1. Introduction
Unclean drinking water is an important source of pathogenic bacteria, viruses and protozoan parasites for 2.7 billion people worldwide, underscoring the need for innovative safe and effective water disinfecting methods [
1]. Current methods of water disinfection broadly include physical or chemical treatment. Among the well-known chemical disinfectants are halogens, which include bromine, fluorine, chlorine and iodine. [
2,
3]. Of these iodine has been widely used because it is generally effective, simple, cost-efficient and its properties for water disinfection are well known [
4].
Iodine has mainly been used in emergencies and by travelers [
4,
5]. Its use as a water disinfectant can be dated back to the early 1900 s when the military first developed a tablet formulation for use in the field [
6]. Like all halogens, iodine is a biocide because it is a strong oxidant [
4]. However, relative to other halogens, complexed iodine has greater chemical stability [
7]. It is also less reactive with organic nitrogenous contaminants and can be retained at a higher residual concentration in water [
7]. These characteristics mean that iodine residuals will persist longer and will be more stable in the presence of organic matter compared to chlorine, the other commonly used halogen for water disinfection [
6,
8]. Compared to chlorine, iodine also has a more acceptable taste in equipotent concentrations and it is effective over a wider pH range [
9,
10,
11]. Although the exact mechanism of action is unknown, iodine is known to rapidly penetrate into microorganisms [
12]. It targets aromatic C–H functions, sulfur-containing amino acids (cysteine, methionine), and unsaturated fatty acids [
13]. As a bactericidal agent, iodine likely penetrates bacterial cell walls, and it’s killing mechanism is likely related to retardation of bacterial protein synthesis, disruption of electron transport, DNA denaturation or membrane destabilization [
3]. In lipid enveloped viruses [
14] it is presumed to attack the surface proteins and destabilize membrane fatty acids by reacting with unsaturated carbon bonds [
15]. Povidone iodine has also been reported as an effective environmental microbicide in the inactivation of coronaviruses [
16].
In water, iodine is minimally soluble (0.03 g/100 g water at 20 °C) compared to other halogens [
17]. When added to water, it may remain unchanged or it may hydrolyze into different species depending on temperature, pH and the initial concentration. These different species include hypoiodous acid (HIO), iodide ion (I
−), hypoiodite ion (IO
−), triiodide ion (I
3−), iodic acid (HIO
3) and iodate (IO
3−) [
18]. It also exists as the diatomic elemental molecule, I
2, momentarily after being oxidized from iodide. Of the species formed, only I
2 and HIO are capable of biocide activity [
8,
9,
17,
19,
20]. A pH near neutral to mildly alkaline (pH 7–7.5) allows for adequate levels of both elemental iodine and hypoiodous acid [
7]. The other iodine species serve as “reservoirs” by providing a source for I
2 [
17].
For practical purposes, iodine is employed as an iodine solution (e.g., tincture of iodine, a 2% iodine solution) and as tablets containing iodine along with carrier and stabilizing agents to enhance dissolvability (e.g., Globaline, composed of tetraglycine hydroperiodide, sodium acid pyrophosphate and talc; [
20]). Iodine resins have also been designed as solid-phase iodine disinfectants were water disinfection occurs through direct contact with the microorganism as water passes through iodine that is adsorbed onto the resin [
4]. The same principle is used in the formulation of iodinated biocides where the degree of disinfection is determined by the availability and regulated release of free elemental iodine [
21].
Based on these characteristics of iodine, we propose that presentation of elemental iodine in an un-complexed form coupled to a delivery method that maximizes distribution and interaction with the target microbes would offer significant and rapid antimicrobial activity in a fluid. We evaluated the effectiveness of an “I
2 vapor infusion” (I
2VP) technology that introduces free iodine into solutions via transitory air bubbles that contain I
2 vapor [
22] The I
2VP technology is an adaptation of the iodine-infused aeration of elemental I
2 for hull fouling prevention. This method uses an infusion of air bubbles containing I
2 to prevent or reduce fouling by inactivating bacteria that create fouling matrixes [
23]. The goal was to determine if I
2VP was capable of general water disinfection by testing the killing of gram-positive and negative bacteria either in free solution or as biofilm communities. This was considered in water of varying quality. We used the technology in two forms; as a hand powered system for use in emergencies, and for applications relevant to both water and wastewater treatment.
