Bayesian Regression Model for a Cost-Utility and Cost-Effectiveness Analysis Comparing Punch Grafting Versus Usual Care for the Treatment of Chronic Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Background
2.1.1. Flowchart and Decision Making
2.1.2. Research “Wound-PRO-Spain”
2.2. Study Design
2.3. Setting and Location
2.4. Target Population
2.5. Study Groups/Comparators
2.6. Data Source
2.7. Study Perspective
2.8. Time Horizon
2.9. Choice and Measurement of Health Outcomes
2.10. Estimating Health Resources Consumed and Related Costs
2.11. Assumptions
2.12. Cost-Utility and Cost-Effectiveness Analysis (CUA and CEA)
β16 × Wound-QoLi + β17 × Treatmenti + ε1i
β26 × Wound-QoLi + β27 × Treatmenti + ε2i
β16 × Wound-QoLi + β17 × Treatmenti + ε1i
β36 × Wound-QoLi + β37 × Treatmenti + ε3i
2.13. Sensitivity Analysis
2.14. Sample Size
2.15. Frequentist Statistical Analyses
2.16. Ethical Issues
3. Results
3.1. Demographic Characteristics
3.2. Cost Analysis
3.3. Health Outcomes
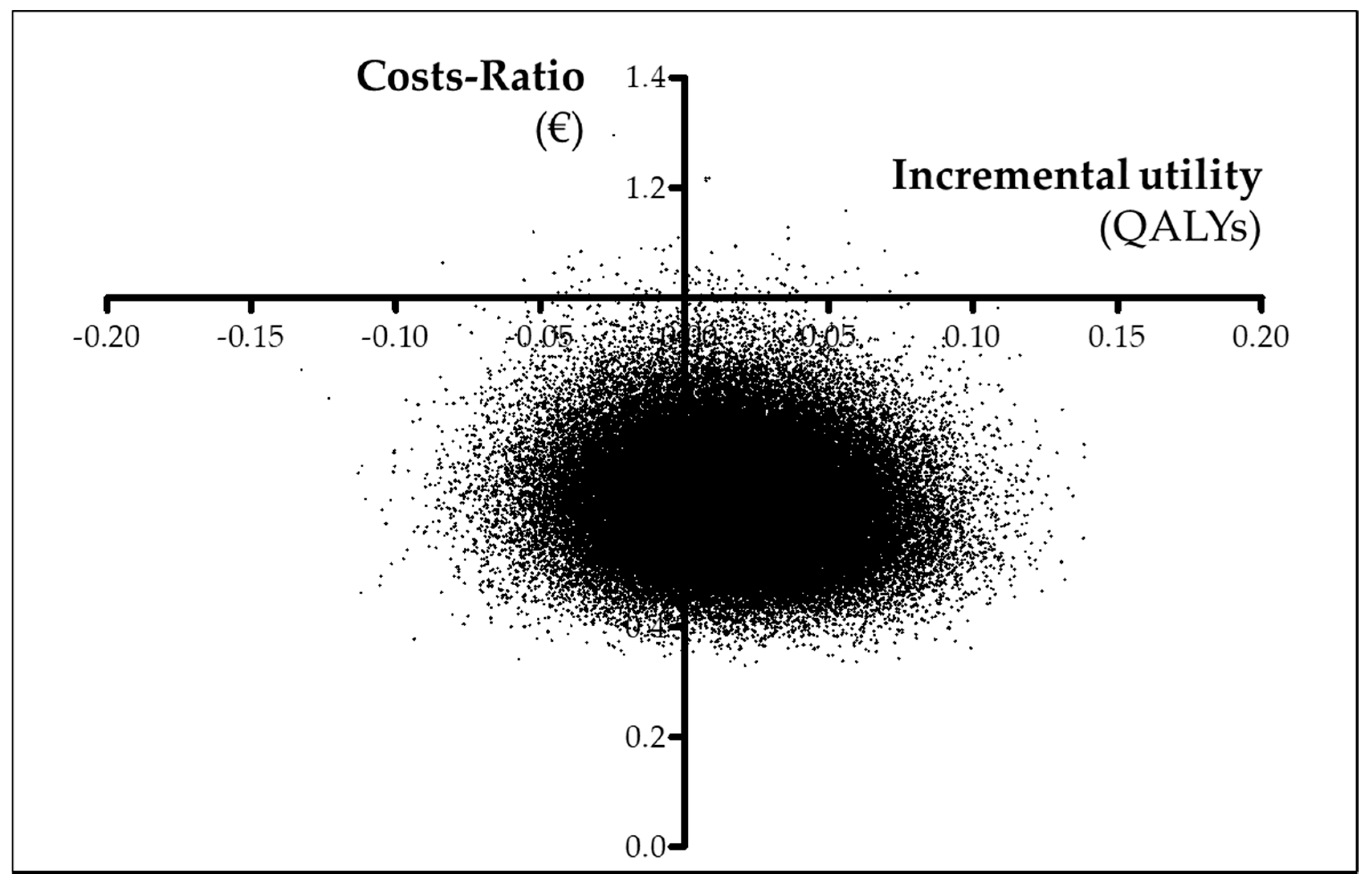
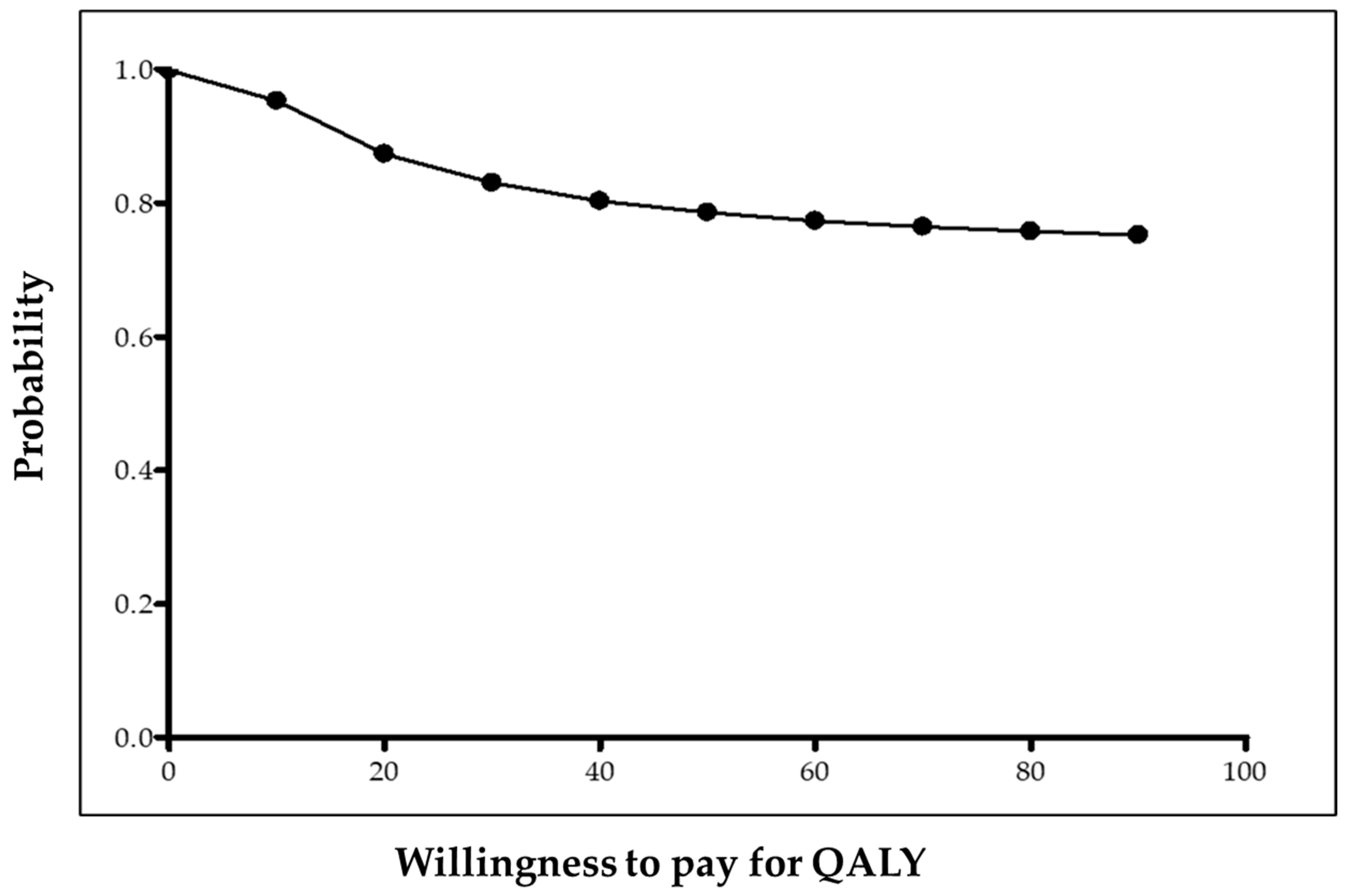
3.4. Cost-Utility Analysis (CUA)
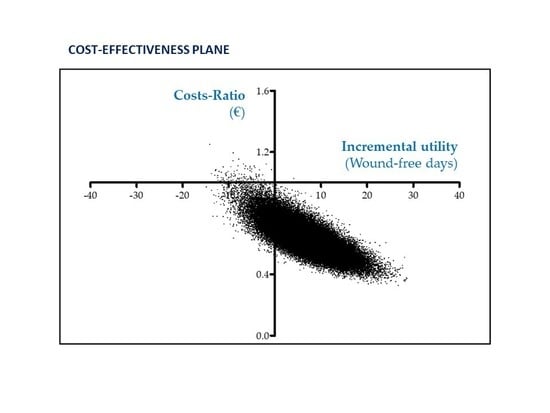
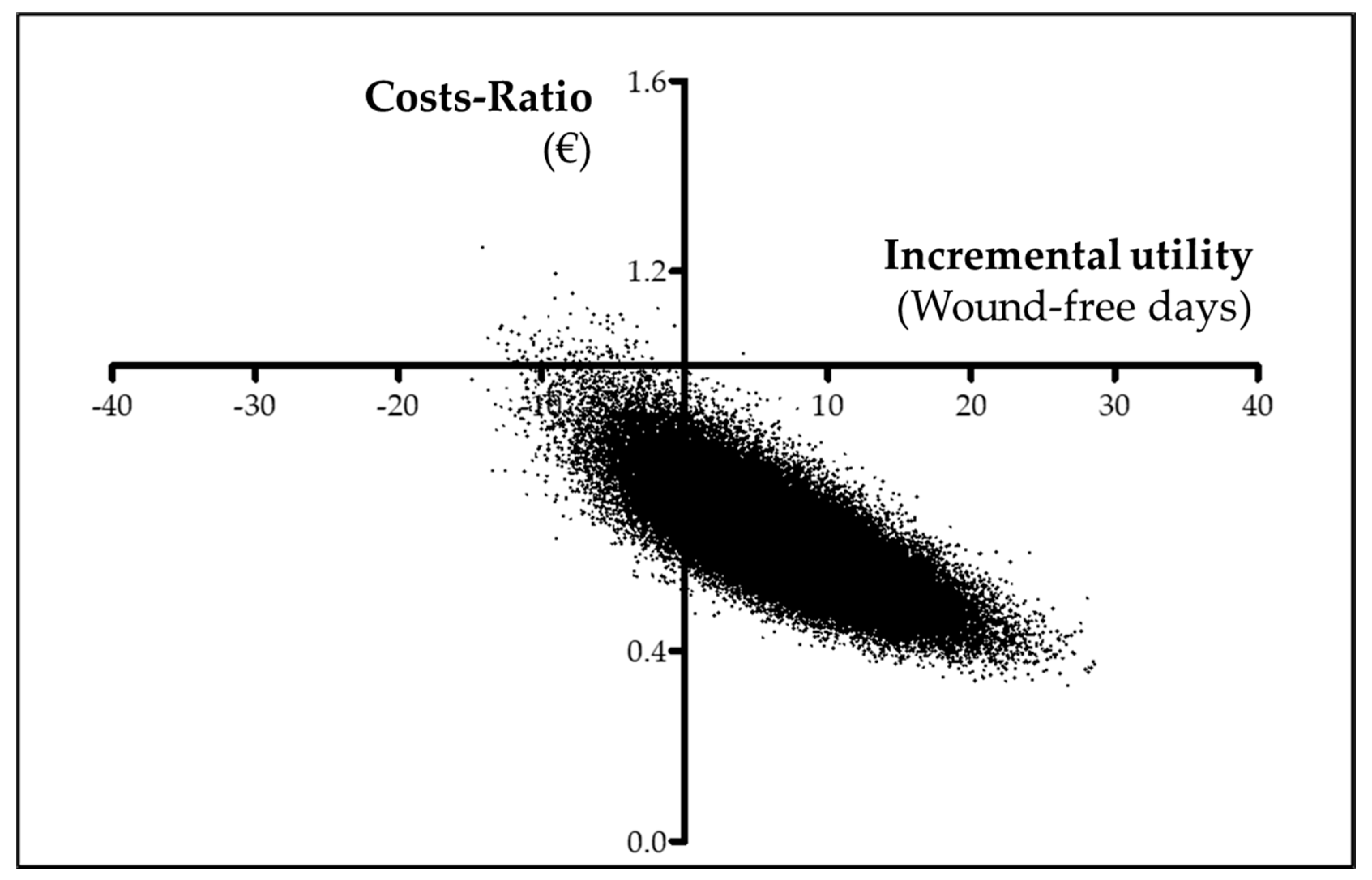
3.5. Cost-effectiveness analysis (CEA)
3.6. Sensitivity Analysis
4. Discussion
4.1. Comparison with Literature
4.2. Study Perspective
4.3. Strengths
- Chronic wounds have been the subject of many CEAs comparing a variety of therapeutic alternatives [31,32], but as far as we know, no CEA comparison of punch grafting versus usual care or another technique had been conducted until now. In general, punch grafting is rarely investigated, and comparative studies of any kind were lacking until now, regardless of the type of wounds treated. Therefore, this research is the first CEA and CUA comparing punch grafting versus usual care.
