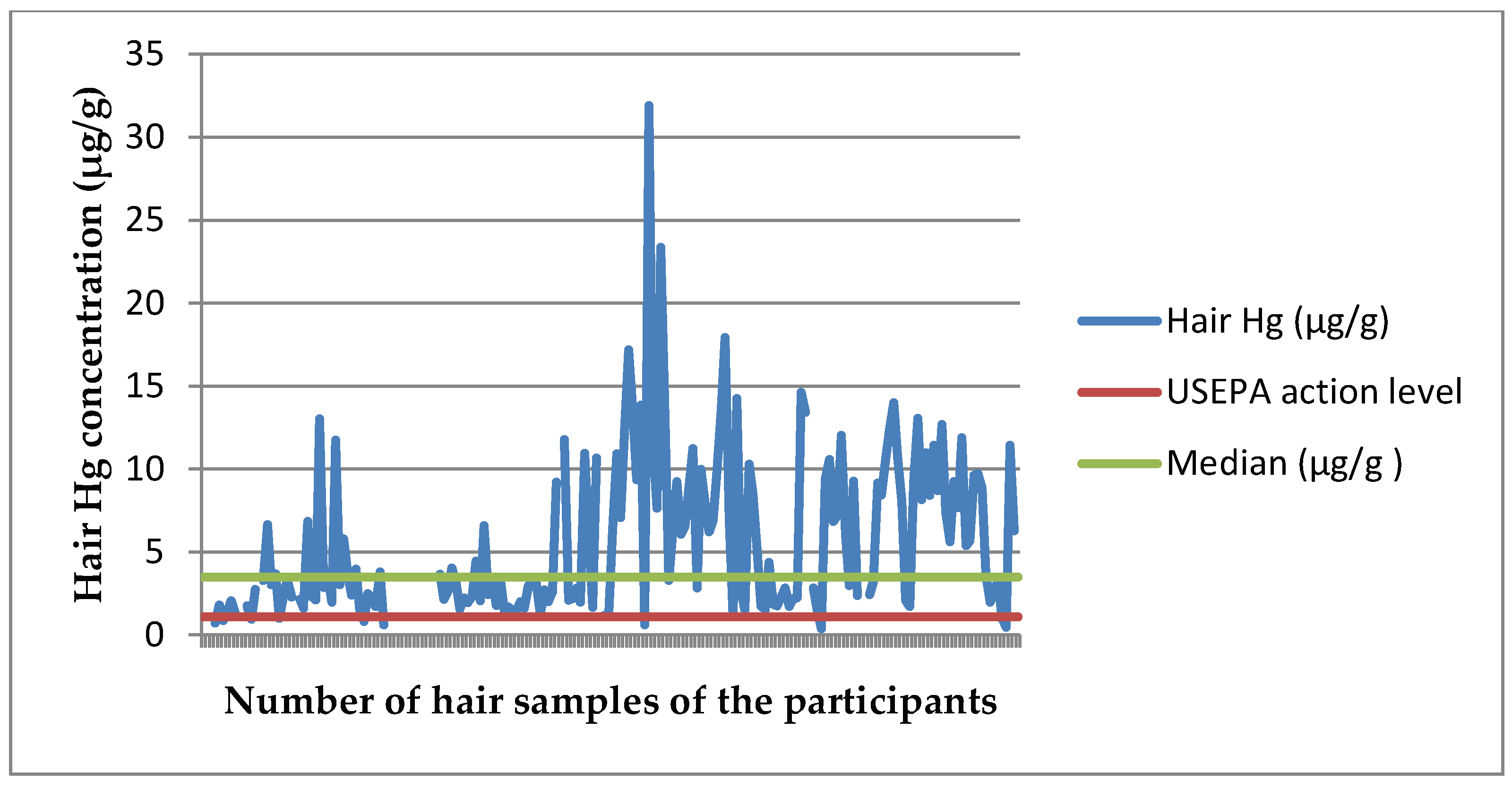
3.1. Mercury and Birth Outcome Analyses
3.1.1. Regression Analyses
3.1.2. Mercury and Gender
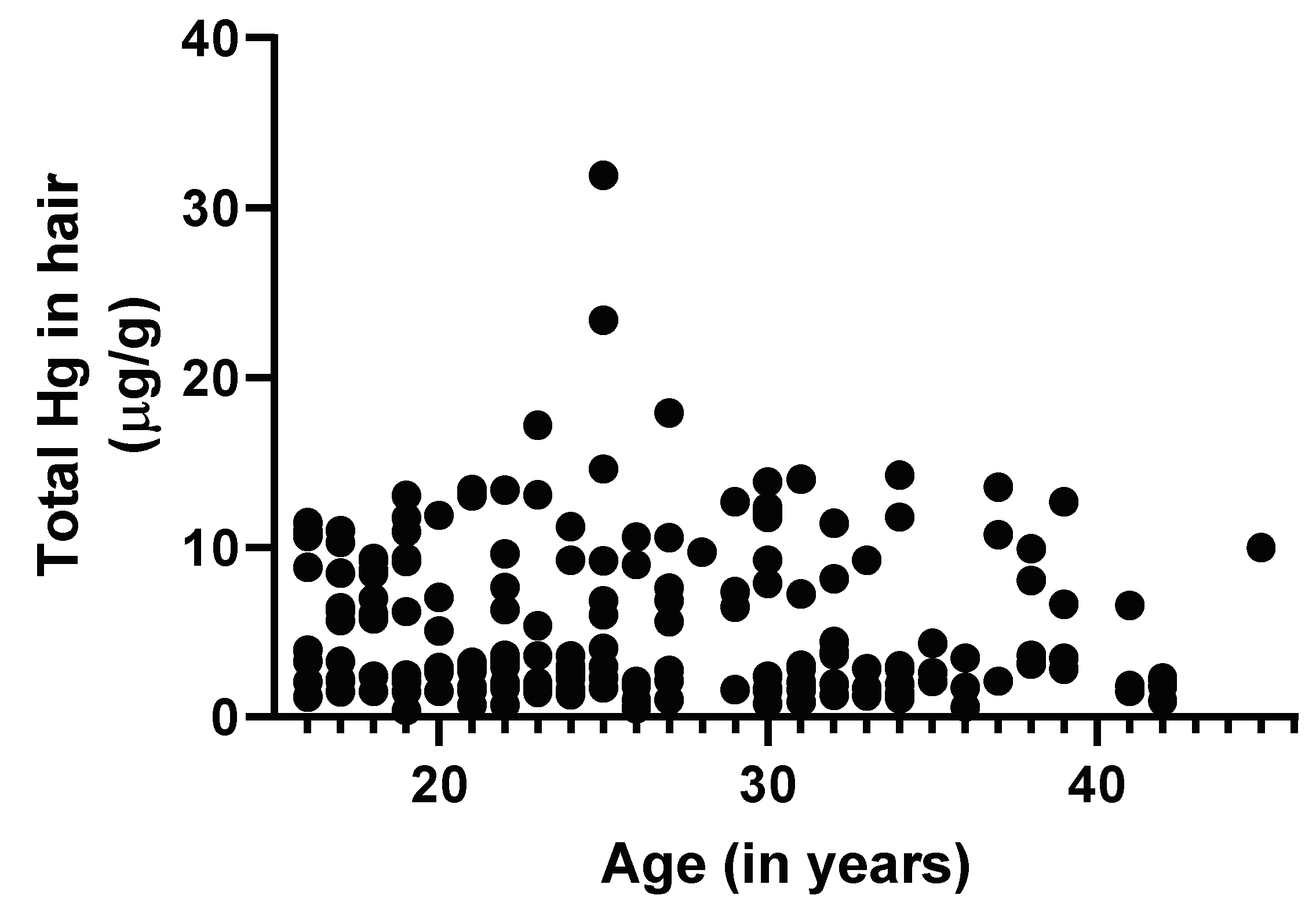
3.2. Maternal Age and Birth Outcome Analysis
Regression Analyses
3.3. Gender and Birth Outcome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bashore, C.; Geer, L.; He, X.; Puett, R.; Parsons, P.; Palmer, C.; Steuerwald, A.; Abulafia, O.; Dalloul, M.; Sapkota, A. Maternal Mercury Exposure, Season of Conception and Adverse Birth Outcomes in an Urban Immigrant Community in Brooklyn, New York, NY, USA. Int. J. Environ. Res. Public Health 2014, 11, 8414–8442. [Google Scholar] [CrossRef] [PubMed]
- Vigeh, M.; Nishioka, E.; Ohtani, K.; Omori, Y.; Matsukawa, T.; Koda, S.; Yokoyama, K. Prenatal mercury exposure and birth weight. Reprod. Toxicol. 2018, 76, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Weihe, P.; Grandjean, P. Cohort studies of Faroese children concerning potential adverse health effects after the mothers’ exposure to marine contaminants during pregnancy. Acta Vet. Scand. 2012, 54, S7. [Google Scholar] [CrossRef]
- Strain, J.; Yeates, A.J.; van Wijngaarden, E.; Thurston, S.W.; Mulhern, M.S.; McSorley, E.M.; Watson, G.E.; Love, T.M.; Smith, T.H.; Yost, K.; et al. Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: Associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 2015, 101, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Ouboter, P.; Landburg, G.; Satnarain, G.; Starke, S.; Nanden, I.; Simon-Friedt, B.; Hawkins, W.; Taylor, R.; Lichtveld, M.; Harville, E.; et al. Mercury Levels in Women and Children from Interior Villages in Suriname, South America. Int. J. Environ. Res. Public Health 2018, 15, 1007. [Google Scholar] [CrossRef] [PubMed]