2. Materials and Methods
2.1. “I2 Vapor Perfusion” (I2VP) System
The I
2 fluid innovation system used in this study is a derivation of the device created by I
2 Air Fluid Innovation as a method for airstream, fluid and vessel decontamination, (Radicone and Miller 2008) [
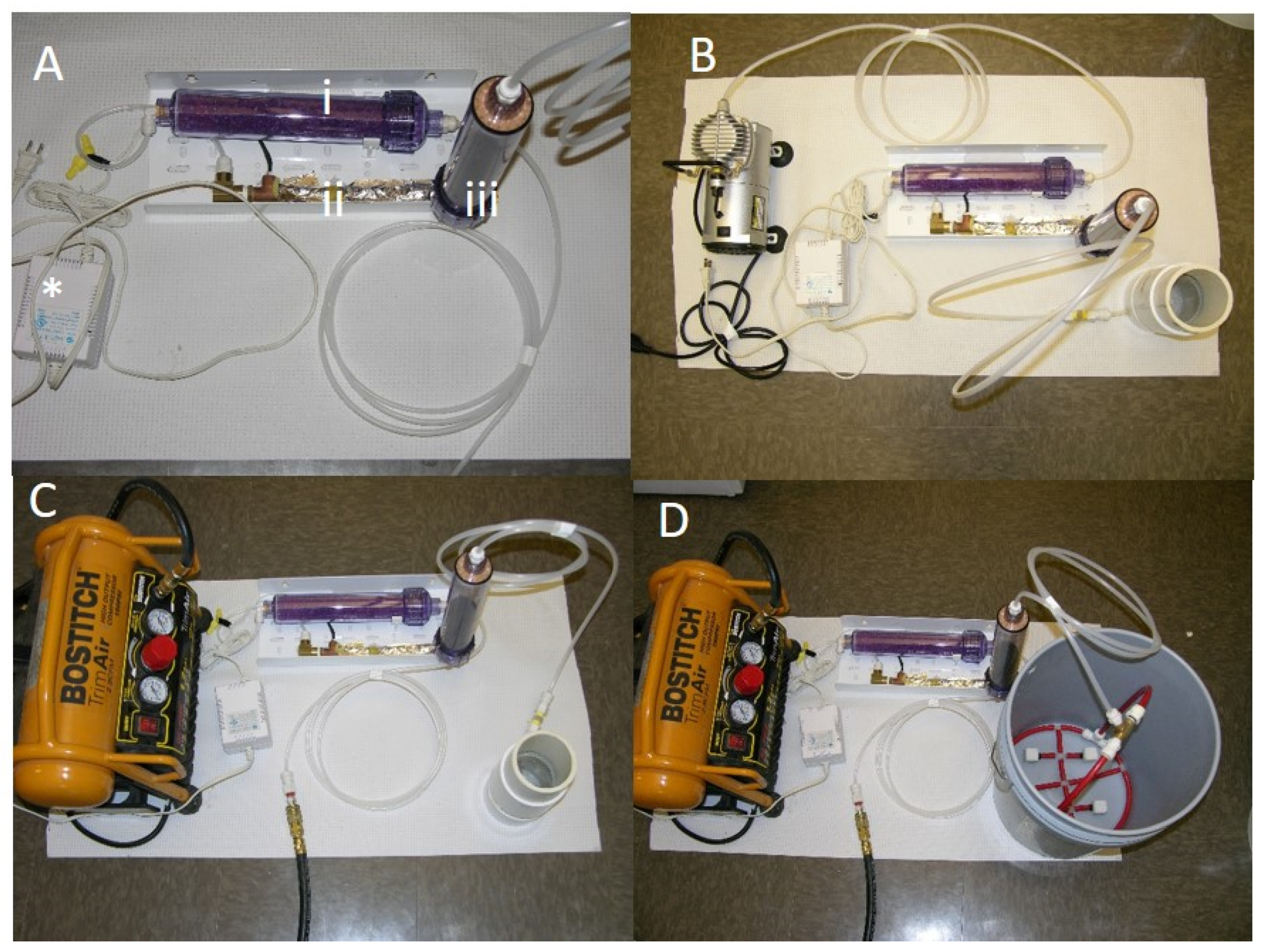
22]. Air was delivered to the I2VP system by either an electric compressor or hand pump. The airstream was first directed to a heater element (
Figure 1A, ii.) warmed to 90 °F to increase sublimation of iodine. The amount of air entering the unit was controlled by a manual valve that was fixed to one position for all the experiments. From the heater element, air then entered the iodine resin encased in a cartridge. (
Figure 1A, iii.). The iodine cartridge comprises of an air-inlet portion, through which air enters. The column of the cartridge is packed with beaded iodine resin. As air passes from the air-inlet portion, into the column, the iodine resin rapidly sublimates iodine ions into the air passing through the column and exists at the distal end connected to a tube. Iodine vapor is then delivered by the tube into a bubble forming element at the bottom of the container (
Figure 1B–D) with water to be disinfected. The bubble forming element is constructed from a porous material and provides active bubble formation. The iodine-laden air bubbles sublimate the iodine into the fluid treating microbial contamination that may be present in the fluid [
22].
