Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment
Abstract
1. Introduction
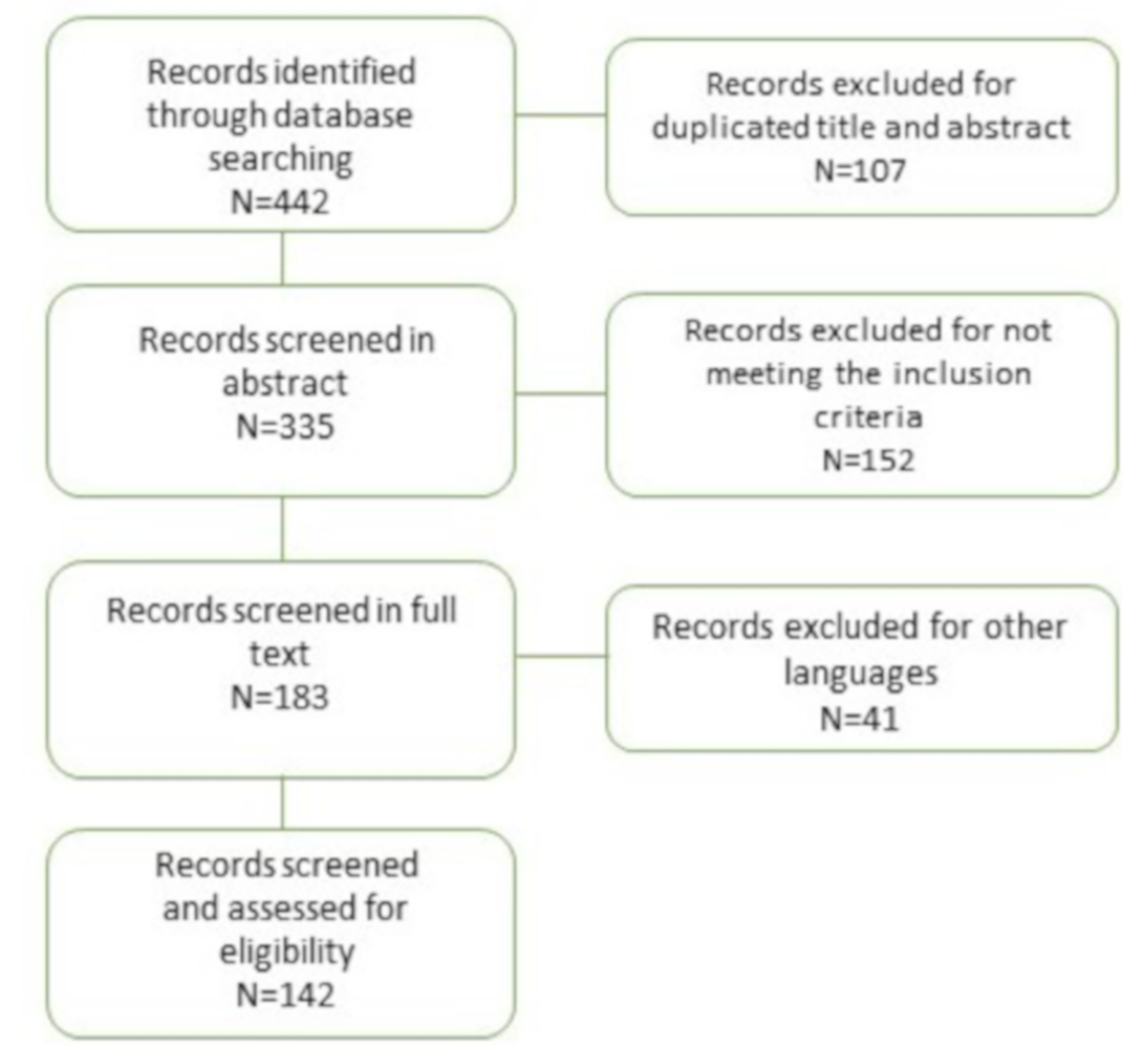
2. Materials and Methods
3. Results
3.1. Infectious Agents
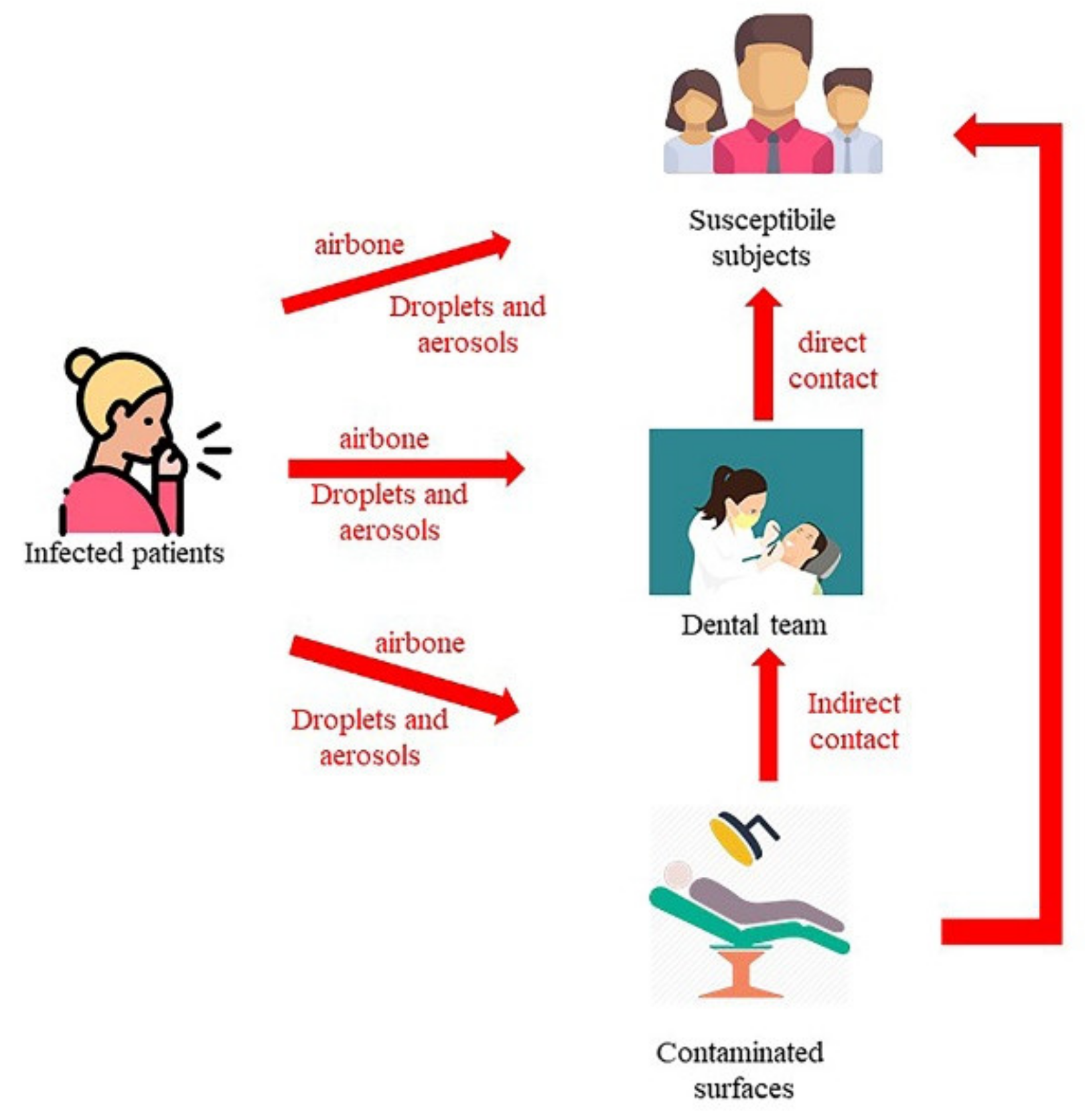
3.1.1. Transmission Mode in Healthcare Settings
- Contact transmission: Contact transmission can be through direct contact and indirect contact.
- ○
- During direct contact transmission, pathogens are transmitted from an infected person to another subject without an intermediate object or person (for example, mucous membrane or breaks contact blood or other blood-containing body fluids infected, or contact HSV lesion without gloves) [3,4,14,15,18,22,24,25].
- ○
- During indirect contact transmission, pathogens are transmitted to the host through objects or human body carrying those pathogens [17,18,22,26,27,28,29,30]. Moreover, all the personal protective equipment (PPE), such as uniforms or isolation gowns, can be contaminated by infectious agents during the treatment of a patient colonized or infected.
- Droplet transmission: Some infectious agents can reach the host through the direct and indirect contact routes or through droplets [3,15,31,32,33]. Droplets can carry infectious pathogens travelling for short distances directly from the respiratory tract of the infectious subjects to host reaching susceptible mucosal surfaces [3,15,31,32,33]. Respiratory droplets are produced during coughs, sneezes, or talks [34] or by airway health procedures. The nasal mucosa, conjunctivae, and mouth are good portals for respiratory viruses [35]. To date, the maximum distance that a droplet can reach is not known, even if pathogens transmitted by a droplet do not run across long distances [19]. The size of droplets has traditionally been defined as being >5 µm [19]. Several types of droplets, including those with diameters of 30 µm or greater, can remain suspended in the air [36]. The sizes of the droplets can determine the maximum distance reached: largest droplets, between 60 and 100 microns, totally evaporate before spontaneously falling 2 m away [37]. For respiratory exhalation flows, the critical factor is the exhalation air velocity: these droplets are carried more than 6 m away by exhaled air at a velocity of 50 m/s (sneezing), more than 2 m away at a velocity of 10 m/s (coughing), and less than 1 m away at a velocity of 1 m/s (breathing) [37].