- The World Bank. Data: Suriname. Available online: https://data.worldbank.org/country/suriname?view=chart (accessed on 30 April 2020).
- Declaration of Alma-Ata. Available online: https://www.who.int/publications/almaata_declaration_en.pdf?ua=1 (accessed on 30 April 2020).
- Mahumud, R.A.; Sultana, M.; Sarker, A.R. Distribution and Determinants of Low Birth Weight in Developing Countries. J. Prev. Med. Public Health 2017, 50, 18–28. [Google Scholar] [CrossRef]
- Country Profile: Suriname. Available online: https://data.unicef.org/country/sur/ (accessed on 30 April 2020).
- Country Nutrition Profile: Suriname. Available online: https://globalnutritionreport.org/media/profiles/v2.1/pdfs/suriname.pdf (accessed on 30 April 2020).
- Global Burden of Disease Profile: Suriname. Available online: http://www.healthdata.org/suriname (accessed on 4 June 2020).
- Medische Zending PHCS Jaarverslag 2016 (Medical Mission Primary Health Care Suriname, Annual Report 2016). Available online: http://www.medischezending.sr/wp-content/uploads/2018/01/Laatste-versie-jaarverslag-2016.pdf (accessed on 30 April 2020).
- Kobayashi, S.; Kishi, R.; Saijo, Y.; Ito, Y.; Oba, K.; Araki, A.; Miyashita, C.; Itoh, S.; Minatoya, M.; Yamazaki, K.; et al. Association of blood mercury levels during pregnancy with infant birth size by blood selenium levels in the Japan Environment and Children’s Study: A prospective birth cohort. Environ. Int. 2019, 125, 418–429. [Google Scholar] [CrossRef]
- Murcia, M.; Ballester, F.; Enning, A.M.; Iñiguez, C.; Valvi, D.; Basterrechea, M.; Rebagliato, M.; Vioque, J.; Maruri, M.; Tardon, A.; et al. Prenatal mercury exposure and birth outcomes. Environ. Res. 2016, 151, 11–20. [Google Scholar] [CrossRef]
