A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Presentation
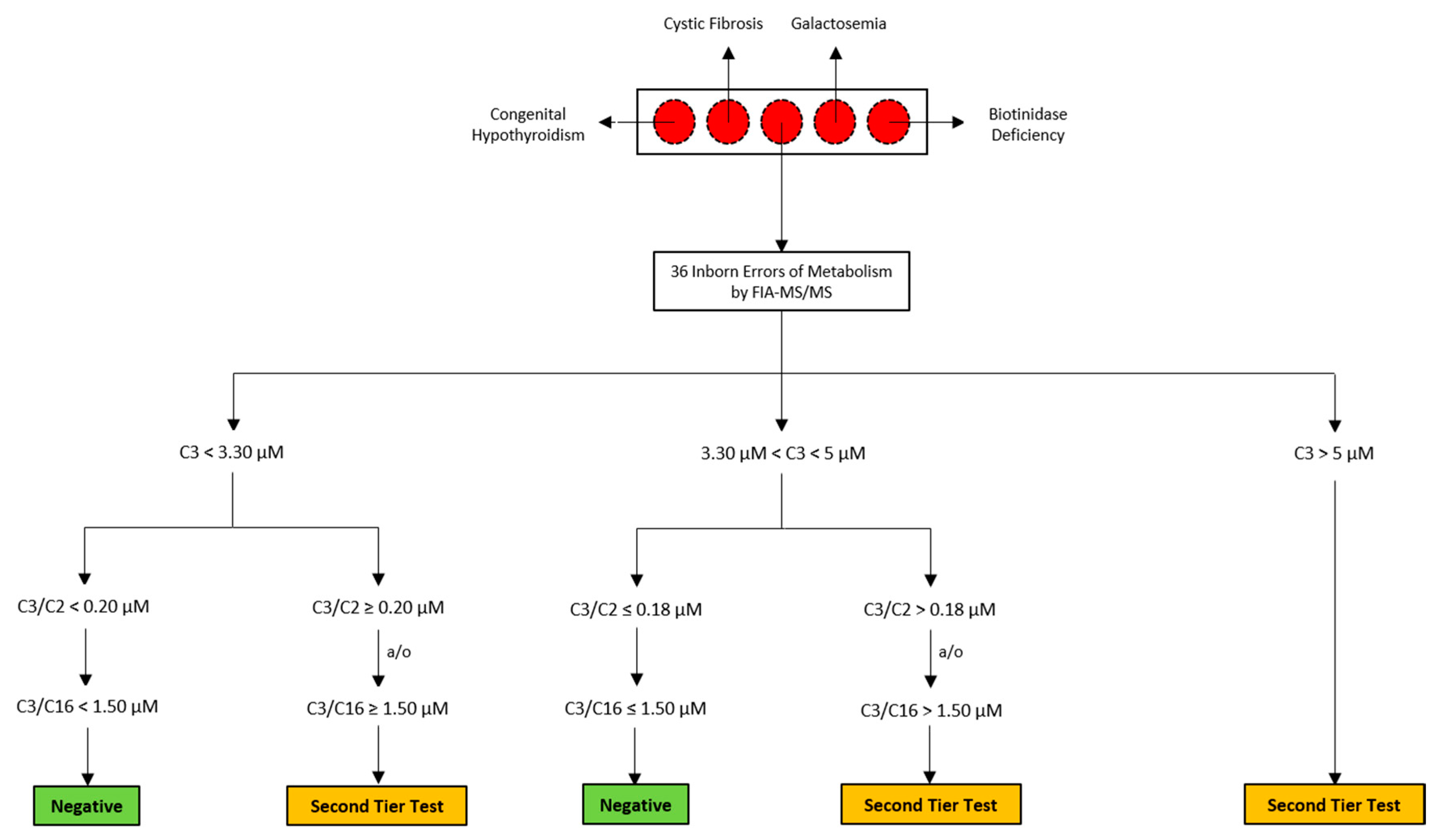
2.2. Routine Newborn Screening Analysis and Second-Tier Testing
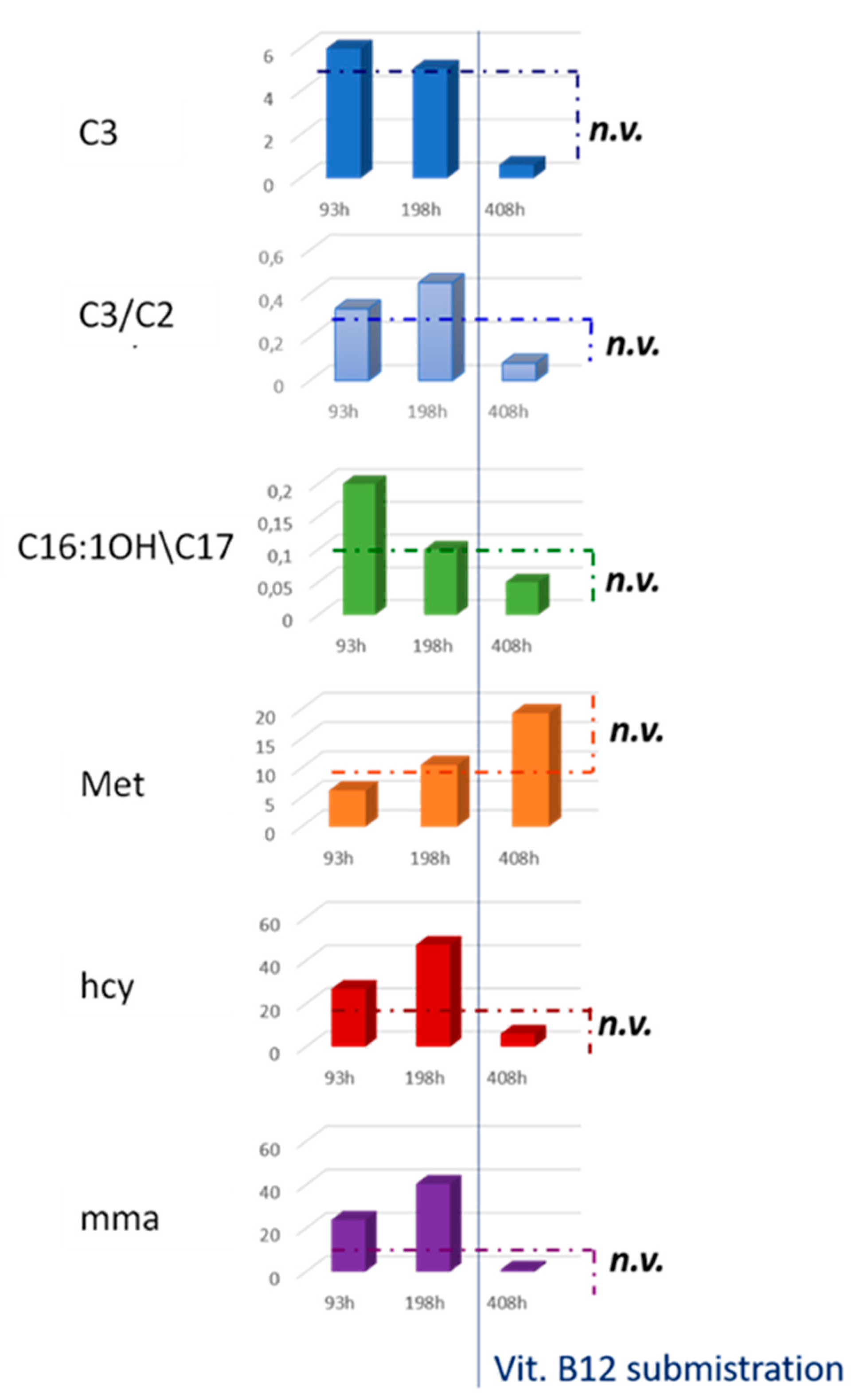
2.3. NBS Analysis and Second-Tier Testing of The Suspected Neonate
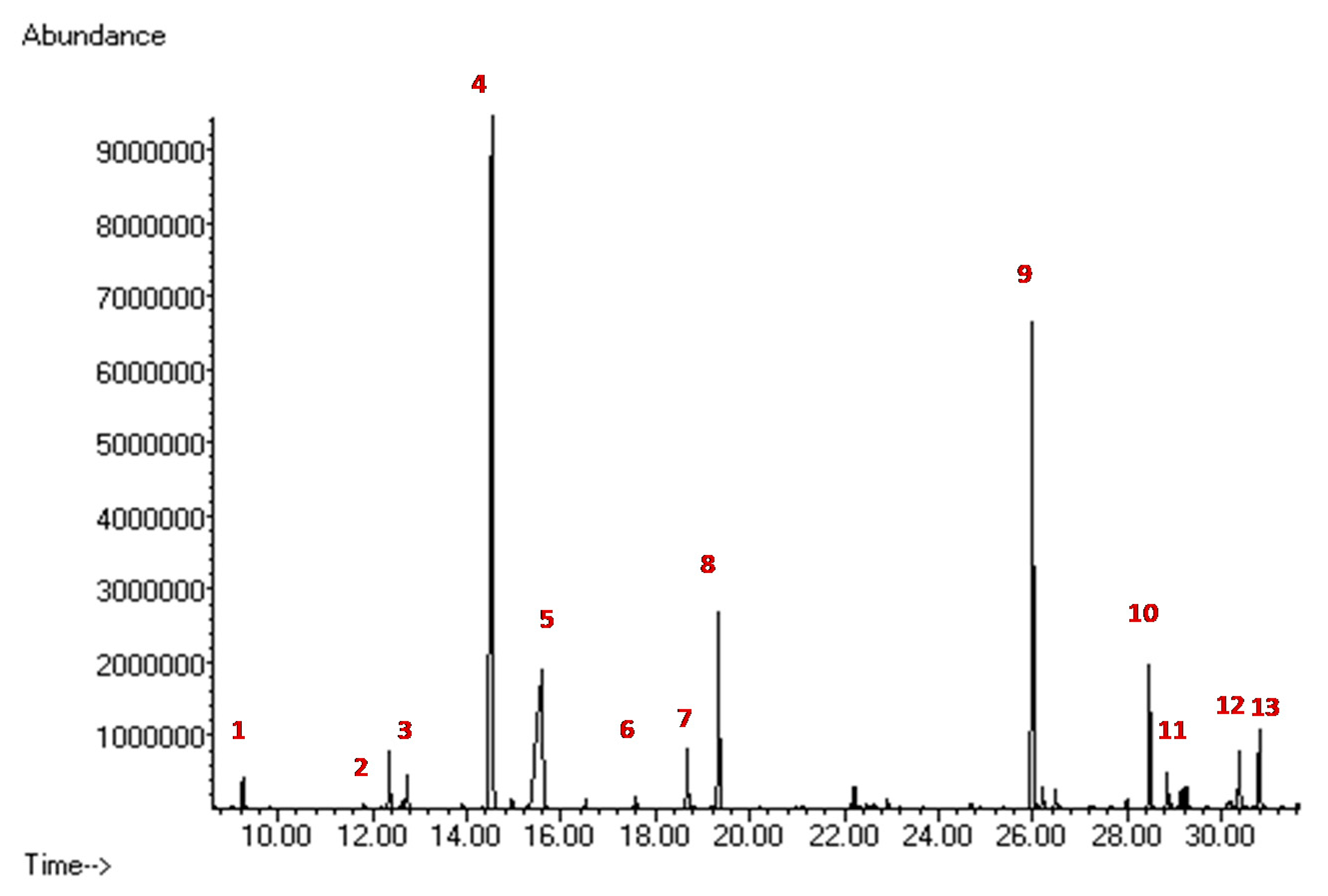
2.4. Biochemical Diagnostic Confirmations
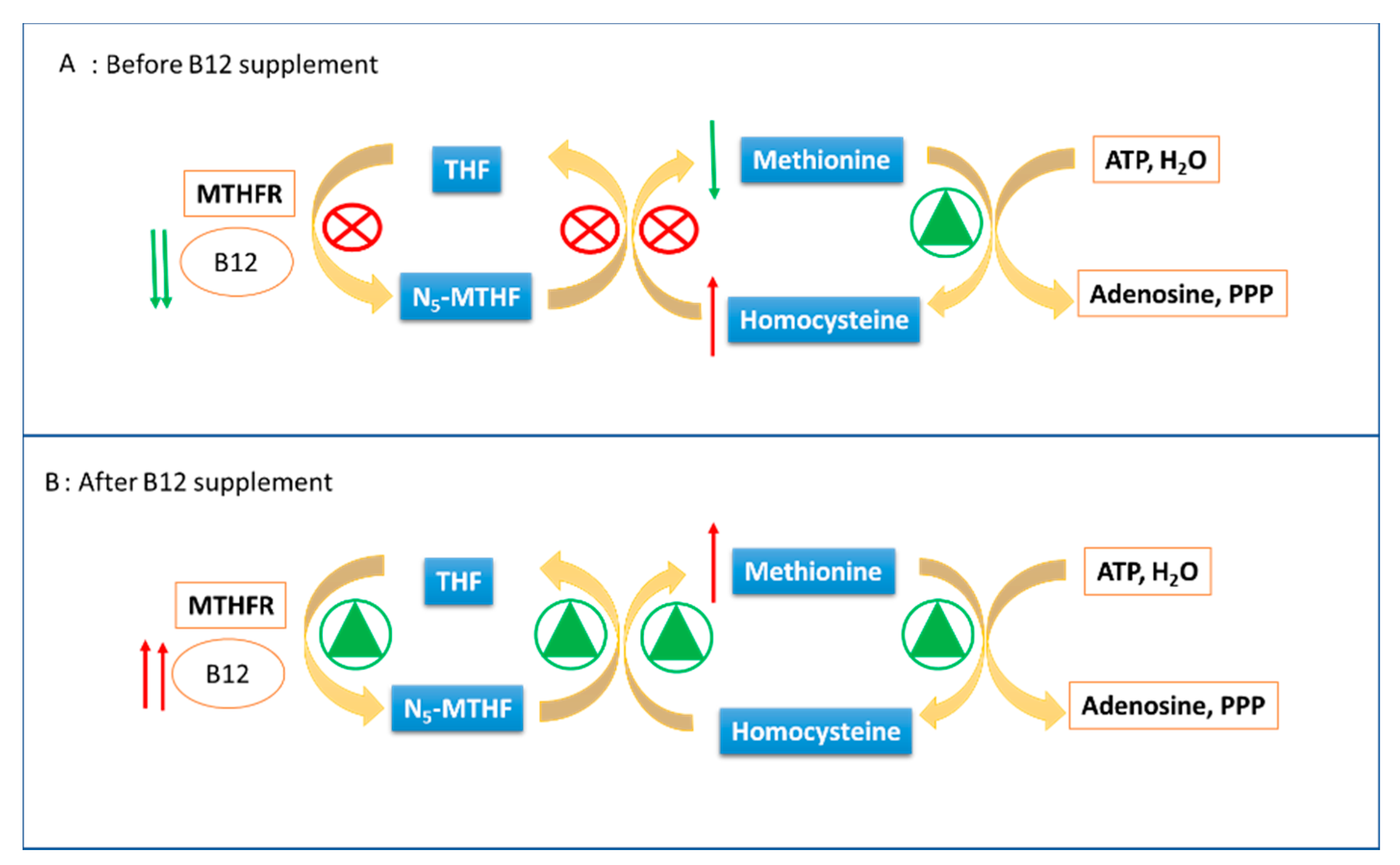
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramsay, J.; Morton, J.; Norris, M.; Kanungo, S. Organic acid disorders. Ann. Transl. Med. 2018, 6, 472. [Google Scholar] [CrossRef]
- Villani, G.R.; Gallo, G.; Scolamiero, E.; Salvatore, F.; Ruoppolo, M. “Classical organic acidurias”: Diagnosis and pathogenesis. Clin. Exp. Med. 2017, 17, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, K.; Narayanan, M.P.; Vasudevan, D.M. Organic acidurias: An updated review. Indian J. Clin. Biochem. 2011, 26, 319–325. [Google Scholar]
- Dionisi-Vici, C.; Deodato, F.; Roschinger, W.; Rhead, W.; Wilcken, B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: Long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J. Inherit. Metab. Dis. 2006, 29, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Almasi, T.; Guey, L.T.; Lukacs, C.; Csetneki, K.; Voko, Z.; Zelei, T. Systematic literature review and meta-analysis on the epidemiology of methylmalonic acidemia (MMA) with a focus on MMA caused by methylmalonyl-CoA