3.1.2. Infectious Agents of Particular Importance in Dentistry Settings
Viral infections
- SARS-COV-2 determines COVID-19 (coronavirus disease 2019), an infectious disease characterized by several important systemic problems such as coronavirus associated pneumonia. The principal symptoms are fever, cough, and breathing difficulties; the most patients have mild symptoms, some progress to severe pneumonia [43]. The diagnosis is performed with the identification of the virus in swabs of patient throat and nose. COVID-19 can involve the respiratory tract determining a mild or highly acute respiratory syndrome due to the production of pro-inflammatory cytokines, such as interleukin (IL)-1beta and IL-6 [44]. One mechanism that can make the coronavirus lethal is the induction of interstitial pneumonia linked to an over-production of IL-6 [44,45]. Based on this principle, several researchers have started to use an anti-arthritis drug, tocilizumab, for its anti-IL-6 action [46,47,48,49].
- Herpes simplex virus (HSV) can determine a primary infection with minor, ulcerative lymphadenopathy gingivostomatitis [50] and a recurrent infection with cold sores. Herpetic whitlow, an HSV infection of the fingers is usually caused by direct contact of the same fingers with infected saliva or a herpetic lesion [51,52,53]. Skin, mucosal lesions, and secretions such as saliva can determine the transmission [54,55]. Lesions are usually characterized by vesicles and sequent crusting. Acyclovir can be used for the treatment of the diseases. It is sufficient to wear gloves in order to avoid the herpetic whitlow when the clinician treats patients with HSV lesions [10,56].
- Varicella zoster virus (VZV) can determine chickenpox (primary disease), usually in children, and shingles, which is very painful (secondary disease), for the reactivation of a virus residing in sensory ganglia during the latency period [57,58,59]. Chickenpox disease is highly contagious and spreads via-airborne routes [60,61,62]. The virus can infect nonimmune dental team via inhalation of aerosols from a patient incubating the disease. Masks and gloves can be not sufficient for complete protection of the healthcare workers [10].
- Rubella (German measles) is a toga virus that can cause cataract, deafness, and other complications which affect developing foetus, so it is particularly dangerous for the female components of a dental team during pregnancy. It can be transmitted by droplets. In order to avoid these problems, dental staff could be vaccinated for MMR (measles, mumps, and rubella) [10,74,75,76].
- Hepatitis B virus (HBV) causes acute hepatitis and it is an important risk-agent for the health care staff [83,84]. The possible ways of transmission are sexual intercourse, through blood, contaminated material injuries, and perinatal way [85,86,87,88]. So, all operators of the dental team should be vaccinated [10,89].
- Hepatitis C virus (HCV) causes “non-A” and “non-B” hepatitis and it is transmitted like HBV [85,86,87,90]. The primary infection is often asymptomatic and the most of infected subjects become carriers of the virus with risk of development of chronic liver disease that could evolve in hepatocellular carcinoma [10].
- Human immunodeficiency virus (HIV) infects the immune system of susceptible subjects, T-helper cells particularly. It can be transmitted like HBV (sexual intercourse, blood borne and perinatal ways) [91,92]. Moreover, this infection have oral manifestations that can help in diagnosis: e.g., oral candidiasis, oral hairy leukoplakia, oral necrotising ulcerative gingivitis and oral Kaposi’s sarcoma [10,93,94,95,96].
- Cytomegalovirus (CMV) is part of the herpes virus family and can cause diseases with several manifestations [97].
- Mumps virus is part of the Paramyxoviridae group. This pathogen often affects the parotid glands, and the consequently characteristic symptom is swelling of these salivary glands [98]. Moreover, this virus can cause inflammation of the ovaries, testis, pancreas or meninges with several complications. After the introduction of the vaccine against measles, mumps, and rubella (MMR), mumps incidence has decreased, even if several mumps cases have recently been reported [99].
Bacterial infections
- Mycobacterium Tuberculosis causes tuberculosis and is a bacterium transmitted by inhalation, ingestion and inoculation. The main symptoms are cervical lymphadenitis and lung infections. In order to prevent infection, the dental team should be adequately vaccinated and wear PPE [100,101,102,103,104,105]. This bacterium is resistant to chemicals and, for this reason, sterilization and disinfection protocols must be rigorously performed [10].
- Legionella spp. is a gram-negative bacterium that causes Legionellosis and generally it resides in water tanks. Legionellosis occurs with pneumonia, sometimes lethal in older people. Since this pathogen lives in water, it can be easily transmitted during dental procedures through aerosols from incorrectly disinfected water circuits [106,107]. In fact, water circuits that remain unused for long periods of time should be checked regularly to prevent Legionella bacteria from residing [106,107].
- Meningococcal spp. are gram-negative bacteria. They are located on the nasopharyngeal mucosa and their presence is generally asymptomatic. The bacterium is easily transmitted, especially during adolescence, when people get together. As already mentioned, colonization of the nasopharynx is common, and while the resulting disease is rare, at times, it can cause death or permanent disability [109,110].
- Staphylococcus Aureus is an important agent involved in nosocomial infections. This bacterium causes a wide range of diseases that can be mild or life-threatening (e.g., bacteraemia, pneumonia, and surgical site infection [111]). In addition, S. Aureus can easily have antimicrobial resistance. This bacterium principally resides on the epithelium of the anterior nares in human beings [112].
- Group A streptococcus (GAS) is a gram-positive, beta-haemolytic bacterium. This pathogen is responsible for several diseases in human beings, such as acute pharyngitis, impetigo and cellulitis. It can also cause serious invasive diseases such as necrotizing fasciitis and toxic shock syndrome (TSS) [113,114,115]. The bacterium mainly resides in human nose, throat and on skin and it is often transmitted without symptoms [116,117,118]. Obviously, asymptomatic subjects are less contagious than the symptomatic carriers of this bacterium. GAS is transmitted through respiratory droplets spread in the air, for example during coughing, sneezing and nasal secretions [117,118]. In addition, this bacterium can spread through close interpersonal contact during a kiss, using the same dishes and sharing the same cigarette.
- Streptococci Mutans mainly colonize dental surfaces after tooth eruption and is associated to the development of caries [119]. This bacterium may be transmitted horizontally between children during the initial phases of the S. Mutans colonization in nursery environments [120]. There is scientific evidence of vertical transmission of S. mutans from mother to child [121].
- Some periodontal bacteria (e.g., A. actinomycetemcomitans, P. gingivalis) are considered person-to-person transmitted, but it is still unclear if transmission is governed only by domestic pathways, without definitive implications for the dental office. Vertical transmission of A. actinomycetemcomitans is between 30% and 60%, while that of P. gingivalis is rarely observed. Horizontal transmission ranges from 14% to 60% for A. actinomycetemcomitans and between 30% and 75% for P. gingivalis [122]. Certainly, by understanding the spread mechanisms of these bacteria, it would also be possible to prevent a number of systemic diseases [123].
3.2. Personal Protective Equipment (PPE)
3.2.1. Mask/Respirators
- protect personnel from contact with patient infectious material;
- protect patients from infectious agents carried by healthcare workers;
- limit the potential spread of infectious respiratory aerosol between patients [19].
- could be flat or pleated (some are like cups) and fixed to the head with straps or elastic bands;
- does not offer complete protection against small particle aerosols (droplet nuclei) and should not be used during contact with patients with diseases caused by airborne pathogens;
- they are not designed to isolate the face and therefore cannot prevent inhalation by the health personnel wearing them;
- they must be replaced if wet or dirty.
- P2 (94%) and P3 (99.95%) in Australia and New Zeland
- II (95%) and I (99%) in China
- CE-certified FFP class 1 (FFP1) (80%), class 2 (FFP2) (95%), or class 3 (FFP3) (99.7%) in European Union
- 2nd class (95%) and 3rd class (99.9%) in Japan
- 1st class (94%) and special respirators (99.95%) in Republic of Korea
- National Institute for Occupational Safety and Health (NIOSH)-certified N95 (95%), N99 (99%) and N100 (99.7%) in United States [126].
- Name of the manufacturer
- Reference standard nuber (e.g., EN 149:2009)
- class (e.g., FFP1, FFP2 or FFP3)
- CE mark
- Possible reuse (NR or R)
3.2.2. Goggles, Face Shields
3.2.3. Gowns or Coveralls
3.2.4. Gloves
- Surgical gloves in general always distinguish the right side from the left, they are long enough to be worn over the cuffs of the gowns and always packaged in sterile pairs,
- The inspection glove is usually an ambidextrous device, shorter and thinner than the previous one and rarely sterile [132].
3.3. Personal Hygiene
- hair, if a doctor hair can touch the patient or dental equipment, should be attached to the back of the head or a surgical cap should be worn [23];
- facial hair should be covered with a mask or shield [23];
- jewels should be removed from the hands, arms, or facial area during the patient treatment [23];
- nails should be kept clean and short to prevent the perforation of the gloves and the accumulation of debris [23];
- full forearm and hand washing are mandatory before and after treatment [23].
Hand Hygiene
- during handwashing, water and soap should be used in order to generate lather that is distributed on all surface of the hands and after rinsed off;
- hand antisepsis, to physically remove microorganisms by antimicrobial soap or to kill microorganisms with an alcohol-based hand rub;
- surgical hand rub procedure that kills transient organisms and reduces resident flora for the duration of a surgical procedure with antimicrobial soap or an alcohol-based hand rub [135].
- plain soap, that have no antimicrobial properties and works physically removing dirt ad microorganism;
- alcohol-based hand rub, used without water, kills microorganism but does not remove soil or organic material physically;antimicrobial soap kills microorganism and removes physically soil and organic material [135].
3.4. Safety of Tools
3.4.1. Sharp Safety
3.4.2. Instrument Sterilization
3.5. Operative Room Protection
3.5.1. Surface Asepsis/Disinfection
- Chlorine, e.g., sodium hypochlorite
- Phenolic compounds
- Water-based, Water with ortho-phenylphenol, tertiary amylphenol, or O-benzyl–p-chlorophenol
- Alcohol-based ethyl or isopropyl alcohol with ortho-phenylphenol or tertiary amylphenol
- Iodophor–butoxy polypropoxy polyethoxy ethanol iodine complex [23].