- Xue, F.; Holzman, C.; Rahbar, M.H.; Trosko, K.; Fischer, L. Maternal Fish Consumption, Mercury Levels, and Risk of Preterm Delivery. Environ. Health Perspect. 2007, 115, 42–47. [Google Scholar] [CrossRef]
- National Research Council. Toxicological Effects of Methylmercury; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Ouboter, P.E.; Landburg, G.A.; Quik, J.H.M.; Mol, J.H.A.; van der Lugt, F. Mercury Levels in Pristine and Gold Mining Impacted Aquatic Ecosystems of Suriname, South America. Ambio 2012, 41, 873–882. [Google Scholar] [CrossRef]
- Mahaffey, K.R.; Sunderland, E.M.; Chan, H.M.; Choi, A.L.; Grandjean, P.; Mariën, K.; Oken, E.; Sakamoto, M.; Schoeny, R.; Weihe, P.; et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption: Nutrition Reviews©. Nutr. Rev. 2011, 69, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Sackett, D.; Cope, W.; Rice, J.; Aday, D. The Influence of Fish Length on Tissue Mercury Dynamics: Implications for Natural Resource Management and Human Health Risk. Int. J. Environ. Res. Public Health 2013, 10, 638–659. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Relationship between Total Mercury Concentration and Fish Size in Two Pelagic Fish Species: Implications for Consumer Health. J. Food Prot. 2006, 69, 1402–1405. [Google Scholar] [CrossRef]
- Bramante, C.T.; Spiller, P.; Landa, M. Fish Consumption During Pregnancy: An Opportunity, Not a Risk. JAMA Pediatrics 2018, 172, 801. [Google Scholar] [CrossRef] [PubMed]