For the compressor system (
Figure 1) air was supplied through a low-capacity compressor, Gilford Vacuum Pump (115V 60Hz 2.3A, 58 max psi) attached to a 250 mL cylinder (
Figure 1B).
For the experiments described this compressor was operated at maximum (58 psi). A high-capacity Bostitch trim Air 2.8CFM High Output compressor attached to a 250 mL cylinder or 5-gallon bucket. This compressor was operated between (75–100 psi).
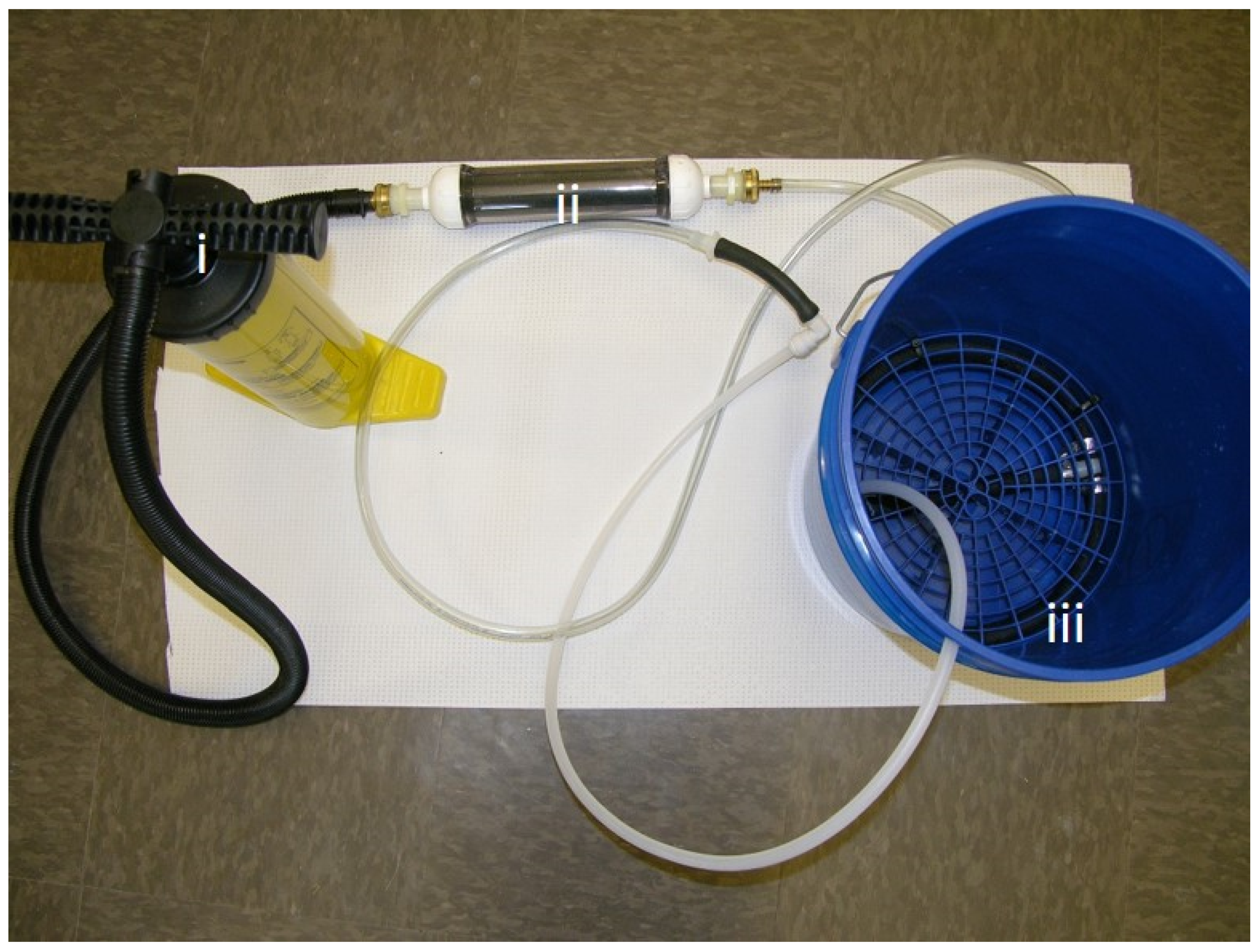
The hand powered (bucket) system (
Figure 2). Following initial testing with the electric system we adapted the iodine cartridge to a simple, hand-powered “bucket system” to determine if the technology could be scaled for use in households that lack access to potable water and electricity. The same iodine cartridge design was used for both the electric and hand powered system. Through the action of the hand pump ambient air was passed through the cartridge and delivered into a 5-gallon bucket through a coiled tube diffuser. Air was not pre-warmed with the bucket system. For the bucket system, the total volume of water used was approximately 3 L.
2.2. Experimental Set Up
Three configurations were used with the two different compressors (
Figure 1), and a fourth configuration deployed a hand-operated recreational inflation pump (
Figure 2). Experiments were done in increasing level of air pressure and decreasing water quality as determined by visual inspection. The first configuration used the low capacity compressor Gilford vacuum pump (115V 60Hz 2.3A, 58 psi) attached to a 250 mL cylinder with distilled water (i) inoculated with common water pathogens and biofilms and (ii) distilled water plus loess soil to simulate water of low quality containing organic matter (
Figure 1B). The total carbon (TC) content was determined with a Leco CNS analyzer (Leco, St. Joseph, MI, USA) described previously [