- establish a protocol for cleaning and disinfection of surfaces and environments of which health personnel must be informed;
- cover with disposable films all the surfaces that are touched during the procedures (for example switches, IT equipment) and change these protections between each patient;
- surfaces that are not protected by a barrier should be cleaned and disinfected with a disinfectant after each patient;
- use a medium level disinfectant (i.e., tuberculocidal indication) if a surface is visibly contaminated with blood;
3.5.2. Dental Unit Waterlines (DUWLs)
- use water compliant with Environmental Protection Agency (EPA) standards for drinking water (i.e., ≤ 500 CFU/mL of heterotrophic water bacteria),
- follow the recommendations for water quality monitoring given by the manufacturer of the unit or waterline treatment product,
- use sterile water or sterile saline for the irrigation during surgical procedures [136].
3.5.3. Waste Management
3.6. Other Precautions
4. From the Literature to a Novel Operative Algorithm
- Prevention of infections must be a priority in any healthcare setting and therefore also in any dental clinic. To do this, staff training and information, adequate management of resources, and use of well-defined operating protocols is necessary.
- Adequate management of the protection for operators (and therefore also for patients) begins with the roles of the secretariat. In order to better organize the workflow, the secretariat must provide a telephone triage. It would be advisable to phone each patient to make sure he is healthy on the day of the appointment. Patients with acute symptoms of any infectious disease should be referred at the time of symptom resolution. The medical history of patients may not reveal asymptomatic infectious disease of which they are affected. This means the operator must adopt the same infection control rules for all patients, as if they were all infective. In addition, the secretariat must organize appointments in order to avoid crowding in the waiting room. It would be advisable for the patient to present himself alone, without companions (only minors, the elderly and patients with psycho-physical conditions can be accompanied). In some urgent and non-deferrable cases, it is necessary to treat the patient despite being in the acute phase of infection with any virus. Examples of urgent treatments are: pulpitis, tooth fracture, and avulsion [2]. In these cases, the operator must implement the maximum individual protection measures.
- In the waiting room all material (e.g., magazines, newspapers, information posters) that can represent a source of contamination must be eliminated so that the room is easy to disinfect.
- Patients are requested to go to the appointment without any superfluous objects. At the entrance of the dental structure, the patient must wear shoe covers, disinfect the hands with hydroalcoholic solution according to the following indications, affix any jacket on a special hanger and disinfect the hands again with hydroalcoholic solution. If there are several patients in the waiting room, they must be at least two meters away from each other. The correct hand disinfection procedure with hydroalcoholic solution is as follows:
- a)
- Apply a squirt of sanitizer in the palm of hand,
- b)
- Rub hands palm against each other,
- c)
- Rub the back of each hands with the palm of the other hand,
- d)
- Rub palms together with your finger interlaced,
- e)
- Rub the back of fingers with the opposite palms,
- f)
- Rotate thumbs in the other hand,
- g)
- Do a circle on palm with finger clasped,
- h)
- Once dry, hands are safe.
- 5.
- The operators must be adequately dressed in the correct PPE. Healthcare professionals will need to remove any jewel before starting dressing procedures. All the necessary PPE must already be positioned clearly visible and intact, in a room that will be distinct from the one where the undressing phase will take place. In both areas, hydroalcoholic solution and/or items necessary for washing hands with soap and water should be available. In the dressing room there must be trays for the collection and subsequent disinfection of the non-disposable PPE and special containers for the collection of waste where to dispose of the disposable PPE. A dressing and undressing procedure is described below, imagining that the dentist has to operate under a high risk of infection. Dressing and undressing procedures must be particularly considered.
- a)
- eliminate jewels and personal items from the pockets of the uniform;
- b)
- long hair must be tied and inserted into a cap not mandatory for single use (no tufts of hair must come out of the cap);
- c)
- wear shoe covers;
- d)
- perform social hand washing or disinfection with antiseptic gel;
- e)
- wear the first pair of gloves of the right size;
- f)
- wear the water repellent gown by tying it on the back without double knots (first the upper part and then the lower part, the latter must be tied on the front) being careful not to leave parts of the uniform exposed;
- g)
- wear the mask (FFP2-FFP3) which must adhere well to both the nose and the mind;
- h)
- put on the disposable water-repellent cap and be tied under the chin, the excess ribbons must be inserted inside the gown;
- i)
- wear glasses/protective screen;
- j)
- wear a second pair of gloves for direct patient assistance. These gloves must cover the cuffs of the disposable gown.
- a)
- remove the second pair of (dirty) gloves being careful not to contaminate the underlying gloves;
- b)
- gloves still worn with a hydroalcoholic solution are disinfected and a new pair of gloves is worn on them;
- c)
- the face shield is removed: if it is disposable it should be trashed, and if it is not disposable, it should be placed in a container with disinfectant;
- d)
- the second pair of gloves is removed without contaminating the underlying gloves;
- e)
- the gloves are rubbed with hydroalcoholic solution and a new pair of gloves is worn;
- f)
- disposable gown removal starting from the top, then the bottom, rolling it up to touch the inside, clean;
- g)
- throw disposable shirts and second pair of gloves;
- h)
- the gloves are rubbed with hydroalcoholic solution and a new pair of gloves is worn;
- i)
- remove the water-repellent cap;
- j)
- the gloves are rubbed with hydroalcoholic solution and a new pair of gloves is worn;
- k)
- remove mask taking it by the elastics with the head bent forward and down;
- l)
- both the first pair and the second pair of gloves are removed;
- m)
- hands are disinfected with hydroalcoholic solution.
- 6.
- Before entering the surgical room, the patient must be dressed in a disposable gown and headgear worn in order to avoid any contagion on clothing and hair.
- 7.
- Before dental session patient should rinse and gargle with a specific mouthwash.Chlorhexidine is commonly used for pre-procedural oral rinses in dental offices, but its capacity of 2019-nCoV destruction has not yet been demonstrated [4]. Instead, pre-procedural oral rinses with oxidizing such as 1% hydrogen peroxide or 0.2% povidone-iodine are recommended [4]. So, the pre-procedural use of mouthwash, especially in cases of inability to use a rubber dam, can significantly reduce the microbial load of oral cavity fluids [3]. In fact, even if oral rinses seem to “limit” the viral load, virus can spread through the complete respiratory tract and it is not scientifically possible to guarantee that this reduction is constant during the operative manoeuvre (e.g., cough, sneezing, runny nose). Then the following pre-operative procedure is recommended to the patient: a) 1% hydrogen peroxide 15" gargle followed by 30” rinse, b) do not rinse with water at the end of the rinse and continue with Chlorhexidine 0.20% 60” rinse with final gargle of 15" [146]. At the end of the procedure, the patient must be appropriately undressed, and have another oral rinse performed before washing hands and face thoroughly.
- 8.
- After every patient, carefully clean all surfaces, starting from the least contaminated to the most potentially infected, taking care not to overlook the handles of the doors and the various drawers, worktops and all the devices used during the treatment and which are not disposable or autoclavable. Cover switches, mice, computer keyboards, and anything else that may be more difficult to clean with disposable film. The worktops must be free from anything that is not strictly necessary to perform the service. An accurate disinfection of the surfaces includes a preventive cleaning of the same in order to eliminate the soil which otherwise would not allow the disinfectant to inactivate the microorganisms [29]. In the same way, if you want to use disinfectant wipes, you must use one to cleanse and after another to disinfect. As regards spray disinfectants, the percentage of dilution and the time of application vary from product to product: you must follow the instructions provided by the company. Moreover, alcohol-based disinfectants (75%), 0.5% hydrogen peroxide, 0.1% sodium hypochlorite are recommended to be left to act on the surfaces for 1 min. Disinfect the circuits of the treatment center at each patient change. Between patients, the tubing of high-volume aspirators and saliva ejectors should be regularly flushed with water and disinfectant such as 0.1% sodium hypochlorite. Always air the rooms after each patient (at least 20–30 min) or use germicidal lamps. Clean floors with bleach at least two times a day.
- 9.
- During every procedure minimize the use of an air/water syringe: dry the site with cotton rollers when possible; use suction at maximum power (it might be an idea to use autoclavable plastic suction cannulas that have a greater suction capacity than normal disposable PVC cannulas) or use two saliva ejectors; in the case of exposed carious dentine, try to remove it as manually as possible using excavators; be sure to first mount the rubber dam, disinfect the crown with pellets soaked in 75% alcohol and recommend with the second operator to position the aspirator as correctly as possible to avoid excessive spraying and/or splashing; do not use air-polishing; avoid intraoral x-rays as they stimulate salivation, coughing and/or vomiting; prefer exams like OPT (orthopantomography) or CBCT (cone beam computed tomography). In case of extractions, it is preferable to use resorbable sutures to seal the post-extraction site. In the case of patients who are definitely positive for any infectious agent or on which there are greater possibilities of positivity highlighted by the medical history, it is necessary to plan their treatment at the end of the day. Do not touch patient card and pens with dirty gloves. It is good practice to cough or sneeze into the elbow. The operator must avoid touching his eyes, nose and mouth with dirty gloves or hands.
- 10.
- Isolation with rubber dam [4]. Isolating the oral cavity with the use of rubber dams greatly reduces (about 70%) the spread of respiratory droplets and aerosols containing saliva or blood coming from the patient and aimed to the operator area of action [4]. After positioning the dam, the operator must provide an efficient high-volume intraoral aspiration in order to prevent the spread of aerosol and spray as much as possible [148]. If rubber dams cannot be used for any reason, the operator should prefer to use manual tools such as hand scalers [4].
- 11.
- Anti-retraction handpiece [4]. During the COVID-19 pandemic, operators should avoid using dental mechanical handpieces that do not have an anti-retraction function [4]. Mechanical handpieces with the anti-retraction system have valves (anti-retraction) that are very important in order to prevent the spread and dispersion of droplets and aerosol [148,149].
- 12.