- Wound healing is frequently selected as an indicator of effectiveness in many reported economic evaluations (44%), which is logical in the field of chronic wounds, whereas wound-free period and QALYs are less frequently selected as indicators (15% and 17%, respectively) [31,32]. In our case, using these two indicators to conduct our CEA and CUA, we simultaneously assessed the influence of punch grafting on healing time of the wounds as well as on the quality of life of patients.
- Regression models were built to explore whether differences in costs, wound-free period, and QALYs between both groups could be explained by other variables different to treatment. Such models allowed us to combine the advantages of incorporating patients’ characteristics through covariates in CUA and CEA, with the advantages of the Bayesian methodology. A remarkable advantage of incorporating covariates was that they allowed for differences between treatment groups in costs, utility, and effectiveness to be modelled as if they were completely homogeneous, except for the treatment received. They also allowed us to identify the part of the difference in costs, utility, and effectiveness that was not attributable to the treatment but to the differences in the characteristics of the patients. In addition, covariates allowed us to reduce the bias and uncertainty of the estimation of the coefficients [47,48,49,50]. Our regression model specification was properly evaluated as it fitted the checklist developed to assess statistical methods for addressing selection bias in CEAs based on observational data [61]. The selection of a Bayesian perspective instead of a standard frequentist one provided two advantages. First, it allowed for the incorporation of a priori information through a dynamic process. Second, it allowed for the results of CUAs and CEAs to be interpreted in terms of probability. More specifically, the cost-utility and cost-effectiveness acceptability curves were able to be interpreted in terms of probability; as stated before, such interpretation is only possible when a Bayesian perspective is adopted [49].
- We conducted a retrospective observational study based on patients who had been treated by their attending physicians according to their best clinical judgement. This kind of observational study based on individual patient data is thought to be more valid than clinical trials to generate real-world data, which, in turn, are thought to provide more of an advantage than a limitation when generalizing the results of specific research to day-to-day practice [62,63]. In addition, working with individual patient data allows real clinical outcomes of treatment to be captured [62]. In our opinion, this realistic approach to the assessment of treatment and outcomes represents an advantage over studies that try to model the natural history of the disease based on population data, as do Markov models, for instance [64].