24]. No carbonates were detected, and soil was measured to have a total organic content of 2.27%. The second configuration used the high capacity compressor Bostitch trim Air 2.8CFM High Output compressor (75–100 psi) attached to a 250 mL cylinder (
Figure 1C) and centrifuged water (with particulate matter removed) from a diary lagoon. The third configuration was composed of the high capacity compressor attached to a 5-gallon bucket and was used to treat water from naturally occurring water bodies (pond, sewer and lagoon) to simulate use of the technology in real settings with high particulate matter or organic matter. The fourth configuration used a hand pump to treat (i) distilled water inoculated with common water pathogens (ii) water from naturally occurring water bodies to simulate use in low resource settings (water was inoculated with pathogenic bacteria to reach high bacterial load). Summary of the experimental set up is shown in
Figure 3.
2.3. Bacterial Strains, Inoculation, Conditions and Enumeration
Bacteria causing diseases transmitted through water were tested, these included two
Escherichia coli strains,
E. coli O157:H7 ((RIMD 0509952) (Sakai strain; [
25,
26]) a concern in outbreaks involving consumption of drinking water contaminated with human sewage or cattle feces. A lab adapted strain, nalidixic-resistant
E. coli K12 (E.
coli K12 Nal
R, [
27]) was included as a control.
Salmonella enterica serovar Typhimurium [
28,
29] an environmental contaminate, commonly found in municipal sewage, agriculture pollution, and storm water;
Enterococcus faecalis an important opportunistic pathogen frequently found in water [
30]. Two biofilm producing strains, multi-drug resistant
Acinetobacter baumannii (ATCC BAA-1605) and methicillin-resistant
Staphylococcus aureus (ATCC BAA-1747) were also included. Strains were tested independently in each experimental run.