- Starling, P.; Charlton, K.; McMahon, A.; Lucas, C. Fish Intake during Pregnancy and Foetal Neurodevelopment—A Systematic Review of the Evidence. Nutrients 2015, 7, 2001–2014. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Golding, J.; Emond, A. Blood mercury levels and fish consumption in pregnancy: Risks and benefits for birth outcomes in a prospective observational birth cohort. Int. J. Hygiene Environ. Health 2016, 219, 513–520. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emmett, P.M.; Emond, A.M.; Golding, J. A review of guidance on fish consumption in pregnancy: Is it fit for purpose? Public Health Nutr. 2018, 21, 2149–2159. [Google Scholar] [CrossRef]
- Caserta, D.; Graziano, A.; Monte, G.L.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharm. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Van Wijngaarden, E.; Harrington, D.; Kobrosly, R.; Thurston, S.W.; O’Hara, T.; McSorley, E.M.; Myers, G.J.; Watson, G.E.; Shamlaye, C.F.; Strain, J.J.; et al. Prenatal exposure to methylmercury and LCPUFA in relation to birth weight. Ann. Epidemiol. 2014, 24, 273–278. [Google Scholar] [CrossRef]
- Ramón, R.; Ballester, F.; Aguinagalde, X.; Amurrio, A.; Vioque, J.; Lacasaña, M.; Rebagliato, M.; Murcia, M.; Iñiguez, C. Fish consumption during pregnancy, prenatal mercury exposure, and anthropometric measures at birth in a prospective mother-infant cohort study in Spain. Am. J. Clin. Nutr. 2009, 90, 1047–1055. [Google Scholar] [CrossRef]
- United Nations Children’s Fund (UNICEF); World Health Organization (WHO). UNICEF-WHO Low Birthweight Estimates: Levels and Trends 2000–2015; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Births: Provisional Data for 2018. Available online: https://www.cdc.gov/nchs/data/vsrr/vsrr-007-508.pdf (accessed on 7 May 2019).