mutase (mut) deficiency. Orphanet. J. Rare Dis. 2019, 14, 84. [Google Scholar] [CrossRef]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and propionic acidemias: Clinical management update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Peng, G.; Tang, Y.; Cowan, T.M.; Enns, G.M.; Zhao, H.; Scharfe, C. Reducing False-Positive Results in Newborn Screening Using Machine Learning. Int. J. Neonatal Screen. 2020, 6, 16. [Google Scholar] [CrossRef]
- La Marca, G. Mass spectrometry in clinical chemistry: The case of newborn screening. J. Pharm. Biomed. Anal. 2014, 101, 174–182. [Google Scholar] [CrossRef]
- Pieragostino, D.; Cicalini, I.; Di Michele, S.; Fusilli, P.; Cotugno, G.; Ferrante, R.; Bucci, I.; Dionisi-Vici, C.; Stuppia, L.; De Laurenzi, V.; et al. A Case of Suspected Hyperphenylalaninemia at Newborn Screening by Tandem Mass Spectrometry during Total Parenteral Nutrition. Metabolites 2020, 10, 44. [Google Scholar] [CrossRef]
- Dietzen, D.J.; Rinaldo, P.; Whitley, R.J.; Rhead, W.J.; Hannon, W.H.; Garg, U.C.; Lo, S.F.; Bennett, M.J. National academy of clinical biochemistry laboratory medicine practice guidelines: Follow-up testing for metabolic disease identified by expanded newborn screening using tandem mass spectrometry; executive summary. Clin. Chem. 2009, 55, 1615–1626. [Google Scholar]