Bacteria were cultured overnight, and the resulting optical density was measured at 600 nm (OD600) to estimate colony forming units (cfu) mL−1 and then inoculated into water at a final concentration of approximately 106 cfu mL−1 (to ensure a high concentration of bacteria). Before inoculation, cultures were centrifuged at 3000x g for 15 min and washed two times by re-suspending in 1X Dulbecco’s Phosphate Buffered saline (PBS; MP Biomedicals LLC, Solon, OH, USA). Inoculum was added directly to water. Before treatment 2 mL samples were collected to determine starting cfu mL−1. Water was then treated with iodine using the I2VP system.
After treatment, 2 mL water samples were collected to determine colony forming units (cfu) mL
−1. Serial dilutions were prepared and cfu mL
−1 was estimated by using a 6X6 drop-plate method [
31]. Lennox Broth (LB) (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) agar with nalidixic acid (32 µg mL
−1; MP Biomedicals LLC, Solon, OH, USA) was used to enumerate nalidixic-resistant
E. coli K12 Nal
R. LB agar without antibiotic was used to enumerate
E. coli O157:H7. mEnterococcus broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) agar plates were used to enumerate
Enterococcus faecalis. Xylose lysine deoxycholate agar (XLD agar; Hardy Diagnostics, Santa Maria, CA, USA) was used to enumerate Salmonella. Unless noted otherwise, experiments were independently repeated three times. To assess the potential for use in turbid liquids,
E. coli K12-Nal
R (10
6 cfu mL
−1) (in 5 mL sterile water) was mixed with 5 g of loess soil [
24] before or after iodine perfusion, followed by bacterial quantification.
For the bucket system the same strains and final concentrations were used. Ambient air was moved through the cartridge and diffuser by hand pumping for 2 min followed by a 5 min break and then for 2 additional min. After treatment, a 2 mL sample was used for bacterial enumeration. Total cfu counts were determined after each two-minute pumping session. The system was rested and after 24 h, a sample was collected before adding respective bacterial strains again at 106 mL−1. After 5 min water samples were again collected for enumeration. The latter procedure was used to determine residual activity of iodine species after 24 h.
2.4. Biofilm Treatment
To assess the efficacy of the I2VP technology on antimicrobial resistant and biofilm forming bacteria, Acinetobacter baumannii (ATCC BAA-1605) and Staphylococcus aureus (ATCC BAA-1747) biofilms were grown separately for 24 h in 3 mL of LB broth. Cultures were incubated in 16 mL polystyrene tubes and grown without shaking at (37 °C). The LB broth was decanted and biofilms were rinsed by gently mixing with sterile water (4 mL). Rinse water was decanted, and the bottom 1 cm of each plastic tube was cut to yield an open-ended column. Control biofilm communities were harvested for enumeration by first inserting the 16 mL open-ended tube into a 50 mL polypropylene tube. Ten sterile glass beads and 3 mL of LB agar were added directly to the 16 mL tube and the entire apparatus was vortexed for 60 s to dislodge and disrupt any biofilm from the surface of the 16 mL tube. Cfu counts were conducted as described above. Immediately after rinsing, biofilm communities from the no-treatment tubes were vortexed and enumerated. Remaining treatments involved submersion of the biofilm tubes into 45 mL water within a 250 mL cylinder. Air with or without iodine vapor was passed through the system so that the biofilm communities were directly exposed to I2 vapor for 90 s. Vortexing with sterile beads and enumeration followed as described above.
2.5. Direct Infusion into Untreated Municipal Wastewater
To test applicability of the device as an alternative method of municipal water treatment, raw wastewater (sewer water) was collected from a municipal wastewater treatment plant (Pullman, WA, USA). Water was collected after removal of solid waste at the plant. A Bostitch trim Air 2.8CFM High Output compressor was used to move air through the iodine matrix followed by diffusion into 3-L wastewater samples (n = 3) for 2, 4, 8 and 16 min. The bucket was thoroughly cleaned with 70% ethanol between each treatment. After treatments serial dilutions were prepared and cfu mL−1 was estimated by using a 6X6 drop-plate method (see above).
2.6. Infusion into River and Pond Water
To assess efficacy of the device to clear naturally occurring bacteria from natural water bodies, we used pond and river water. We used the Bostitch trim Air 2.8 CFM High Output compressor with pumping for 2, 4, 8 and 16 min followed by enumeration by using both LB and MacConkey agar plates (Hardy Diagnostics, Santa Maria, CA, USA). MacConkey agar plates were used to selectively detect lactose and non-lactose fermenting Gram-negative bacteria. The same water samples were also treated using the hand pump. To challenge this system water was inoculated with three of the mentioned bacterial strains E. coli K-12, Salmonella enterica and Enterococcus faecalis as described above.