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability
Acknowledgments
Conflicts of Interest
Appendix A. Sharp Debridement
Appendix B. Punch Grafting
References
- Cheng, Q.; Graves, N.; Pacella, R.E. Economic Evaluations of Guideline-Based Care for Chronic Wounds: A Systematic Review. Appl. Health Econ. Health Policy 2018, 16, 633–651. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Waycaster, C.; Schaum, K.; Gilligan, A.M. Cost-Effectiveness of Three Adjunct Cellular/Tissue-Derived Products Used in the Management of Chronic Venous Leg Ulcers. Value Health 2014, 17, 801–813. [Google Scholar] [CrossRef]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-illness studies in chronic ulcers: A systematic review. J. Wound Care 2017, 26, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Vandeput, J.; Nelissen, M.; Tanner, J.C.; Boswick, J. A review of skin meshers. Burns 1995, 21, 364–370. [Google Scholar] [CrossRef]
- Nordström, A.; Hansson, C. Punch-grafting to enhance healing and to reduce pain in complicated leg and foot ulcers. Acta Derm. Venereol. 2008, 88, 381–391. [Google Scholar] [CrossRef]
- Fourgeaud, C.; Mouloise, G.; Michon-Pasturel, U.; Bonhomme, S.; Lazareth, I.; Meaume, S.; Priollet, P. Interest of punch skin grafting for the treatment of painful ulcers. J. Mal. Vasc. 2016, 41, 329–334. [Google Scholar] [CrossRef]
- Conde-Montero, E.; de Farias Khayat, Y.; Pérez Jerónimo, L.; Vázquez, A.P.; Marín, L.R.; Guisado, S.; de la Cueva Dobao, P. Punch grafting for pain reduction in hard-to-heal ulcers. J. Wound Care 2020, 29, 194–197. [Google Scholar] [CrossRef]
- Öien, R.F.; Håkansson, A.; Ahnlide, I.; Bjellerup, M.; Hansen, B.U.; Borgquist, L. Pinch grafting in hospital and primary care: A cost analysis. J. Wound Care 2001, 10, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Castanedo, L.; Recarte-Marín, L.; Pérez-Jerónimo, L.; Conde-Montero, E.; Cueva-Dobao, P. Ulcerative necrobiosis lipoidica diabeticorum successfully treated with topical sevoflurane and punch grafting. Int. Wound J. 2019, 16, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Ahnlide, I.; Bjellerup, M. Efficacy of pinch grafting in leg ulcers of different aetiologies. Acta Derm. Venereol. 1997, 77, 144–145. [Google Scholar] [CrossRef]
- Christiansen, J.; Ek, L.; Tegner, E. Pinch grafting of leg ulcers: A retrospective study of 412 treated ulcers in 146 patients. Acta Derm. Venereol. 1997, 77, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Öien, R.F.; Håkansson, A.; Hansen, B.U.; Bjellerup, M. Pinch Grafting of Chronic Leg Ulcers in Primary Care: Fourteen Years’ Experience. Acta Derm. Venereol. 2002, 82, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Hjerppe, A.; Sane, M.; Huhtala, H.; Vaalasti, A. Pinch grafting of chronic leg ulcers: A retrospective analysis of 104 patients with 169 ulcers. J. Wound Care 2010, 19, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Groening, L.; Holthuis, L.; Polesie, S.; Sönnergren, H. Clinical Outcomes of Punch-grafting for Chronic Leg and Foot Ulcers: A Retrospective Non-comparative Cohort Study. Acta Derm. Venereol. 2017, 97, 131–132. [Google Scholar] [CrossRef]
- Guisado Muñoz, S.; Conde Montero, E.; de la Cueva Dobao, P. Tratamiento de la úlcera isquémica hipertensiva de Martorell con microinjertos autólogos en sello. Actas Dermo-Sifiliogr. 2019, 110, 689–690. [Google Scholar] [CrossRef]
- Grado Sanz, R.; Conde-Montero, E.; Pérez Jerónimo, L.; Peral Vázquez, A.; Recarte Marín, L.; Galindo Carlos, A.; Martín Navarro, J.A. Clinically inactive pyoderma gangrenosum successfully treated with negative pressure therapy and punch grafting. Int. Wound J. 2020, 17, 516–518. [Google Scholar] [CrossRef]
- Seo, J.; Kim, J.; Nam, K.A.; Zheng, Z.; Oh, B.H.; Chung, K.Y. Reconstruction of large wounds using a combination of negative pressure wound therapy and punch grafting after excision of acral lentiginous melanoma on the foot. J. Dermatol. 2016, 43, 79–84. [Google Scholar] [CrossRef]
- Green, J.; Jester, R.; McKinley, R.; Pooler, A. The impact of chronic venous leg ulcers: A systematic review. J. Wound Care 2014, 23, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and Elaboration: A Report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013, 16, 231–250. [Google Scholar] [CrossRef] [PubMed]
- McGhan, W.F.; Al, M.; Doshi, J.A.; Kamae, I.; Marx, S.E.; Rindress, D. The ISPOR Good Practices for Quality Improvement of Cost-Effectiveness Research Task Force Report. Value Health 2009, 12, 1086–1099. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.L.; Mamdani, M.; Atkins, D.; Johnson, M.L. Good Research Practices for Comparative Effectiveness Research: Defining, Reporting and Interpreting Nonrandomized Studies of Treatment Effects Using Secondary Data Sources: The ISPOR Good Research Practices for Retrospective Database Analysis Task Force. Value Health 2009, 12, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.L.; Sox, H.; Willke, R.; Brixner, D.; Eichler, H.-G.; Goettsch, W.; Madigan, D.; Makady, A.; Schneeweiss, S.; Tarricone, R.; et al. Recommendations for Good Procedural Practices for Real-World Data Studies of Treatment Effectiveness and/or Comparative Effectiveness Designed to Inform Health Care Decisions: Report of the Joint ISPOR-ISPE Special Task Force on Real-World Evidence in Hea. Value Health 2017, 20, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Blome, C.; Baade, K.; Sebastian Debus, E.; Price, P.; Augustin, M. The “wound-QoL”: A short questionnaire measuring quality of life in patients with chronic wounds based on three established disease-specific instruments. Wound Repair Regen. 2014, 22, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Conde-Montero, E.; Sommer, R.; Augustin, M.; Blome, C.; Cabeza Martínez, R.; Horcajada Reales, C.; Alsina Gibert, M.; Ramón Sapena, R.; Peral Vázquez, A.; Montoro López, J.; et al. Validation of the Spanish Wound-QoL Questionnaire. Actas Dermo-Sifiliogr. (under review).