- Semeraro, M.; Boenzi, S.; Carrozzo, R.; Diodato, D.; Martinelli, D.; Olivieri, G.; Antonetti, G.; Sacchetti, E.; Catesini, G.; Rizzo, C.; et al. The urinary organic acids profile in single large-scale mitochondrial DNA deletion disorders, Clinica chimica. Acta Int. J. Clin. Chem. 2018, 481, 156–160. [Google Scholar] [CrossRef] [PubMed]
- La Marca, G.; Malvagia, S.; Pasquini, E.; Innocenti, M.; Donati, M.A.; Zammarchi, E. Rapid 2nd-tier test for measurement of 3-OH-propionic and methylmalonic acids on dried blood spots: Reducing the false-positive rate for propionylcarnitine during expanded newborn screening by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2007, 53, 1364–1369. [Google Scholar] [CrossRef]
- Malvagia, S.; Haynes, C.A.; Grisotto, L.; Ombrone, D.; Funghini, S.; Moretti, E.; McGreevy, K.S.; Biggeri, A.; Guerrini, R.; Yahyaoui, R.; et al. Heptadecanoylcarnitine (C17) a novel candidate biomarker for newborn screening of propionic and methylmalonic acidemias, Clinica chimica. Acta Int. J. Clin. Chem. 2015, 450, 342–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebastiani, G.; Herranz Barbero, A.; Borras-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernandez, V.; Pascual Tutusaus, M.; Ferrero Martinez, S.; Gomez Roig, M.D.; Garcia-Algar, O. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef]
- Pitt, J.J. Newborn screening. Clin. Biochem. Rev. 2010, 31, 57–68. [Google Scholar] [PubMed]