2.7. Infusion of Iodine Vapor into Dairy Lagoon Water
To assess the feasibility of using iodine to treat agricultural water, we collected water from the animal waste lagoon at Knott’s Dairy, Washington State University, Pullman, WA. Water was divided into two groups containing 20 mL aliquots. Water from the first group was sieved to remove bulk debris and then centrifuged at 3000x g for 15 min with the supernatant being subsequently treated as described above. The second group was not centrifuged. Experiments were conducted using the Bostitch trim Air 2.8 CFM High Output compressor with pumping for 4 and 8 min (air alone or with iodine vapor) followed by enumeration by using both LB and MacConkey agar plates (Hardy Diagnostics, Santa Maria, CA, USA). MacConkey agar plates were used to selectively detect lactose and non-lactose fermenting Gram-negative bacteria. This experiment was then repeated with 1 L of lagoon water to observe the effect of volume and continuous iodine exposure on bacteria recovery. Pumping was extended to 16 min.
2.8. Iodine Residue Testing
Water samples were collected, before pumping, at 2 and 4 min, and 24 h after infusion of iodine by either the compressor or hand pump in 25 mL glass tubes that were filled completely to exclude any air. These samples were then shipped by overnight courier to the Diagnostic Center for Population and Animal Health at Michigan State University for quantification of iodine species by using gas chromatography.
4. Discussion
This study describes the potential application of a newly patented technology (I
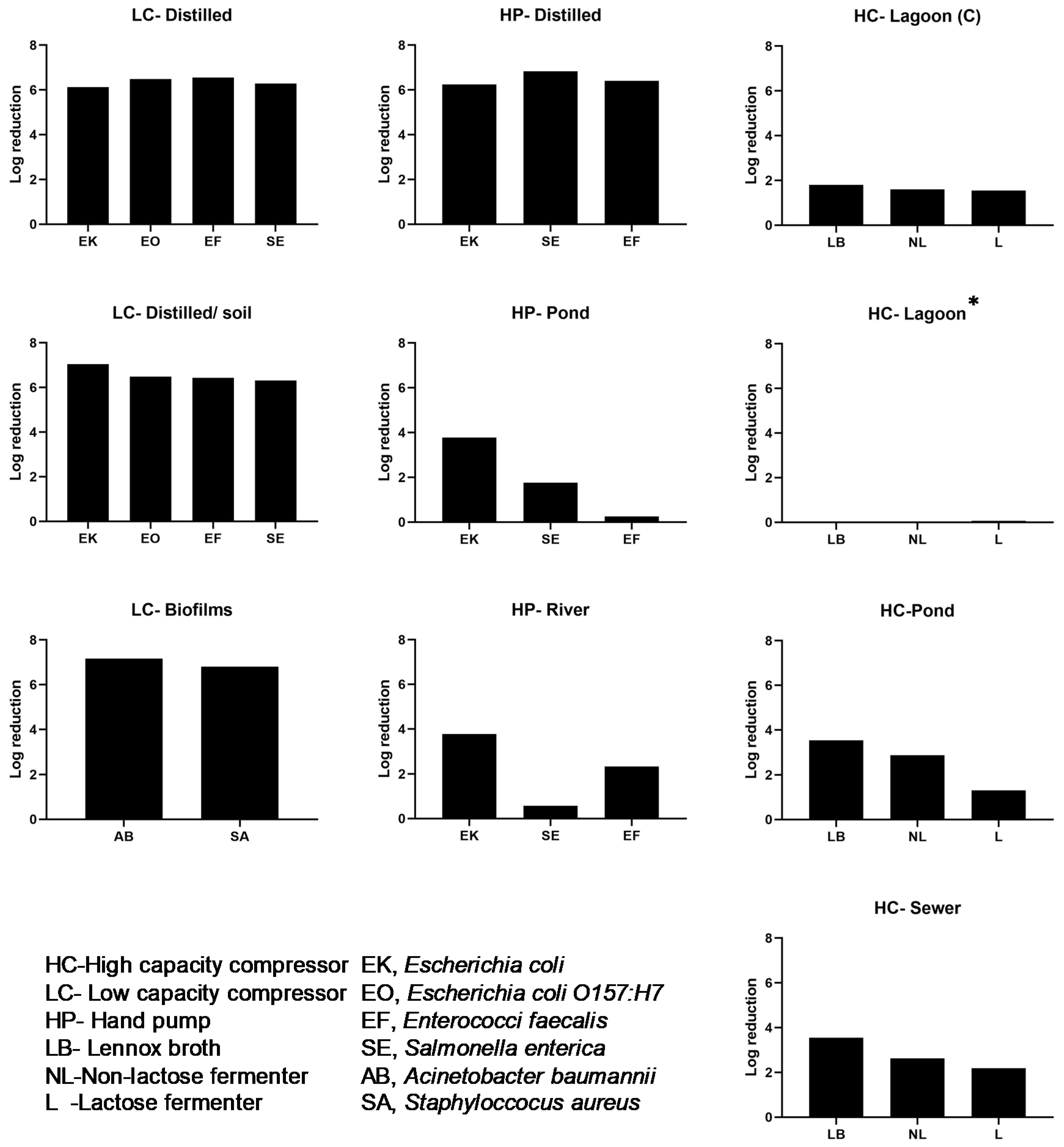
2VP) that delivers iodine vapor for water disinfection. The technology was effective when sterilizing distilled water that was inoculated with enteric organisms (
Escherichia,
Salmonella and
Enterococcus). This worked well when distilled water was pre-infused with iodine before adding bacteria, but also when water was infused. In drinking water, coliforms,
Escherichia coli and enterococci have been used as the primary method of assessing contamination (representative strains were used in this study [
32]. We show that the I2VP would be effective in sterilizing these coliforms by a 6-log reduction. The device was also effective against drug resistant pathogens tested i.e., methicillin-resistant
Staphylococcus and multi-drug resistant
Acinetobacter biofilm communities. This is also consistent with published reports of its potential use in controlling biofouling or for sanitation of interior surfaces of hoses [
22].
The last comprehensive study of the disinfection efficacy of iodine for water treatment showed that concentrations in the range 5–10 ppm were effective against different types of microorganisms within 10 min at room temperature [