- EuroQol Group: EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [CrossRef]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish version of EuroQol: A description and its applications. European Quality of Life scale. Med. Clin. (Barc.) 1999, 112 (Suppl. 1), 79–85. [Google Scholar]
- Augustin, M.; Conde Montero, E.; Zander, N.; Baade, K.; Herberger, K.; Debus, E.S.; Diener, H.; Neubert, T.; Blome, C. Validity and feasibility of the wound-QoL questionnaire on health-related quality of life in chronic wounds. Wound Repair Regen. 2017, 25, 852–857. [Google Scholar] [CrossRef]
- Tricco, A.C.; Cogo, E.; Isaranuwatchai, W.; Khan, P.A.; Sanmugalingham, G.; Antony, J.; Hoch, J.S.; Straus, S.E. A systematic review of cost-effectiveness analyses of complex wound interventions reveals optimal treatments for specific wound types. BMC Med. 2015, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J. Economic evaluations of guideline-based or strategic interventions for the prevention or treatment of chronic wounds. Appl. Health Econ. Health Policy 2014, 12, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.; Manca, A. Calculation of quality adjusted life years in the published literature: A review of methodology and transparency. Health Econ. 2004, 13, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Boletín Oficial de la Comunidad de Madrid. Available online: https://www.bocm.es/boletin/CM_Orden_BOCM/2017/08/21/BOCM-20170821-1.PDF (accessed on 1 December 2019).
- Baio, G.; Berardi, A.; Heath, A. Bayesian Cost-Effectiveness Analysis with the R Package BCEA; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Moreno, E.; Vázquez-Polo, F.J.; Negrín-Hernández, M.Á. Bayesian Cost—Effectiveness Analysis of Medical Treatments; Chapman & Hall/CRC: Boca Raton, FL, USA, 2019. [Google Scholar]
- Zhang, H.; Huo, M.; Chao, J.; Liu, P. Application of Bayesian Approach to Cost-Effectiveness Analysis of Antiviral Treatments in Chronic Hepatitis B. PLoS ONE 2016, 11, e0161936. [Google Scholar] [CrossRef]
- Heitjan, D.F.; Moskowitz, A.J.; Whang, W. Bayesian estimation of cost-effectiveness ratios from clinical trials. Health Econ. 1999, 8, 191–201. [Google Scholar] [CrossRef]
- Briggs, A.H. A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ. 1999, 8, 257–261. [Google Scholar] [CrossRef]
- O’Hagan, A.; Stevens, J.W. A framework for cost-effectiveness analysis from clinical trial data. Health Econ. 2001, 10, 303–315. [Google Scholar] [CrossRef]
- O’Hagan, A.; Stevens, J.W.; Montmartin, J. Bayesian cost-effectiveness analysis from clinical trial data. Stat. Med. 2001, 20, 733–753. [Google Scholar] [CrossRef]
- O’Hagan, A.; Stevens, J.W. The probability of cost-effectiveness. BMC Med. Res. Methodol. 2002, 2, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hoch, J.S.; Briggs, A.H.; Willan, A.R. Something old, something new, something borrowed, something blue: A framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ. 2002, 11, 415–430. [Google Scholar] [CrossRef]