- Papp, F.; Racz, G.; Lenart, I.; Kobor, J.; Bereczki, C.; Karg, E.; Barath, A. Maternal and neonatal vitamin B12 deficiency detected by expanded newborn screening. Orv. Hetil. 2017, 158, 1909–1918. [Google Scholar]
- Scolamiero, E.; Villani, G.R.; Ingenito, L.; Pecce, R.; Albano, L.; Caterino, M.; di Girolamo, M.G.; Di Stefano, C.; Franzese, I.; Gallo, G.; et al. Maternal vitamin B12 deficiency detected in expanded newborn screening. Clin. Biochem. 2014, 47, 312–317. [Google Scholar]
- Hinton, C.F.; Ojodu, J.A.; Fernhoff, P.M.; Rasmussen, S.A.; Scanlon, K.S.; Hannon, W.H. Maternal and neonatal vitamin B12 deficiency detected through expanded newborn screening-United States, 2003−2007. J. Pediatr. 2010, 157, 162–163. [Google Scholar] [CrossRef]
- Ficicioglu, C. New tools and approaches to newborn screening: Ready to open Pandora’s box? Cold Spring Harb. Mol. Case Stud. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, L.; Rocha, H.; Sousa, C.; Marcao, A.; Fonseca, H.; Bogas, M.; Osorio, R.V. Four years of expanded newborn screening in Portugal with tandem mass spectrometry. J. Inherit. Metab. Dis. 2010, 33, 133–138. [Google Scholar]
- El-Hattab, A.W.; Li, F.Y.; Shen, J.; Powell, B.R.; Bawle, E.V.; Adams, D.J.; Wahl, E.; Kobori, J.A.; Graham, B.; Scaglia, F.; et al. Maternal systemic primary carnitine deficiency uncovered by newborn screening: Clinical, biochemical, and molecular aspects, Genetics in medicine. Off. J. Am. Coll. Med. Genet. 2010, 12, 19–24. [Google Scholar] [CrossRef]