6], another group showed that iodine tablets needed a contact time of 5–25 min depending on temperature [
11] and up to 30 min in the presence of organic matter [
33]. In this study, we were able to show efficacy of less than 90 s at room temperature with residues of 12 ppm with the electric power compressor and 4 min with residues less than 1 ppm following use of the hand pump. The electric powered compressor was superior in terms of time and effort required to deliver I
2 vapor compared to the manual version. However, a 4 min pump time with the manual device was still less than half of the 10 min reportedly necessary to kill microorganism using aqueous iodine. Our findings suggest that the I
2VP technology greatly reduces contact time necessary for water disinfection.
As has been also shown previously [
33], application of this technology is limited when water contains a high level of suspended solids and organic materials such as manually agitated dairy waste lagoon water that we tested. The presence of natural organic substances is associated with iodine demand and reduced efficacy [
7]. Even though this occurs, iodine shows appreciable lower reactivity when compared to chlorine, which is widely used for water disinfection [
7]. Despite this limitation, we found the technology was effective against bacteria found naturally in municipal sewer water and in a sample of pond water provided that enough volume of I
2 vapor is introduced into the water column. Depending on the situation, pre-filtration using a gravity-feed sand filter or using flocculation could be used as simple methods to reduce the concentration of suspended particles prior to I
2 vapor treatment. Use of sequential UV radiation can also be considered as it has been shown to increase efficacy of chlorine in the presence of suspended solids [
34]. Coupling iodine use with alternative disinfectants such as Peracetic acid (PAA) may also be explored [
35].
We hypothesize that the technology works well because vaporized I
2 is protected within the air bubbles long enough to be dispersed throughout a water column where it diffuses into the water and interacts with bacterial membranes. If correct, this is also consistent with reduced performance with increasing load of dissolved solids with which the iodine can interact. It also suggests that there will be optimal performance conditions that match the rate of infusion with water volume and concentration of interfering contaminants. Others have shown that iodine disinfection can be very sensitive to organic nitrogenous contaminants [
10,
11] and we surmise that the failures that we observed were due to the presence of organic compounds in the culture media. If correct, empirical testing will be needed to validate potential applications of this technology for different waste streams.