- Woodhouse, C.; Lopez Camelo, J.; Wehby, G.L. A Comparative Analysis of Prenatal Care and Fetal Growth in Eight South American Countries. PLoS ONE 2014, 9, e91292. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Dewailly, É.; Muckle, G.; Ayotte, P.; Bruneau, S.; Gingras, S.; Rhainds, M.; Holub, B.J. Gestational age and birth weight in relation to n−3 fatty acids among inuit (Canada). Lipids 2004, 39, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Lederman, S.A.; Jones, R.L.; Caldwell, K.L.; Rauh, V.; Sheets, S.E.; Tang, D.; Viswanathan, S.; Becker, M.; Stein, J.L.; Wang, R.Y.; et al. Relation between Cord Blood Mercury Levels and Early Child Development in a World Trade Center Cohort. Environ. Health Perspect. 2008, 116, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Drouillet-Pinard, P.; Huel, G.; Slama, R.; Forhan, A.; Sahuquillo, J.; Goua, V.; Thiébaugeorges, O.; Foliguet, B.; Magnin, G.; Kaminski, M.; et al. Prenatal mercury contamination: Relationship with maternal seafood consumption during pregnancy and fetal growth in the ‘EDEN mother–child’ cohort. Br. J. Nutr. 2010, 104, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Voskamp, B.J.; Peelen, M.J.C.S.; Ravelli, A.C.J.; Lee, R.; Mol, B.W.J.; Pajkrt, E.; Ganzevoort, W.; Kazemier, B.M. Association between fetal sex, birthweight percentile and adverse pregnancy outcome. Acta Obstet. Gynecol Scand. 2020, 99, 48–58. [Google Scholar] [CrossRef]