- Schimmenti, L.A.; Crombez, E.A.; Schwahn, B.C.; Heese, B.A.; Wood, T.C.; Schroer, R.J.; Bentler, K.; Cederbaum, S.; Sarafoglou, K.; McCann, M.; et al. Expanded newborn screening identifies maternal primary carnitine deficiency. Mol. Genet. Metab. 2007, 90, 441–445. [Google Scholar] [CrossRef]
- Crombez, E.A.; Cederbaum, S.D.; Spector, E.; Chan, E.; Salazar, D.; Neidich, J.; Goodman, S. Maternal glutaric acidemia, type I identified by newborn screening. Mol. Genet. Metab. 2008, 94, 132–134. [Google Scholar] [CrossRef]
- Cho, K.L.; Kim, Y.J.; Yang, S.H.; Kim, G.H.; Lee, J.H. Maternal 3-methylcrotonyl-coenzyme A carboxylase deficiency with elevated 3-hydroxyisovalerylcarnitine in breast milk. Korean J. Pediatr. 2016, 59, 41–44. [Google Scholar] [CrossRef]
- Rips, J.; Almashanu, S.; Mandel, H.; Josephsberg, S.; Lerman-Sagie, T.; Zerem, A.; Podeh, B.; Anikster, Y.; Shaag, A.; Luder, A.; et al. Primary and maternal 3-methylcrotonyl-CoA carboxylase deficiency: Insights from the Israel newborn screening program. J. Inherit. Metab. Dis. 2016, 39, 211–217. [Google Scholar] [CrossRef]
| Analyte | Age at Birth (Hours) | Normal Value | ||
|---|---|---|---|---|
| 93 h | 198 h | 408 h | ||
| C3 | 5.99 | 5.06 | 0.62 | <3.3 |
| C3/C2 | 0.33 | 0.45 | 0.08 | <0.18 |
| C16:1OH\C17 | 0.2 | 0.1 | 0.05 | <0.09 |
| Met | 6.16 | 10.5 | 19.3 | 7-37 |
| hcy | 26.9 | 47.4 | 6.1 | <10 |
| mma | 23.9 | 40.5 | 1 | <4 |