- Willan, A.R.; Briggs, A.H.; Hoch, J.S. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ. 2004, 13, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.M.; Thompson, S.G. Methods for incorporating covariate adjustment, subgroup analysis and between-centre differences into cost-effectiveness evaluations. Health Econ. 2005, 14, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Rice, N.; Sculpher, M.J.; Briggs, A.H. Assessing generalisability by location in trial-based cost-effectiveness analysis: The use of multilevel models. Health Econ. 2005, 14, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Polo, F.-J.V.; Negrin, M.; Badia, X.; Roset, M. Bayesian regression models for cost-effectiveness analysis. Eur. J. Health Econ. 2005, 6, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Polo, F.J.; Negrín Hernández, M.A.; López-Valcárcel, B.G. Using covariates to reduce uncertainty in the economic evaluation of clinical trial data. Health Econ. 2005, 14, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Negrín, M.A.; Vázquez-Polo, F.-J. Incorporating model uncertainty in cost-effectiveness analysis: A Bayesian model averaging approach. J. Health Econ. 2008, 27, 1250–1259. [Google Scholar] [CrossRef]
- Negrín, M.A.; Vázquez-Polo, F.J.; Martel, M.; Moreno, E.; Girón, F.J. Bayesian Variable Selection in Cost-Effectiveness Analysis. Int. J. Environ. Res. Public Health 2010, 7, 1577–1596. [Google Scholar] [CrossRef]
- Geman, S.; Geman, D. Stochastic Relaxation, Gibbs Distributions, and the Bayesian Restoration of Images. In Readings in Computer Vision; Elsevier: Amsterdam, The Netherlands, 1987; pp. 564–584. [Google Scholar]
- Gilks, W.; Richardson, S.; Spiegelhalter, D. Markov Chain Monte Carlo in Practice; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Agro, K.E.; Bradley, C.A.; Mittmann, N.; Iskedjian, M.; Lane, A.; Einarson, T.R. Sensitivity Analysis in Health Economic and Pharmacoeconomic Studies. Pharmacoeconomics 1997, 11, 75–88. [Google Scholar] [CrossRef]
- Briggs, A.H.; Gray, A.M. Handling uncertainty when performing economic evaluation of healthcare interventions. Health Technol. Assess. (Rockv.) 1999, 3, 1–134. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Atkin, L.; Bucko, Z.; Conde Montero, E.; Cutting, K.; Moffatt, C.; Probst, A.; Romanelli, M.; Schultz, G.S.; Tettelbach, W. Implementing TIMERS: The race against hard-to-heal wounds Inflammation/infection Social factors Edge Regeneration Moisture Tissue. J. Wound Care 2019, 28, S1–S50. [Google Scholar] [CrossRef]
- Öien, R.F.; Åkesson, N.; Forssell, H. Assessing quality of life in patients with hard-to-heal ulcers using the EQ-5D questionnaire. J. Wound Care. 2013, 22, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Öien, R.F.; Forssell, H.; Tennvall, G.R. Cost consequences due to reduced ulcer healing times—Analyses based on the Swedish Registry of Ulcer Treatment. Int. Wound J. 2016, 13, 957–962. [Google Scholar] [CrossRef]
- Barshes, N.R.; Chambers, J.D.; Cohen, J.; Belkin, M. Cost-effectiveness in the contemporary management of critical limb ischemia with tissue loss. J. Vasc. Surg. 2012, 56, 1015.e1–1024.e1. [Google Scholar] [CrossRef] [PubMed]
- Purwins, S.; Herberger, K.; Debus, E.S.; Rustenbach, S.J.; Pelzer, P.; Rabe, E.; Schäfer, E.; Stadler, R.; Augustin, M. Cost-of-illness of chronic leg ulcers in Germany. Int. Wound J. 2010, 7, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kreif, N.; Grieve, R.; Sadique, M.Z. Statistical methods for cost-effectiveness analyses that use observational data: A critical appraisal tool and review of current practice. Health Econ. (UK) 2013, 22, 486–500. [Google Scholar] [CrossRef]