- McGregor, J.; Leff, M.; Orleans, M.; Baron, A. Fetal Gender Differences in Preterm Birth: Findings in a North American Cohort. Am. J. Perinatol. 1992, 9, 43–48. [Google Scholar] [CrossRef]
- Zeitlin, J.; Ancel, P.-Y.; Larroque, B.; Kaminski, M.; the EPIPAGE group. Fetal sex and indicated very preterm birth: Results of the EPIPAGE study. Am. J. Obstet. Gynecol. 2004, 190, 1322–1325. [Google Scholar] [CrossRef]
- McDowell, M.A.; Dillon, C.F.; Osterloh, J.; Bolger, P.M.; Pellizzari, E.; Fernando, R.; de Oca, R.M.; Schober, S.E.; Sinks, T.; Jones, R.L.; et al. Hair Mercury Levels in U.S. Children and Women of Childbearing Age: Reference Range Data from NHANES 1999–2000. Environ. Health Perspect. 2004, 112, 1165–1171. [Google Scholar] [CrossRef]
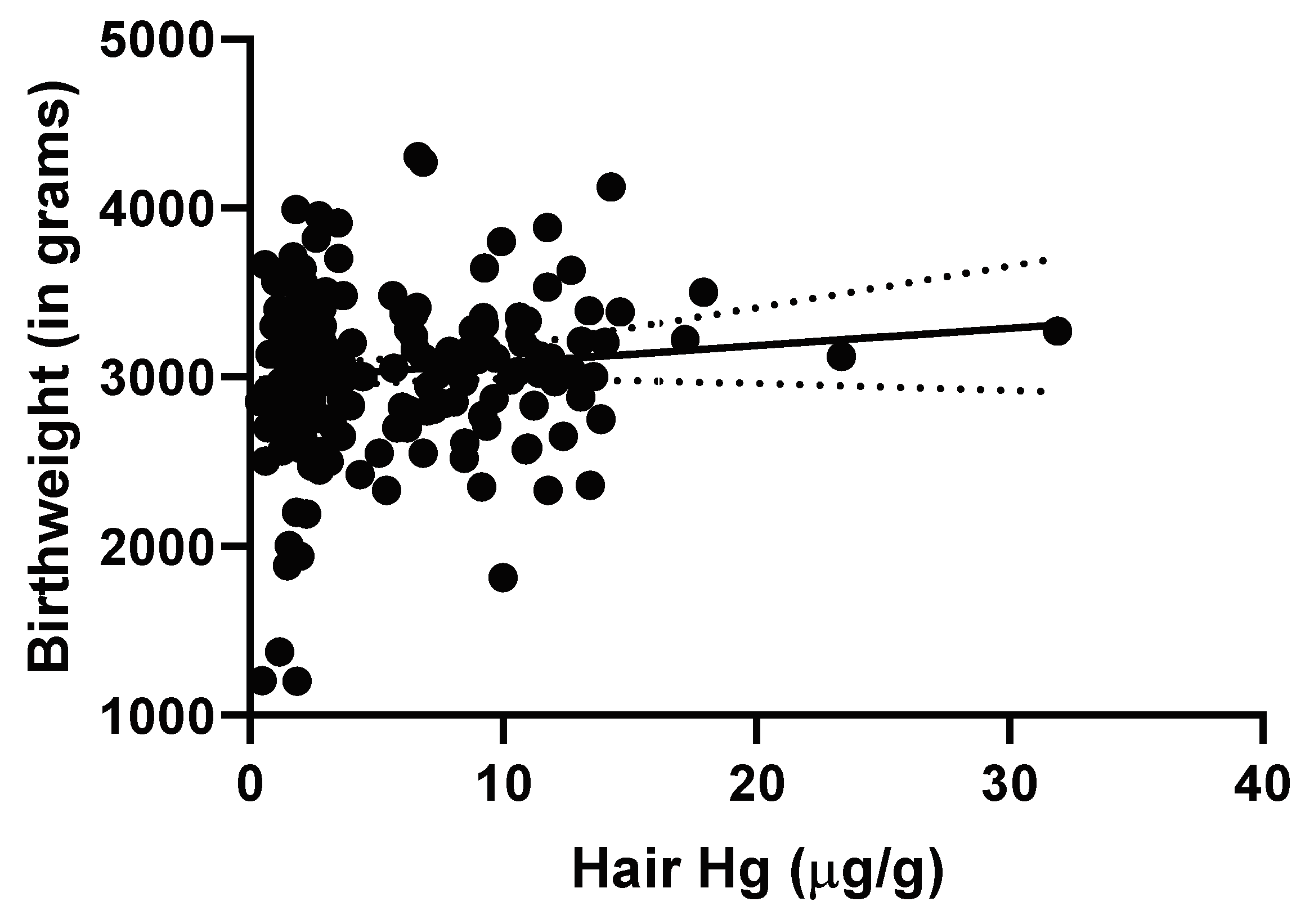
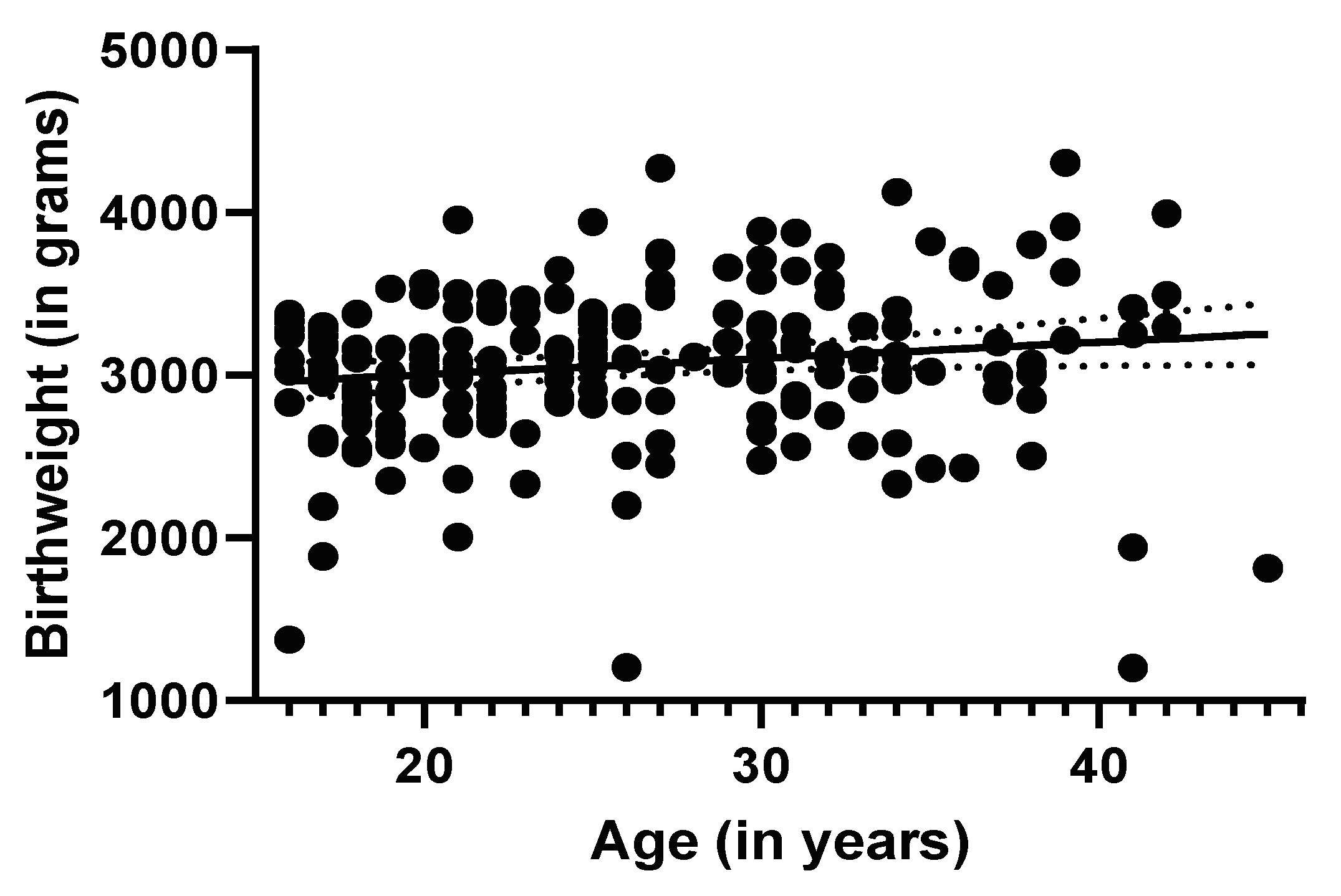
| Maternal Characteristics | N (%) | Tribal (n = 100) N (%) | Indigenous (n = 104) N (%) |
|---|---|---|---|
| Age (years) | |||
| 16–19 | 46 (22.5) | 16 (34.8) | 30 (65.2) |
| 20–34 | 125 (61.3) | 63 (50.4) | 62 (49.6) |
| ≥35 | 33 (16.2) | 21 (63.6) | 12 (36.4) |
| Parity | |||
| ≤1 | 74 (36.3) | 31 (41.9) | 43 (58.1) |
| 1–4 | 54 (26.5) | 24 (44.4) | 30 (55.6) |
| ≥5 | 74 (36.3) | 44 (59.5) | 30 (40.5) |
| Missing | 2 (1.0) | 1 (50.0) | 1 (50.0) |
| Gestational age (wks.) | |||
| <37 | 32 (15.7) | 11 (34.4) | 21 (65.6) |
| 37–40 | 148 (72.6) | 77 (52.0) | 71 (48.0) |
| ≥41 | 22 (10.8) | 12 (54.5) | 10 (45.5) |
| Missing | 2 (0.9) | 2 (100) | |
| Birth weight (grams) | |||
| <2500 | 16 (7.8) | 8 (50.0) | 8 (50.0) |
| ≥2500 | 182 (89.6) | 88 (48.4) | 94 (51.6) |