| mca | <1 | 1.2 | <1 | <1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, C.; Cicalini, I.; Rizzo, C.; Zucchelli, M.; Consalvo, A.; Valentinuzzi, S.; Semeraro, D.; Gasparroni, G.; Brindisino, P.; Gazzolo, D.; et al. A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 3601. https://doi.org/10.3390/ijerph17103601
Rossi C, Cicalini I, Rizzo C, Zucchelli M, Consalvo A, Valentinuzzi S, Semeraro D, Gasparroni G, Brindisino P, Gazzolo D, et al. A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. International Journal of Environmental Research and Public Health. 2020; 17(10):3601. https://doi.org/10.3390/ijerph17103601
Chicago/Turabian StyleRossi, Claudia, Ilaria Cicalini, Cristiano Rizzo, Mirco Zucchelli, Ada Consalvo, Silvia Valentinuzzi, Daniela Semeraro, Giorgia Gasparroni, Patrizia Brindisino, Diego Gazzolo, and et al. 2020. "A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy" International Journal of Environmental Research and Public Health 17, no. 10: 3601. https://doi.org/10.3390/ijerph17103601
APA StyleRossi, C., Cicalini, I., Rizzo, C., Zucchelli, M., Consalvo, A., Valentinuzzi, S., Semeraro, D., Gasparroni, G., Brindisino, P., Gazzolo, D., Dionisi-Vici, C., De Laurenzi, V., & Pieragostino, D. (2020). A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. International Journal of Environmental Research and Public Health, 17(10), 3601. https://doi.org/10.3390/ijerph17103601