- All instruments which have been used for the treatment of a patient or which have only been touched by operators during a session and which cannot be sterilized according to standard protocols, must be disinfected (e.g., immersed in a container with phenol) [23]. This tools bagged in disinfection solution must remain in solution for about 10 min [23]. Some materials, such as polysulphide, polyvinylsiloxane, impression compound, and ZOE impressing materials, after being in the patient mouth, are rinsed with water and immersed in a 5.25% sodium hypochlorite solution for about 10 min [23]. The alginate or polyether impressions are also rinsed with water, sprayed with a 5.25% sodium hypochlorite solution and placed in a container for about 10 min [23]. Wax, resin centric relation records, and ZOE are rinsed with water and sprayed with a 5.25% sodium hypochlorite solution and placed in a plastic bag for about 10 min [23]. Provisional restorations and complete dentures removed from the patient mouth are immersed in a 5.25% sodium hypochlorite solution for 10 min [23]. Otherwise, removable partial prostheses with metal bases are treated with 2% glutaraldehyde solution and placed in a plastic bag for 10 min [23].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Izzetti, R.; Nisi, M.; Gabriele, M.; Graziani, F. COVID-19 Transmission in Dental Practice: Brief Review of Preventive Measures in Italy. J. Dent. Res. 2020. [Google Scholar] [CrossRef]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus Disease 2019 (COVID-19): Emerging and Future Challenges for Dental and Oral Medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 9. [Google Scholar] [CrossRef]
- Fallahi, H.R.; Keyhan, S.O.; Zandian, D.; Kim, S.-G.; Cheshmi, B. Being a front-line dentist during the Covid-19 pandemic: A literature review. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 12. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Jouhar, R.; Ahmed, N.; Adnan, S.; Aftab, M.; Zafar, M.; Khurshid, Z. Fear and Practice Modifications among Dentists to Combat Novel Coronavirus Disease (COVID-19) Outbreak. Int. J. Environ. Res. Public Health 2020, 17, 2821. [Google Scholar] [CrossRef]
- Kamate, S.K.; Sharma, S.; Thakar, S.; Srivastava, D.; Sengupta, K.; Hadi, A.J.; Chaudhary, A.; Joshi, R.; Dhanker, K. Assessing Knowledge, Attitudes and Practices of dental practitioners regarding the COVID-19 pandemic: A multinational study. Dent. Med. Probl. 2020, 57, 11–17. [Google Scholar] [CrossRef]
- Yonis, O.B.; Alyahya, M.; Khader, Y.; Al Nsour, M.; Al-Batayneh, O.B.; Saadeh, R.; Bashier, H.; Alfaqih, M.; Al-Azzam, S.; Alshurman, B.A. Dentists’ Awareness, Perception, and Attitude Regarding COVID-19 and Infection Control: Cross-Sectional Study Among Jordanian Dentists. JMIR Public Health Surveill. 2020, 6, e18798. [Google Scholar] [CrossRef]
- Mallineni, S.K.; Innes, N.P.; Raggio, D.P.; Araujo, M.P.; Robertson, M.D.; Jayaraman, J. Coronavirus disease (COVID-19): Characteristics in children and considerations for dentists providing their care. Int. J. Paediatr. Dent. 2020, 30, 245–250. [Google Scholar] [CrossRef]
- Mupparapu, M. Editorial: Dental practitioners’ role in the assessment and containment of coronavirus disease (COVID-19): Evolving recommendations from the Centers for Disease Control. Quintessence Int. 2020, 51, 349–350. [Google Scholar]
- Infection Control - Updates. Infection Control—Updates; IntechOpen: Rijeka, Croatia, 2012; p. 2251. [Google Scholar]
- Esen, E. Personnel protective measures for infection control in dental health care settings. Turk. J. Hosp. Infect. 2007, 11, 143–146. [Google Scholar]
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial Cells Lining Salivary Gland Ducts Are Early Target Cells of Severe Acute Respiratory Syndrome Coronavirus Infection in the Upper Respiratory Tracts of Rhesus Macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef]
- Chen, J. Pathogenicity and transmissibility of 2019-nCoV—A quick overview and comparison with other emerging viruses. Microbes Infect. 2020, 22, 69–71. [Google Scholar] [CrossRef]
- Cleveland, J.L.; Gray, S.K.; Harte, J.A.; Robison, V.A.; Moorman, A.; Gooch, B.F. Transmission of blood-borne pathogens in US dental health care settings: 2016 update. J. Am. Dent. Assoc. 2016, 147, 729–738. [Google Scholar] [CrossRef]
- Harrel, S.K.; Molinari, J. Aerosols and splatter in dentistry. J. Am. Dent. Assoc. 2014, 135, 429–437. [Google Scholar] [CrossRef]
- Coulthard, P. Dentistry and coronavirus (COVID-19)—Moral decision-making. Br. Dent. J. 2020, 228, 503–505. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Otter, J.; Donskey, C.; Yezli, S.; Douthwaite, S.; Goldenberg, S.D.; Weber, D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: The possible role of dry surface contamination. J. Hosp. Infect. 2016, 92, 235–250. [Google Scholar] [CrossRef]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Health Care Infection Control Practices Advisory Committee 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am. J. Infect. Control. 2007, 35, S65–S164. [Google Scholar] [CrossRef]
- Thomsen, R.W.; Hundborg, H.H.; Lervang, H.-H.; Johnsen, S.P.; Schønheyder, H.C.; Sørensen, H.T. Risk of community-acquired pneumococcal bacteremia in patients with diabetes: A population-based case-control study. Diabetes Care 2004, 27, 1143–1147. [Google Scholar] [CrossRef]
- Antonovics, J.; Wilson, A.; Forbes, M.R.; Hauffe, H.C.; Kallio, E.R.; Leggett, H.C.; Longdon, B.; Okamura, B.; Sait, S.M.; Webster, J.P. The evolution of transmission mode. Philos. Trans. R. Soc. B Boil. Sci. 2017, 372, 20160083. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Sharma, V.; Kaur, S. “Hepatitis”—Prevention and management in dental practice. J. Educ. Health Promot. 2015, 4, 33. [Google Scholar]
- Upendran, A.; Geiger, Z. Dental Infection Control; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Beltrami, E. Transmission of HIV and hepatitis C virus from a nursing home patient to a health care worker. Am. J. Infect. Control. 2003, 31, 168–175. [Google Scholar] [CrossRef]
- Kessler, C.; McGuinn, M.; Spec, A.; Christensen, J.; Baragi, R.; Hershow, R.C. Underreporting of blood and body fluid exposures among health care students and trainees in the acute care setting: A 2007 survey. Am. J. Infect. Control. 2011, 39, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.; Pultz, N.J.; Gries, D.M.; Ray, A.J.; Eckstein, E.C.; Aron, D.C.; Donskey, C.J. Acquisition of Nosocomial Pathogens on Hands After Contact with Environmental Surfaces Near Hospitalized Patients. Infect. Control. Hosp. Epidemiol. 2004, 25, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.D.; Wang, Z.Y.; Zhang, S.F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.B.; Dong, Y.Z.; Chi, X.Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef]
- Mupparapu, M.; Kothari, K.R.M. Review of surface disinfection protocols in dentistry: A 2019 update. Quintessence Int. 2019, 50, 58–65. [Google Scholar] [CrossRef]
- Sandle, T. Cleaning and disinfection of dental practice surfaces. Dent. Nurs. 2017, 13, 86–87. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Bahl, P.; Doolan, C.; de Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or droplet precautions for health workers treating COVID-19? J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Meselson, M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef]
- Morawska, L.; Cao, J. Airborne transmission of SARS-CoV-2: The world should face the reality. Environ. Int. 2020, 139, 105730. [Google Scholar] [CrossRef] [PubMed]
- Papineni, R.S.; Rosenthal, F.S. The Size Distribution of Droplets in the Exhaled Breath of Healthy Human Subjects. J. Aerosol Med. 1997, 10, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Douglas, R.G.; Schnabel, K.C.; Geiman, J.M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect. Immun. 1981, 33, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.C.; Cook, C.E. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am. J. Infect. Control. 1998, 26, 453–464. [Google Scholar] [CrossRef]
- Xie, X.; Li, Y.; Chwang, A.T.Y.; Ho, P.-L.; Seto, W.H. How far droplets can move in indoor environments ? revisiting the Wells evaporation?falling curve. Indoor Air 2007, 17, 211–225. [Google Scholar] [CrossRef]
- Faridi, S.; Niazi, S.; Sadeghi, K.; Naddafi, K.; Yavarian, J.; Shamsipour, M.; Jandaghi, N.Z.S.; Sadeghniiat, K.; Nabizadeh, R.; Yunesian, M.; et al. A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Sci. Total. Environ. 2020, 725, 138401. [Google Scholar] [CrossRef]
- England, J.H.; Byrne, D.W.; Harris, B.D.; Talbot, T.R. Use of airborne infection isolation in potential cases of pulmonary tuberculosis. Infect. Control. Hosp. Epidemiol. 2020, 41, 505–509. [Google Scholar] [CrossRef]
- Torres, M.; Carranza, C.; Sarkar, S.; Gonzalez, Y.; Osornio-Vargas, A.R.; Black, K.; Meng, Q.; Quintana-Belmares, R.; Hernandez, M.; Garcia, J.J.F.A.; et al. Urban airborne particle exposure impairs human lung and blood Mycobacterium tuberculosis immunity. Thorax 2019, 74, 675–683. [Google Scholar] [CrossRef]