- Garrison, L.P.; Neumann, P.J.; Erickson, P.; Marshall, D.; Mullins, C.D. Using Real-World Data for Coverage and Payment Decisions: The ISPOR Real-World Data Task Force Report. Value Health 2007, 10, 326–335. [Google Scholar] [CrossRef]
- Gilligan, A.M. Health Economics and Outcomes Research of Wound Care: Overview of Methodology. Adv. Wound Care 2018, 7, 380–386. [Google Scholar] [CrossRef]
- Briggs, A.H.; Claxton, K.; Sculpher, M.J. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Blanke, W.; Hallern, B.V. Sharp wound debridement in local anaesthesia using EMLA cream: 6 years?? experience in 1084 patients. Eur. J. Emerg. Med. 2003, 10, 229–231. [Google Scholar] [CrossRef]
- Atkin, L. Understanding methods of wound debridement. Br. J. Nurs. 2014, 23, S10–S15. [Google Scholar] [CrossRef]
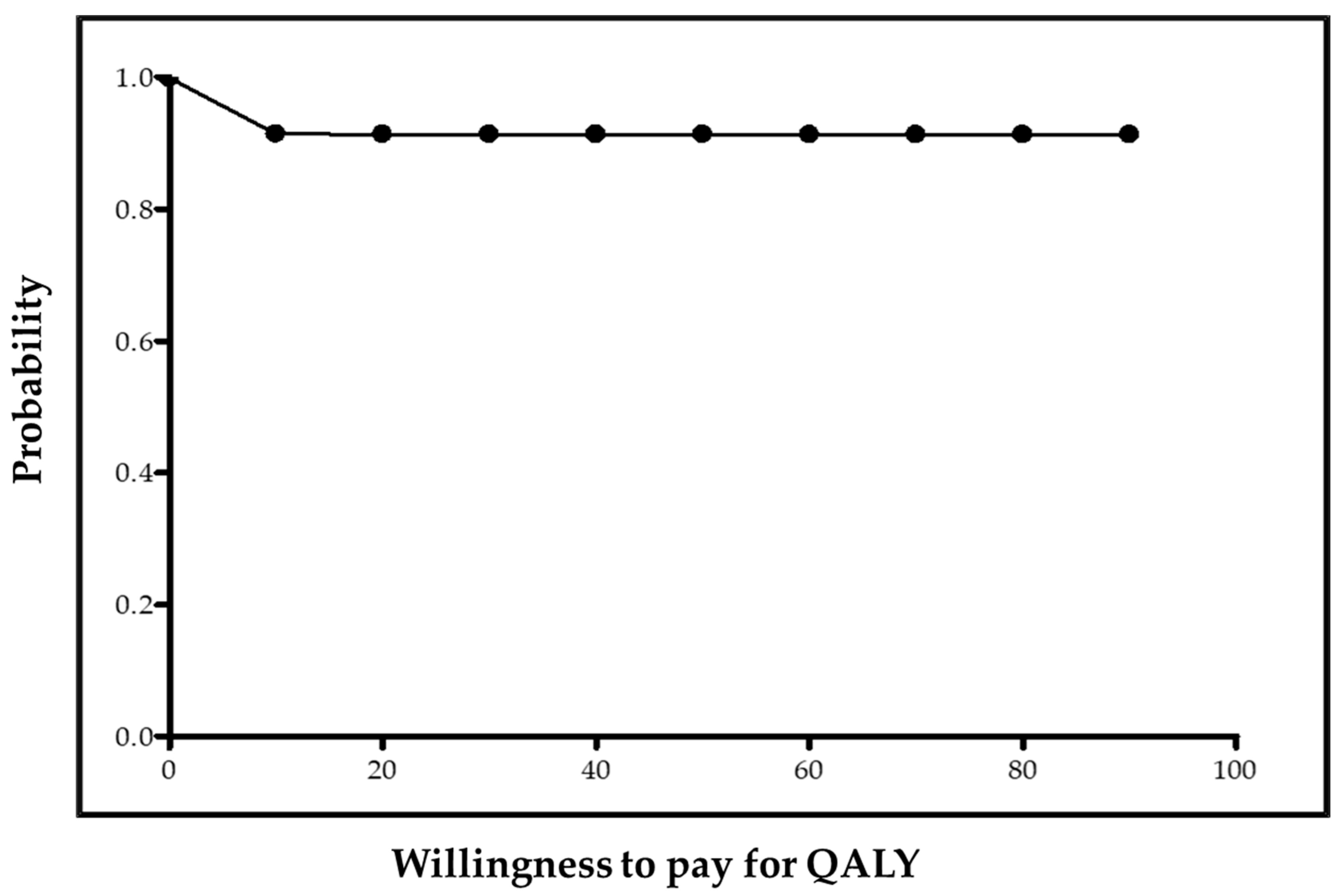
| PUNCH n = 46 | NoPUNCH n = 34 | p-Value | ||
|---|---|---|---|---|
| Patients | Age (years) | 68.4 (16.9) | 72.8 (12.9) | 0.447 a |
| Sex, Female (%) | 56.5 | 71.5 | 0.118 b | |
| Diabetes (%) | 32.6 | 44.1 | 0.293 b | |
| Hypertension (%) | 69.6 | 88.2 | 0.048 b | |
| Smoker (%) | 13.0 | 11.8 | 1.000 c | |
| Pain caused by ulcers (NRS) | 5.6 (3.2) | 5.7 (3.5) | 0.864 a | |
| Wounds | Etiology, vascular (%) | 39.1 | 76.5 | 0.001 b |
| Location, legs (%) | 78.3 | 97.1 | 0.020 c | |
| Duration (months) | 9.2 (10.7) | 8.4 (10.3) | 0.584 a | |
| Size (cm2) | 9.81 (14.98) | 14.07 (22.82) | 0.248 a | |
| Deepness, limited to dermis (%) | 76.1 | 67.7 | 0.503 b | |
| Health Resources (n) | Unit Costs (€) | Costs (€) | |||||
|---|---|---|---|---|---|---|---|
| PUNCH (n = 46) | NoPUNCH (n = 34) | p-Value | PUNCH (n = 46) | NoPUNCH (n = 34) | p-Value | ||
| Surgical visit | 3.5 (3.1) | 9.9 (5.7) | <0.001 | 145 | 501.54 (455.16) | 1431.41 (817.67) | <0.001 |
| Standard visit | 7.7 (6.4) | 4.7 (2.1) | 0.080 | 71 | 538.41 (446.09) | 327.41 (146.72) | 0.127 |
| Total | 11.2 (7.7) | 14.6 (7.3) | 0.031 | --- | 1040 (705) | 1759 (930) | <0.001 |
| PUNCH n = 46 | No PUNCH n = 34 | p-Value for Differences | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow Up | Difference | Baseline | Follow Up | Difference | ||
| EQ-5D utility score | 0.5494 (0.2571) | 0.8383 (0.2528) | 0.2889 (0.2716) | 0.4633 (0.2463) | 0.7138 (0.2526) | 0.2505 (0.2876) | 0.368 |
| EQ-5D VAS score | 63.37 (24.81) | 81.09 (17.06) | 17.72 (14.49) | 61.15 (21.00) | 77.50 (12.81) | 16.35 (18.64) | 0.777 |
| Wound-QoL score | 2.05 (0.96) | 0.52 (0.64) | −1.53 (1.07) | 2.30 (1.20) | 0.98 (1.12) | −1.32 (1.50) | 0.129 |
| Ulcer pain (NRS) | 5.6 (3.2) | 0.7 (1.4) | −4.9 (3.4) | 5.7 (3.5) | 0.7 (1.4) | −5.0 (3.8) | 0.864 |
| Ulcer size (cm2) | 9.81 (14.98) | 0.18 (0.58) | −9.64 (14.94) | 14.07 (22.81) | 2.09 (10.36) | −11.98 (17.50) | 0.275 |
| PUNCH | NoPUNCH | Incremental Difference | |
|---|---|---|---|
| CUA-Model | |||
| Costs (€) | 1074 ± 112.2 (906.8; 1271.0) | 1737 ± 209.4 (1426.0; 2105.0) | 0.63 ± 0.10 (0.48; 0.80) |
| Utility (QALYs) | 0.0523 ± 0.0187 (0.0218; 0.0830) | 0.0364 ± 0.0220 (0.0002; 0.0724) | 0.02 ± 0.03 (−0.03; 0.06) |
| CEA-Model | |||