| Stillbirth | 5 (2.5) | 3 (66.7) | 2 (33.3) |
| Miscarriage | 1 (0.1) | 1 (100.0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldewsingh, G.K.; Wickliffe, J.K.; van Eer, E.D.; Shankar, A.; Hindori-Mohangoo, A.D.; Harville, E.W.; Covert, H.H.; Shi, L.; Lichtveld, M.Y.; Zijlmans, W.C.W.R. Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 4032. https://doi.org/10.3390/ijerph17114032
Baldewsingh GK, Wickliffe JK, van Eer ED, Shankar A, Hindori-Mohangoo AD, Harville EW, Covert HH, Shi L, Lichtveld MY, Zijlmans WCWR. Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes. International Journal of Environmental Research and Public Health. 2020; 17(11):4032. https://doi.org/10.3390/ijerph17114032
Chicago/Turabian StyleBaldewsingh, Gaitree K., Jeffrey K. Wickliffe, Edward D. van Eer, Arti Shankar, Ashna D. Hindori-Mohangoo, Emily W. Harville, Hannah H. Covert, Lizheng Shi, Maureen Y. Lichtveld, and Wilco C.W.R. Zijlmans. 2020. "Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes" International Journal of Environmental Research and Public Health 17, no. 11: 4032. https://doi.org/10.3390/ijerph17114032
APA StyleBaldewsingh, G. K., Wickliffe, J. K., van Eer, E. D., Shankar, A., Hindori-Mohangoo, A. D., Harville, E. W., Covert, H. H., Shi, L., Lichtveld, M. Y., & Zijlmans, W. C. W. R. (2020). Prenatal Mercury Exposure in Pregnant Women from Suriname’s Interior and Its Effects on Birth Outcomes. International Journal of Environmental Research and Public Health, 17(11), 4032. https://doi.org/10.3390/ijerph17114032