- Sornboot, J.; Aekplakorn, W.; Ramasoota, P.; Bualert, S.; Tumwasorn, S.; Jiamjarasrangsi, W. Detection of airborne Mycobacterium tuberculosis complex in high-risk areas of health care facilities in Thailand. Int. J. Tuberc. Lung Dis. 2019, 23, 465–473. [Google Scholar] [CrossRef]
- Küsel, R.R.; Craig, I.; Stoltz, A.C. Modeling the Airborne Infection Risk of Tuberculosis for a Research Facility in eMalahleni, South Africa. Risk Anal. 2018, 39, 630–646. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A.; Chavez-Valencia, V. High IL-6/IFN-gamma ratio could be associated with severe disease in COVID-19 patients. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Fox, B.A.; Urba, W.J.; Anderson, A.C.; Atkins, M.B.; Borden, E.C.; Brahmer, J.R.; Butterfield, L.H.; Cesano, A.; Chen, D.S.; et al. Insights from immuno-oncology: The Society for Immunotherapy of Cancer Statement on access to IL-6-targeting therapies for COVID-19. J. Immunother. Cancer 2020, 8, e000878. [Google Scholar] [CrossRef]
- Capecchi, P.L.; Lazzerini, P.E.; Volterrani, L.; Mazzei, M.A.; Rossetti, B.; Zanelli, G.; Bennett, D.; Bargagli, E.; Franchi, F.; Cameli, M.; et al. Antirheumatic agents in COVID-19: Is IL-6 the right target? Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Monti, S.; Montecucco, C. The conundrum of COVID-19 treatment targets: The close correlation with rheumatology. Response to: ’Management of rheumatic diseases in the time of covid-19 pandemic: Perspectives of rheumatology pracitioners from India’ by Gupta et al and ’Antirheumatic agents in covid-19: Is IL-6 the right target?’ by Capeechi et al. Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents 2020, 105954. [Google Scholar] [CrossRef]
- Davidovici, B.B.; Balicer, R.D.; Klement, E.; Green, M.S.; Mendelson, E.; Smetana, Z.; Cohen, D. Comparison of the dynamics and correlates of transmission of Herpes Simplex Virus-1 (HSV-1) and Varicella-Zoster Virus (VZV) in a sample of the Israeli population. Eur. J. Epidemiol. 2007, 22, 641–646. [Google Scholar] [CrossRef]
- Cunha, N.; Simões, P.; Serrão, V. Extensive Atypical HSV-2 Ulceration of the Finger. Acta Médica Port. 2017, 30, 587. [Google Scholar] [CrossRef]
- Sehayik, R.I.; Bassett, F.H. Herpes Simplex Infection Involving the Hand. Clin. Orthop. Relat. Res. 1982, 138–140. [Google Scholar] [CrossRef]
- Malik, N. Textbook of Oral and Maxillofacial Surgery; Jaypee Brothers Medical Publishing: New Delhi, India, 2016. [Google Scholar]
- Caliento, R.; Sarmento, D.J.D.S.; Silva Érika, M.P.; Tozetto-Mendoza, T.R.; Tobouti, P.L.; Benini, V.; Braz-Silva, P.H.; Gallottini, M. Oral shedding of HSV-1 and EBV and oral manifestations in paediatric chronic kidney disease patients and renal transplant recipients. Acta Odontol. Scand. 2018, 76, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Hyland, P.; Coulter, W.; Abu-Ruman, I.; Fulton, C.; O’Neill, H.; Coyle, P.; Lamey, P.-J. Asymptomatic shedding of HSV-1 in patients undergoing oral surgical procedures and attending for noninvasive treatment. Oral Dis. 2007, 13, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.S. Infection control of herpes simplex virus infections in obstetrics and gynecology. J. Reprod. Med. 1986, 31, 395–398. [Google Scholar] [PubMed]
- Gould, D. Varicella zoster virus: Chickenpox and shingles. Nurs. Stand. 2014, 28, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, A. Varicella-zoster virus, shingles and postherpetic neuralgia. Prof. Nurse 2003, 19, 195–196. [Google Scholar]
- Juel-Jensen, B.E. The natural history of shingles. Events associated with reactivation of varicella-zoster virus. J. R. Coll. Gen. Pr. 1970, 20, 323–327. [Google Scholar]
- Scheifele, D.; Bonner, M. Airborne transmission of chickenpox. N. Engl. J. Med. 1980, 303, 281–282. [Google Scholar]
- Riley, R.L. Airborne Transmission of Chickenpox. N. Engl. J. Med. 1980, 303, 281. [Google Scholar] [CrossRef]
- LeClair, J.M.; Zaia, J.A.; Levin, M.J.; Congdon, R.G.; Goldmann, N.A. Airborne Transmission of Chickenpox in a Hospital. N. Engl. J. Med. 1980, 302, 450–453. [Google Scholar] [CrossRef]
- Kuhara, T.; Watanabe, D.; Ishida, N.; Tamada, Y.; Matsumoto, Y.; Ihira, M.; Fukaya, S.; Yoshida, S.; Yoshikawa, T.; Asano, Y. Quantitative analysis of shedding of Epstein-Barr virus in saliva from patients with connective tissue diseases: A pilot study. Int. J. Dermatol. 2013, 52, 887–890. [Google Scholar] [CrossRef]
- Huynh, G.T.; Rong, L. Modeling the dynamics of virus shedding into the saliva of Epstein-Barr virus positive individuals. J. Theor. Boil. 2012, 310, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, M.R.; Beitollahi, J.; Nikoobakht, N.; Aloosh, M.; Sahebjamee, M.; Rezaeidanesh, M.; Biniaz, F. Evaluation of Epstein–Barr Virus Load in Saliva Before and After Renal Transplantation. Transplant. Proc. 2011, 43, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Mbulaiteye, S.M.; Walters, M.; Engels, E.A.; Bakaki, P.M.; Ndugwa, C.M.; Owor, A.M.; Goedert, J.J.; Whitby, D.; Biggar, R.J. High Levels of Epstein-Barr Virus DNA in Saliva and Peripheral Blood from Ugandan Mother-Child Pairs. J. Infect. Dis. 2006, 193, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Idesawa, M.; Suzuki, N.; Ikeda, K.; Oshikawa, M.; Takane, M.; Seki, K.; Ito, K. Detection of Epstein-Barr virus in saliva by real-time PCR. Oral Microbiol. Immunol. 2004, 19, 230–232. [Google Scholar] [CrossRef]
- Niederman, J.C.; Miller, G.; Pearson, H.A.; Pagano, J.S.; Dowaliby, J.M. Infectious Mononucleosis. N. Engl. J. Med. 1976, 294, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Caserta, M.T.; McDermott, M.P.; Dewhurst, S.; Schnabel, K.; Carnahan, J.A.; Gilbert, L.; Lathan, G.; Lofthus, G.K.; Hall, C.B. Human herpesvirus 6 (HHV6) DNA persistence and reactivation in healthy children. J. Pediatr. 2004, 145, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.A. Three new human herpesviruses (HHV6, 7, and 8). Lancet 1997, 349, 558–563. [Google Scholar] [CrossRef]
- Gwaltney, J.M.; Moskalski, P.B.; Hendley, J.O. Hand-to-Hand Transmission of Rhinovirus Colds. Ann. Intern. Med. 1978, 88, 463. [Google Scholar] [CrossRef] [PubMed]
- Gwaltney, J.M.; Hendley, J.O. Rhinovirus Transmission. Am. J. Epidemiol. 1978, 107, 357–361. [Google Scholar] [CrossRef]
- Douglas, R.G.; Rossen, R.D.; Butler, W.T.; Couch, R.B. Rhinovirus neutralizing antibody in tears, parotid saliva, nasal secretions and serum. J. Immunol. 1967, 99, 297–303. [Google Scholar]
- Jusko, T.A.; Singh, K.; Greener, E.A.; Feiler, M.O.; Thevenet-Morrison, K.; Lawrence, B.P.; Wright, R.O.; Thurston, S.W. Blood Lead Concentrations and Antibody Levels to Measles, Mumps, and Rubella among U.S. Children. Int. J. Environ. Res. Public Health 2019, 16, 3035. [Google Scholar] [CrossRef]
- Chamat, S.; Salameh, P.; Haddad, N.; Berry, A.; Chedid, P.; Bouharoun-Tayoun, H. Protection of medical and paramedical university students in Lebanon against measles, mumps, rubella and varicella: Active measures are needed. J. Infect. Public Health 2011, 4, 125–134. [Google Scholar] [CrossRef][Green Version]
- Yerkovich, S.; Rowe, J.; Richmond, P.; Suriyaarachchi, D.; Heaton, T.; Hollams, E.; Ladyman, C.; Serralha, M.; Sadowska, A.; Loh, R.; et al. Assessment of the potency and potential immunomodulatory effects of the measles mumps rubella and varicella vaccine in infants. Vaccine 2007, 25, 1764–1770. [Google Scholar] [CrossRef]
- Lamey, P.J.; Lewis, M.A. Oral medicine in practice: Viral infection. Br. Dent. J. 1989, 167, 269–274. [Google Scholar] [CrossRef]
- Madonia, J.V.; Bahn, A.N.; Calandra, J.C. Salivary Excretion of Coxsackie B-1 Virus in Rabbits. Appl. Microbiol. 1966, 14, 394–396. [Google Scholar] [CrossRef]
- Donegan, E.; Pell, P.; Shaw, G.; Mosley, J.; Lee, H. The Transfusion Safety Study Group Transmission of human T?lymphotropic virus type I by blood components from a donor lacking anti?p24: A case report. Transfusion 1992, 32, 68–71. [Google Scholar] [CrossRef]
- Gasmi, M.; D’Incan, M.; Desgranges, C. Transfusion transmission of human T?lymphotropic virus type I (HTLV?I) from an asymptomatic blood donor: Conservation of LTR U3, env, and tax nucleotide sequences in a recipient with HTLV?I?associated myelopathy. Transfusion 1997, 37, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Herr, V.; Ambruso, D.; Fairfax, M.; Neumann, A.; Swanson, P.; Lee, H. Transfusion-associated transmission of human T-lymphotropic virus types I and II: Experience of a regional blood center. Transfusion 1993, 33, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C.; Kao, C.L.; Chin, L.T.; Chen, J.W.; Yang, C.M.; Chang, A.C.; Chen, B.H. Intrafamilial transmission and risk assessment of HTLV-I among blood donors in southern Taiwan. Kaohsiung J. Med. Sci. 2001, 17, 126–132. [Google Scholar] [PubMed]
- Kohn, W.G.; Collins, A.S.; Cleveland, J.L.; Harte, J.A.; Eklund, K.J.; Malvitz, D.M. Guidelines for infection control in dental health-care settings—2003. MMWR. Recomm. Rep. 2003, 52, 1–61. [Google Scholar]
- Beltrami, E.M.; Williams, I.T.; Shapiro, C.N.; Chamberland, M.E. Risk and Management of Blood-Borne Infections in Health Care Workers. Clin. Microbiol. Rev. 2000, 13, 385–407. [Google Scholar] [CrossRef]