| Costs (€) | 1075 ± 111.7 (908.1; 1271.0) | 1737.0 ± 207.7 (1428.0; 2102.0) | 0.63 ± 0.10 (0.48; 0.80) |
| Effectiveness (Wound-free period) | 34.16 ± 3.35 (28.65; 39.63) | 26.98 ± 3.93 (20.53; 33.42) | 7.18 ± 5.30 (−1.57; 15.89) |
| Mean (SD) | 95% CI | ||
|---|---|---|---|
| CUA-Model (DIC = 4.695) | |||
| Costs | β11 intercept | 6.40 (0.35) | (5.82; 6.97) |
| β12 WoundDuration | 0.01 (0.01) | (−0.01; 0.02) | |
| β13 WoundLeg | 0.56 (0.23) | (0.19; 0.92) | |
| β14 WoundSize | 0.01 (0.00) | (−0.00; 0.01) | |
| β15 EQ-5D | 0.12 (0.31) | (−0.39; 0.64) | |
| β16 Wound-QoL | 0.09 (0.07) | (−0.03; 0.21) | |
| β17 Treatment | −0.48 (0.15) | (−0.73; –0.22) | |
| Costs-Ratio (exp β17) | 0.63 (0.10) | (0.48; 0.80) | |
| Utility | β21 intercept | 0.09 (0.07) | (−0.02; 0.20) |
| β22 WoundDuration | −0.00 (0.00) | (−0.00; 0.00) | |
| β23 WoundLeg | −0.03 (0.04) | (−0.10; 0.04) | |
| β24 WoundSize | −0.00 (0.00) | (−0.00; 0.00) | |
| β25 EQ-5D | −0.08 (0.06) | (−0.18; 0.02) | |
| β26 Wound-QoL | 0.01 (0.01) | (−0.02; 0.03) | |
| β27 Treatment | 0.02 (0.03) | (−0.03; 0.06) | |
| CEA-Model (DIC = 810.2) | |||
| Costs | β11 intercept | 6.40 (0.35) | (5.82; 6.97) |
| β12 WoundDuration | 0.01 (0.01) | (−0.01; 0.02) | |
| β13 WoundLeg | 0.56 (0.23) | (0.19; 0.92) | |
| β14 WoundSize | 0.01 (0.00) | (−0.00; 0.01) | |
| β15 EQ-5D | 0.12 (0.31) | (−0.39; 0.64) | |
| β16 Wound-QoL | 0.09 (0.07) | (−0.03; 0.21) | |
| β17 Treatment | −0.48 (0.15) | (−0.73; –0.22) | |
| Costs-Ratio (exp β17) | 0.63 (0.10) | (0.48; 0.80) | |
| Effectiveness | β31 intercept | 57.54 (12.25) | (37.41; 77.62) |
| β32 WoundDuration | −0.39 (0.25) | (−0.80; 0.02) | |
| Β33 WoundLeg | −18.66 (7.86) | (−31.56; –5.76) | |
| β34 WoundSize | −0.35 (0.14) | (−0.57; –0.12) | |
| β35 EQ-5D | 3.44 (10.91) | (−14.49; 21.33) | |
| β36 Wound-QoL | −4.06 (2.60) | (−8.35; 0.23) | |
| β37 Treatment | 7.18 (5.30) | (−1.57; 15.89) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selva-Sevilla, C.; Conde-Montero, E.; Gerónimo-Pardo, M. Bayesian Regression Model for a Cost-Utility and Cost-Effectiveness Analysis Comparing Punch Grafting Versus Usual Care for the Treatment of Chronic Wounds. Int. J. Environ. Res. Public Health 2020, 17, 3823. https://doi.org/10.3390/ijerph17113823
Selva-Sevilla C, Conde-Montero E, Gerónimo-Pardo M. Bayesian Regression Model for a Cost-Utility and Cost-Effectiveness Analysis Comparing Punch Grafting Versus Usual Care for the Treatment of Chronic Wounds. International Journal of Environmental Research and Public Health. 2020; 17(11):3823. https://doi.org/10.3390/ijerph17113823
Chicago/Turabian StyleSelva-Sevilla, Carmen, Elena Conde-Montero, and Manuel Gerónimo-Pardo. 2020. "Bayesian Regression Model for a Cost-Utility and Cost-Effectiveness Analysis Comparing Punch Grafting Versus Usual Care for the Treatment of Chronic Wounds" International Journal of Environmental Research and Public Health 17, no. 11: 3823. https://doi.org/10.3390/ijerph17113823
APA StyleSelva-Sevilla, C., Conde-Montero, E., & Gerónimo-Pardo, M. (2020). Bayesian Regression Model for a Cost-Utility and Cost-Effectiveness Analysis Comparing Punch Grafting Versus Usual Care for the Treatment of Chronic Wounds. International Journal of Environmental Research and Public Health, 17(11), 3823. https://doi.org/10.3390/ijerph17113823