- Yooda, A.P.; Sawadogo, S.; Soubeiga, S.T.; Obiri-Yeboah, D.; Nebie, K.; Ouattara, A.K.; Diarra, B.; Simpore, A.; Yonli, Y.D.; Sawadogo, A.-G.; et al. Residual risk of HIV, HCV, and HBV transmission by blood transfusion between 2015 and 2017 at the Regional Blood Transfusion Center of Ouagadougou, Burkina Faso. J. Blood Med. 2019, 10, 53–58. [Google Scholar] [CrossRef] [PubMed]
- López-Menchero, C.; Alvarez, M.; Fernández, P.; Guzmán, M.; Ortiz-De-Salazar, M.I.; Arbona, C. Evolution of the residual risk of HBV, HCV and HIV transmission through blood transfusion in the Region of Valencia, Spain, during a 15-year period (2003–2017). Blood Transfus. 2019, 17, 418–427. [Google Scholar] [PubMed]
- Lee, J.H.; Cho, J.; Kim, Y.J.; Im, S.H.; Jang, E.S.; Kim, J.W.; Kim, W.J.; Jeong, S.-H. Occupational blood exposures in health care workers: Incidence, characteristics, and transmission of bloodborne pathogens in South Korea. BMC Public Health 2017, 17, 827. [Google Scholar] [CrossRef]
- Borg, M. Hepatitis B transmission through blood and body fluids exposure of school personnel. Occup. Med. 2005, 55, 133–135. [Google Scholar] [CrossRef][Green Version]
- Kohn, W.G.; Harte, J.A.; Malvitz, D.M.; Collins, A.S.; Cleveland, J.L.; Eklund, K.J. Cover Story Guidelines for infection control in dental health care settings—2003. J. Am. Dent. Assoc. 2004, 135, 33–47. [Google Scholar] [CrossRef]
- Klevens, R.M.; Moorman, A. Hepatitis C virus. J. Am. Dent. Assoc. 2013, 144, 1340–1347. [Google Scholar] [CrossRef]
- Yang, R.; Gui, X.; Benoit, J.-L.; Xiong, Y. The comparison of human immunodeficiency virus type 1 transmission between couples through blood or sex in central China. Jpn. J. Infect. Dis. 2010, 63, 283–285. [Google Scholar]
- Klein, R.S.; Friedland, G.H. Transmission of Human Immunodeficiency Virus Type 1 (HIV-1) by Exposure to Blood: Defining the Risk. Ann. Intern. Med. 1990, 113, 729. [Google Scholar] [CrossRef]
- Ranganathan, K.; Umadevi, K.M.R. Common oral opportunistic infections in Human Immunodeficiency Virus infection/Acquired Immunodeficiency Syndrome: Changing epidemiology; diagnostic criteria and methods; management protocols. Periodontology 2000 2019, 80, 177–188. [Google Scholar] [CrossRef]
- Sanadhya, Y.K.; Sanadhya, S.; Nagarajappa, R.; Jain, S.; Aapaliya, P.; Sharma, N. Correlation between oral lesions and opportunistic infections among human immunodeficiency virus—Infected individuals in Indian population. Int. Marit. Health 2014, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Del Mistro, A.; Baboci, L.; Frayle, H.; Trevisan, R.; Bergamo, E.; Lignitto, L.; Sasset, L.; Cecchetto, M.G.; Cattelan, A.M.; Calabrò, M.L. Oral Human Papillomavirus and Human Herpesvirus-8 Infections Among Human Immunodeficiency Virus Type 1–Infected Men and Women in Italy. Sex. Transm. Dis. 2012, 39, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, C.; D’Souza, G.; Sugar, E.; Weber, K.; Goshu, E.; Minkoff, H.; Wright, R.; Seaberg, E.; Gillison, M. Relationship between Prevalent Oral and Cervical Human Papillomavirus Infections in Human Immunodeficiency Virus-Positive and -Negative Women. J. Clin. Microbiol. 2006, 44, 4479–4485. [Google Scholar] [CrossRef]
- Sinnott, J.T.; Cancio, M.R. Cytomegalovirus. Infect. Control. 1987, 8, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.G. Salivary IgA Antibodies to Mumps Virus During and After Mumps. J. Infect. Dis. 1981, 143, 617. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Vermeire, T.; Van Gucht, S.; Martens, L.; Hutse, V.; Cremer, J.; Rota, P.A.; Leroux-Roels, G.; Koopmans, M.P.; Van Binnendijk, R.; et al. Differences in antigenic sites and other functional regions between genotype A and G mumps virus surface proteins. Sci. Rep. 2018, 8, 13337. [Google Scholar] [CrossRef]
- Samaranayake, L.P. Re-emergence of tuberculosis and its variants: Implications for dentistry. Int. Dent. J. 2002, 52, 330–336. [Google Scholar] [CrossRef]
- Murphy, D.C.; Younai, F.S. Risk of tuberculosis transmission in dentistry. Results of a retrospective chart review. AAOHN J. 1997, 45, 377–385. [Google Scholar] [CrossRef]
- Molinari, J.A. Tuberculosis infection control: A reasonable approach for dentistry. Compend. Contin. Educ. Dent. 1995, 16, 1080–1082. [Google Scholar]
- Riben, P.D.; Epstein, J.B.; Mathias, R.G. Dentistry and tuberculosis in the 1900s. J. Canadian Dent. Assoc. 1995, 61, 495–498. [Google Scholar]
- Molinari, J.A.; Cottone, J.A.; Chandrasekar, P.H. Tuberculosis in the 1990s: Current implications for dentistry. Compendium 1993, 14, 280–282. [Google Scholar]
- Faecher, R.S.; Thomas, J.E.; Bender, B.S. Tuberculosis: A Growing Concern for Dentistry? J. Am. Dent. Assoc. 1993, 124, 94–104. [Google Scholar] [CrossRef]
- Ajami, B.; Ghazvini, K.; Movahhed, T.; Ariaee, N.; Shakeri, M.; Makarem, S. Contamination of a Dental Unit Water Line System by Legionella Pneumophila in the Mashhad School of Dentistry in 2009. Iran. Red. Crescent Med. J. 2012, 14, 376–378. [Google Scholar] [PubMed]
- Borneff, M. Legionella in dentistry equipment. Schr. Ver. Wasser Boden Lufthyg. 1993, 91, 183–201. [Google Scholar]
- Street, R.T. Syphilis in relation to dentistry. J. Mo. State Dent. Assoc. 1949, 29, 360. [Google Scholar]
- Pizza, M.; Rappuoli, R. Neisseria meningitidis: Pathogenesis and immunity. Curr. Opin. Microbiol. 2015, 23, 68–72. [Google Scholar] [CrossRef]
- Stuart, J.M.; Gilmore, A.B.; Ross, A.; Patterson, W.; Kroll, J.S.; Kaczmarski, E.B.; MacQueen, S.; Keady, P.; Monk, P. Preventing secondary meningococcal disease in health care workers: Recommendations of a working group of the PHLS meningococcus forum. Commun. Dis. Public Health 2001, 4, 102–105. [Google Scholar]
- Slingerland, B.C.G.C.; Vos, M.C.; Bras, W.; Kornelisse, R.F.; De Coninck, D.; Van Belkum, A.; Reiss, I.K.M.; Goessens, W.H.F.; Klaassen, C.H.W.; Verkaik, N.J. Whole-genome sequencing to explore nosocomial transmission and virulence in neonatal methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob. Resist. Infect. Control. 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Denis, O. Route of transmission of Staphylococcus aureus. Lancet Infect. Dis. 2016, 17, 124–125. [Google Scholar] [CrossRef]
- Inoue, M.; Kako, E.; Kinugasa, R.; Sano, F.; Iguchi, H.; Sobue, K. Necrotizing fasciitis following primary peritonitis caused by Streptococcus pyogenes with covS mutation in a healthy woman: A case report. JA Clin. Rep. 2019, 5, 29–36. [Google Scholar] [CrossRef]
- Deneubourg, D.L.; Catherine, Z.; Lejuste, P.; Breton, P. Periorbital Necrotizing Fasciitis Induced by Streptococcus pyogenes: A Case Report and Clarification. J. Oral Maxillofac. Surg. 2018, 76, 154.e1–154.e5. [Google Scholar] [CrossRef]
- Minami, S.; Nakanishi, T.; Kishita, M.; Chang, B.; Eguchi, Y.; Tanaka, T.; Fujimoto, N. Necrotizing fasciitis caused by Streptococcus pneumoniae. Eur. J. Dermatol. EJD 2017, 27, 326–328. [Google Scholar] [CrossRef]
- Moore, J.; Koerner, R. Preventing group A streptococcus cross-infection on ear, nose and throat wards. J. Hosp. Infect. 2014, 88, 180. [Google Scholar] [CrossRef]
- Mahida, N.; Beal, A.; Trigg, D.; Vaughan, N.; Boswell, T. Outbreak of invasive group A streptococcus infection: Contaminated patient curtains and cross-infection on an ear, nose and throat ward. J. Hosp. Infect. 2014, 87, 141–144. [Google Scholar] [CrossRef]
- Hava, D.L.; Lemieux, J.; Camilli, A. From nose to lung: The regulation behind Streptococcus pneumoniae virulence factors. Mol. Microbiol. 2003, 50, 1103–1110. [Google Scholar] [CrossRef]
- Heilmann, A.; Tsakos, G.; Watt, R.G. Oral Health Over the Life Course. In Revisiting Economic Vulnerability in Old Age; Springer: Berlin/Heidelberg, Germany, 2015; Volume 4, pp. 39–59. [Google Scholar]
- Alves, A.C.; Nogueira, R.D.; Stipp, R.N.; Pampolini, F.; Moraes, A.B.A.; Gonçalves, R.B.; Höfling, J.F.; Li, Y.; Mattos-Graner, R.D.O. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J. Med. Microbiol. 2009, 58, 476–481. [Google Scholar] [CrossRef]
- Binks, C.; Duane, B. Mother-to-child transmission of Streptococcus mutans. Evid. Based Dent. 2015, 16, 39–40. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J.; Boutaga, K. Transmission of periodontal bacteria and models of infection. J. Clin. Periodontol. 2005, 32, 16–27. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef]
- Kulekci, G.; Cintan, S.; Dulger, O. Infection control from the point of dentistry. J. Turk. Dent. Assoc. 2000, 58, 91–93. [Google Scholar]
- Honda, H.; Iwata, K. Personal protective equipment and improving compliance among healthcare workers in high-risk settings. Curr. Opin. Infect. Dis. 2016, 29, 400–406. [Google Scholar] [CrossRef]
- World Health Organization. Infection Prevention and Control of Epidemic-and Pandemic-Prone Acute Respiratory Diseases in Health Care; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Lee, S.-A.; Hwang, D.-C.; Li, H.-Y.; Tsai, C.-F.; Chen, C.-W.; Chen, J.-K. Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects. J. Health Eng. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kähler, C.J.; Hain, R. Flow Analyses to Validate SARS-CoV-2 Protective Masks. Available online: https://www.unibw.de/lrt7/report_mask-investigation_unibw_lrt7_06_04_2020.pdf2020 (accessed on 1 April 2020).
- Arnold, F. Eye Safety. Am. Assoc. Ind. Nurses J. 1964, 12, 9–10. [Google Scholar] [CrossRef]
- NIOSH. Considerations for Selecting Protective Clothing Used in Healthcare for Protection Against Microorganisms in Blood and Body Fluids; NIOSH: Cincinnati, OI, USA, 2018. [Google Scholar]
- Molinari, J.A. Updated CDC Infection Control Guidelines for Dental Health Care Settings: 1 Year Later. Compend. Contin. Educ. Dent. 2005, 26, 192–194. [Google Scholar]
- Montevecchi, M.; Checchi, V.; Felice, P.; Checchi, L. Le regole di gestione dello studio odontoiatrico: Dispositivi di protezione individuale (DPI). Dent. Cadmos 2012, 80, 247–263. [Google Scholar] [CrossRef]
- Partecke, L.I.; Goerdt, A.-M.; Langner, I.; Jaeger, B.; Assadian, O.; Heidecke, C.-D.; Kramer, A.; Huebner, N.-O. Incidence of Microperforation for Surgical Gloves Depends on Duration of Wear. Infect. Control. Hosp. Epidemiol. 2009, 30, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, A.; Kralj, N.; Köver, J.; Gasthaus, K.; Hofmann, F. Study of Blood Contact in Simulated Surgical Needlestick Injuries with Single or Double Latex Gloving. Infect. Control. Hosp. Epidemiol. 2009, 30, 53–56. [Google Scholar] [CrossRef]
- Bolon, M.K. Hand Hygiene. Infect. Dis. Clin. North Am. 2016, 30, 591–607. [Google Scholar] [CrossRef]
- CDC. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care and Human Services; CDC: Atlanta, GA, USA, 2016. [Google Scholar]
- Infection Control Committee. Guidelines on Infection Control Practice in the Clinic Settings of Department of Health; Infection Control Committee: Hong Kong, 2019. [Google Scholar]
- Infection Control Committee of DH Guidelines on Infection Control in Dental Clinics 1993. Available online: https://www.aids.gov.hk/pdf/g15.pdf (accessed on 1 March 1993).
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.; Moschella, A.; Bruna, A.; Romano, G.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef]
- Andersen, B.; Bånrud, H.; Bøe, E.; Bjordal, O.; Drangsholt, F. Comparison of UV C Light and Chemicals for Disinfection of Surfaces in Hospital Isolation Units. Infect. Control. Hosp. Epidemiol. 2006, 27, 729–734. [Google Scholar] [CrossRef]
- Casini, B.; Tuvo, B.; Cristina, M.L.; Spagnolo, A.; Totaro, M.; Baggiani, A.; Privitera, G. Evaluation of an Ultraviolet C (UVC) Light-Emitting Device for Disinfection of High Touch Surfaces in Hospital Critical Areas. Int. J. Environ. Res. Public Health 2019, 16, 3572. [Google Scholar] [CrossRef]
- Lindblad, M.; Tano, E.; Lindahl, C.; Huss, F. Ultraviolet-C decontamination of a hospital room: Amount of UV light needed. Burns 2019. [Google Scholar] [CrossRef] [PubMed]
- Mišović, M.; Milenkovic, D.; Martinović, T.; Ćirić, D.; Bumbasirevic, V.; Kravic-Stevovic, T. Short-term Exposure to UV-A, UV-B, and UV-C Irradiation Induces Alteration in Cytoskeleton and Autophagy in Human Keratinocytes. Ultrastruct. Pathol. 2013, 37, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.; Greenwood, M.; Meechan, J.G. General medicine and surgery for dental practitioners: Part Infections and infection control. Br. Dent. J. 2014, 217, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Jarboe, G.; Frazer, R.Q. Infection Control in the Dental Office. Dent. Clin. North Am. 2008, 52, 609–628. [Google Scholar] [CrossRef]
- Kissler, S.M.; Tedijanto, C.; Goldstein, E.; Grad, Y.H.; Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020, 368, 860–868. [Google Scholar] [CrossRef]
- Samaranayake, L.P.; Reid, J.; Evans, D. The efficacy of rubber dam isolation in reducing atmospheric bacterial contamination. ASDC J. Dent. Child. 1989, 56, 442–444. [Google Scholar]
- Samaranayake, L.; Peiris, M. Severe acute respiratory syndrome and dentistry. J. Am. Dent. Assoc. 2014, 135, 1292–1302. [Google Scholar] [CrossRef]
- Zimmermann, M.; Nkenke, E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J. Cranio-Maxillofac. Surg. 2020, 48, 521–526. [Google Scholar] [CrossRef]
- ADA. ADA Interim Guidance for Management of Emergency and Urgent Dental Care; ADA: Niagara Falls, NY, USA, 2020. [Google Scholar]
- Organización Colegial de Dentistas de España. Plan Estratégico De Acción Para El Periodo Posterior A La Crisis Creada Por El Covid-19; Consejo Dentistas Madrid: Madrid, Spain, 2020. [Google Scholar]
| Infective agent | Modes of Transmission Direct Contact and/or Blood and/or Droplet | Detectable in Aerosols | Persistence on Inanimate Surfaces |
|---|---|---|---|
| Adenoviruses | Direct contact/Droplet | 1–3 days | |
| Coxsackievirus | Direct contact/droplet | 7–10 days, up to >2 weeks | |
| Cytomegalovirus | Direct contact with saliva/urine /droplet | few hours–7 days | |
| Epstein-Barr Virus | Direct contact/droplet | few hours–7 days | |
| Hepatitis B Virus | Direct contact with Blood | approx. 1 week | |
| Hepatitis C Virus | Direct contact with blood | approx. 1 week | |
| Herpes Simplex Virus | Direct contact/droplet | few hours–7 days | |
| Human betaherpesvirus 6 | Direct contact/droplet | ||
| Human Immunodeficiency Virus | Direct contact | approx. 1 week | |
| Human Rubulavirus (Mumps) | Droplet | ||
| Human T-Lymphotropic Virus | Direct contact | ||
| Influenza A-B Virus | Droplet (Airborne) | up to 3 h | 8 h –3 days |
| MERS-CoV | Droplet (Airborne) | up to 1–3 h | 1–3 days |
| Rhinovirus | Droplet | 1–3 days | |
| Rubella virus | Airborne | ||
| SARS-CoV | Droplet (Airborne) | up to 1–3 h | 1–5 days |
| SARS-CoV-2 | Droplet (Airborne) | up to 1–3 h | up to 3 days |
| Varicella Zoster Virus | Airborne | ||
| Legionella pneumophila | Small droplets of water in the air | ||
| Mycobacterium tuberculosis | Airborne | 30 min–24 h | 1–4 moths |
| Neisseria meningitidis | Droplet | 1–3 days | |
| Staphylococcus aureus | Direct contact | 7 days–7 months | |
| Streptococcus spp. | Droplet | 3 days–6.5 months | |
| Treponema pallidum (Syphilis) | Direct contact |
| TYPE OF PPE | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Medical mask | Easy to wear, disposable, comfortable compared withN95, N99 respirator or PAPR | Controversial adequacy against novel influenza or highly virulent droplet pathogens, not indicated when operator is in contact with highly virulent pathogens during aerosol-generating procedure |
| Particulate respirators (FFP2, FFP3, N95…) | Indicated for airborne pathogens, able to protect from virulent pathogens during aerosol-generating procedure, disposable | Less comfortable, facial hair and facial deformity prevent sealing mask to face |
| Powered air purifying respirator (PAPR) | Desired for high-risk aerosol-generating procedures, half or full face piece provides facial protection | Unwieldy, battery-operated, not disposable |
| Gown | Easy to put on and take off, not causing heat, disposable, more available | Have more openings than coveralls |
| Coverall | Covers large part of surface area | Causes heat stress unwieldy |
| Apron | Additional protection when using gowns or coveralls | Disinfection is needed with apron not disposable |
| Goggles | Easy to wear, Protection to eyes | Affect visibility with fogging, some parts of face may not be protected |
| Face shield | Less fogging, Easy to wear, covers larger part of face | |
| Gloves (double gloving) | Reduction of the risk of transmission for high virulent pathogens through glove holes, reduction of contamination risk for hands when removing gloves | Reduction tactile sensation, unwieldy removal process |
| Head and neck cover | Protects head, neck skin and hair | No evidence about protection in high-risk |
| Boots | Easy to disinfect, considered a standard equipment in high-risk procedures | Lack of information in comparison boots vs shoes with covers |
| Shoes with covers | Easy to wear | Not optimal when floors is wet |
| Oil Resistance | NIOSH Class | Filtration Percentage % Filtration of Airborne Particles |
|---|---|---|
| Not oil resistant (N) | N95 N99 N100 | 95% 99% 99.97% |
| Somewhat resistant to oil (R) | R95 R99 R100 | 95% 99% 99.97% |
| Strongly resistant to oil (P) | P95 P99 P100 | 95% 99% 99.97% |
| Concentration of the Preparate | Level of Activity on Target Agents | Other Characteristics | Recommended Uses | |
|---|---|---|---|---|
| Alcohol |
|
|
|
|
| Diguanides |
|
|
|
|
| Glutaraldehyde |
|
|
|
|
| Hypochlorites |
|
|
|
|
| ITEM | RECOMMENDED METHOD | ALTERNATIVE METHOD |
|---|---|---|
| Articulators | scrub with 70% ethyl alcohol | |
| Burs–diamond | Clean with metallic brush and detergent, autoclave | |
| Burs–steel tungsten-carbide | Clean with metallic brush and detergent, rinse, dry and dry heat | Clean with metallic brush and detergent, rinse, dry and immerse in 2% glutaraldehyde for 10 h, rinse |
| composite carriers | Wipe with 70% ethyl alcohol | |
| Dental mirrors | Clean with detergent and water, autoclave, store in covered pack or container | |
| Denture | Clean with detergent and water | |
| If contaminated with blood, immerse in 0.1% sodium hypochlorite for 10 min and rinse | ||
| Extraction Forceps | Clean with detergent and water, autoclave, store in covered pack or container | |
| Handpieces Air motor for slow speed handpieces | Flush for 30 s, clean with detergent and water, oil, autoclave | Flush for 30 s, clean with detergent and water, oil, surrounding the handpiece by a gauze pad soaked in 2% glutaraldehyde for 10 min, rinse with water |
| Impressions–Alginate (plastic trays) | Rinse, spray with 0.1% sodium hypochlorite, put in closed container for 10 min. | |
| Zinc-oxide eugenol paste | Rinse, spray with 0.1% sodium hypochlorite, put in closed container for 10 min. | |
| Alginate (metallic trays) | Rinse, spray with 2% glutaraldehyde, put in closed container for 10 min. | |
| Rubber base | Rinse, immerse in 2% glutaraldehyde for 10 min, rinse | |
| Instrument trays | Clean with detergent and water, autoclave | |
| Orthodontic bands | Clean with detergent and water, autoclave | |
| Orthodontic pliers | Clean with detergent and water, autoclave | |
| Polishing stones | Clean with detergent and water, autoclave | |
| Prophylactic cups and brushes | Disposable | Clean with detergent and water autoclave |
| Protective, plastic glasses and shields | scrub with 0.1% sodium hypochlorite | |
| Root canal instruments | Clean with detergent and water, autoclave, store in covered container | |
| Rubber dam clamps | Clean with detergent and water, autoclave | |
| Rubber dam forceps | Clean and autoclave | Clean, immerse in 2% glutaraldehyde for 10 min, rinse |
| Rubber dam punches | Clean with detergent and water | |
| Saliva ejectors, metallic | Clean with detergent and water, autoclave | |
| Stainless steel instruments | Clean with water and detergent, autoclave, store in covered pack or container | Dry heat |
| Suction tube adaptors | Wipe with 70% alcohol after each use. Autoclave weekly | |
| Surgical instruments | Clean with water and detergent, autoclave, store in covered pack or container | Dry heat |
| Syringe–local anaesthetic | Clean with water and detergent, autoclave, store in covered pack or container | Dry heat |
| Ultrasonic scaler tips and inserts | Clean with water and detergent, autoclave, store in covered pack or container | |
| Wax bite block, wafer | Rinse, immersion in 0.1% sodium hypochlorite for 10 min, rinse |
| Item | Recommended Method | Alternative Method |
|---|---|---|
| Attachments dental units | Clean with 2% glutaraldehyde and then rinse | Clean with 70% alcohol |
| Bracket tables | Clean with 70% ethyl alcohol | |
| If there is blood or pus clean, disinfect with 0.5% sodium hypochlorite and rinse | ||
| Dental chairs | Clean with detergent and water | |
| If there is blood or pus clean, disinfect with 0.5% sodium hypochlorite or 2% glutaraldehyde and rinse | ||
| Dental service unit | Wipe with detergent and water | |
| If there is blood or pus clean, disinfect with 0.5% sodium hypochlorite or 2% glutaraldehyde and rinse |
| Procedure | Dental Specialty | Pre-COVID | POST-COVID Risk-Level |
|---|---|---|---|
| Checks in Restraint or Post-Restraint | Orthodontics | Low | Low |
| Dental structure tests | Prosthodontics | Low | Low |
| Manual reduction of dislocation of the jaw | Gnathology | Low | Low |
| Mobile/fixed orthodontic appliance positioning | Orthodontics | Low | Low |
| Radiographic examination | Diagnosis | Low | Low |
| Topical periodontal therapy | Periodontics | Low | Low |
| Topical treatment of dental hypersensitivity and caries prophylaxis | Hygiene and prevention | Low | Low |
| Test of night guard/bite | Gnathology | Low | Low |
| Dental impression | Diagnosis | Low | Low |
| Prosthetic tests, positioning and adaptation (temporary/definitive, removable/fixed) | Prosthodontics | Low | Low |
| Biopsy | Surgery | High | Low |
| Bone graft (autogenous/biocompatible material) without rotating tools | Surgery | Hgh | Low |
| Mucogingival surgery (quadrant) | Periodontics | High | Low |
| Open air curettage without rotating tools (quadrant) | Periodontics | High | Low |
| Removal of cysts or small benign neoplasms | Surgery | High | Low |
| Surgical medication | Surgery | High | Low |
| Oral minor surgery (e.g., abscess incision, frenulectomy, frenulotomy) | Surgery | High | Low |
| Salivary stone removal | Surgery | High | Low |
| Extraction without rotating tools | Surgery | High | Low |
| Gingivectomy /gingivoplasty | Periodontics | High | Low |
| Endodontic treatment (1 root) with rubber dum (in subsequent appointment after access cavity) | Endodontics | Low | Low |
| Pulp hooding, pulpotomy, pulpectomy (in subsequent appointment after access cavity) with rubber dum | Endodontics | Low | Low |
| Bleaching | Hygiene and prevention | Low | Medium |
| Splinting | Hygiene and prevention | Low | Medium |
| Visit | Diagnosis | Low | Medium |
| Tartar scaling | Hygiene and prevention | Low | High |
| Extraction with rotating tools | Surgery | High | High |
| Sinus lift | Surgery | High | High |
| Access cavity (rotating instruments) | Endodontics | Medium | High |
| Implantology | Surgery | High | High |
| Open air curettage (quadrant) (rotating tools) | Periodontics | High | High |
| Resective/regenerative bone surgery (rotating tools) | Periodontics | High | High |
| Rhizectomy / rhizotomy (rotating tools) | Periodontics | High | High |
| Sealing of dental grooves | Hygiene and prevention | Low | High |
| Apicectomy with retrograde filling | Surgery | Medium | High |
| Autologous bone harvest (rotating tools) | Surgery | High | High |
| Abutment tooth preparation | Prosthodontics | Low | High |
| Odontoplasty (1 tooth) | Gnathology | Low | High |
| Simple / complex filling using rotating tools | Conservative | Low | High |
| Pre-COVID | Post-COVID | |
|---|---|---|
| Low risk | sterilizable headgear Protective goggles Surgical mask disposable latex gloves | Disposable or sterilizable headgear Protective goggles Surgical mask Disposable or sterilizable gown Double disposable latex gloves |
| Medium risk | Disposable headgear Disposable/sterilizable visor to remove immediately Surgical mask Protective goggles disposable latex gloves | Disposable headgear Disposable/sterilizable visor to remove immediately Protective respirator (FFP2) Disposable gown Double disposable latex gloves |
| High risk | Disposable headgear Disposable/sterilizable visor to remove immediately Surgical mask Disposable gown disposable latex gloves | Disposable headgear Disposable/sterilizable visor to remove immediately FPP3 / Powered air purifying respirator (PAPR) Disposable protective suit Double disposable latex gloves Cover shoes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzoca, M.E.; Campisi, G.; Lo Muzio, L. Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment. Int. J. Environ. Res. Public Health 2020, 17, 3793. https://doi.org/10.3390/ijerph17113793
Bizzoca ME, Campisi G, Lo Muzio L. Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment. International Journal of Environmental Research and Public Health. 2020; 17(11):3793. https://doi.org/10.3390/ijerph17113793
Chicago/Turabian StyleBizzoca, Maria Eleonora, Giuseppina Campisi, and Lorenzo Lo Muzio. 2020. "Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment" International Journal of Environmental Research and Public Health 17, no. 11: 3793. https://doi.org/10.3390/ijerph17113793
APA StyleBizzoca, M. E., Campisi, G., & Lo Muzio, L. (2020). Covid-19 Pandemic: What Changes for Dentists and Oral Medicine Experts? A Narrative Review and Novel Approaches to Infection Containment. International Journal of Environmental Research and Public Health, 17(11), 3793. https://doi.org/10.3390/ijerph17113793