Hydrolysis and the subsequent equilibrium between elemental iodine and hypoiodous acid are pH-dependent, but the effect is not as pronounced as with chlorine [
6], which allow iodine to be used across a wide range of pH. For our experiments the pH was neutral to mildly alkaline (pH 7–7.5), which is reportedly compatible with both elemental iodine and hypoiodous acid [
7]. Elemental iodine is primarily effective against bacterial spores and protozoan cysts, whereas hypoiodous acid is known to be an effective bactericide and virucide [
36]. We surmise that the I
2VP technology can effectively deliver iodine to kill viruses and cysts, but this will need to be investigated further.
In addition, iodine is reportedly less reactive than chlorine and consequently chlorine is relatively less effective in the presence of organic material [
37]. Because chlorine interacts with dissolved organic matter, it potentially forms harmful disinfection byproducts (e.g., trihalomethanes and haloacetic acids), most of which are regulated by the U.S. Environmental Protection Agency [
37,
38]. With respect to toxicity, recent research performed during a Navy Environmental Sustainability Development to Integration (NESDI) program compared the toxicity of aqueous chlorine and iodine by evaluating the relative effects on fertilization rates of the
Trepnuestes gratilla, the Hawaiian collector sea urchin. Results were expressed as the no observable effect concentration (NOEC) and lowest observable effect concentration (LOEC). The NOEC for iodine and chlorine was 0.01 ppm and 0.002 ppm, respectively, while the LOEC for iodine and chlorine was 0.014 ppm and 0.003 ppm, respectively. These data are consistent with chlorine toxicity being an order-of-magnitude greater than that of iodine. Formation of DBPs from a number of iodine-based disinfectants was compared and it was found to be dependent on method of delivery method [
39]. This report suggested that use of iodine tincture was associated with higher levels of iodoforms. This will require further investigation for the I
2VP device. This suggests that perfecting iodine delivery will allow its use as a safer alternative to chlorine for water treatment.
Our results are consistent with scalability of the I2VP system, which is not surprising given that delivery and dispersion will be concentration and volume dependent. Larger diffusers, more air flow and longer exposures could be used to improve performance against larger volumes of water or when organic loads are higher. Nevertheless, under ideal conditions (i.e., with no visible contaminants) sanitation can be successful at a scale (3 L) that can be processed using a manually operated air pump for as little as 4 min. Given longer exposures and smaller volumes, the total residue concentration increases; hand-pumped bucket system (0.183 ug mL−1 for 3 L and 4 min), compressor pumped bucket system (3.47 ug mL−1 for 3 L; 16 min), 250 mL compressor cylinder (12 ug mL−1 for 250 mL, 16 min).
Iodine is an essential nutrient required for development and functioning of the thyroid gland, but excess iodine may lead to thyroid disease [
40,
41]. Toxicity to iodine has been linked to genetic or physiological predisposition (Rose et al., 2002) and to the use of iodine-based products as disinfectants [
4] and medications [
42]. Earlier work suggested that consumption of conventionally iodinated drinking-water did not cause adverse health effects in people [
43]. More recent reports indicate that iodine toxicity is uncommon [
44] and people appear to have a high tolerance to iodine given doses <2 mg day
−1 [
42]. The World Health Organization estimates that oral doses of 2000–3000 mg iodine (about 30–40 mg kg
−1 of body weight) are lethal to people [
45] while chronic ingestion of 2 mg of iodide per day (0.03 mg kg
−1 of body weight per day) is considered excessive. Others have reported that daily doses of 50–80 mg (0.8–1.3 mg kg
−1 of body weight per day) can be consumed without ill effect [
46]. For the current study, if we assume that water consumption for an adult person is 4 L day
−1 then water prepared by using the hand-pumped bucket system would deliver a daily dose of 0.73 mg of iodine that is well below suggested limits for chronic exposure. At the very least, the technology should be suitable for emergency water treatment in the absence of electricity assuming that the treated water has limited suspended solids. If higher air volume methods are used, real-time testing for iodine residues or secondary sequestration (e.g., activated charcoal) may be needed to guard against excessive iodine exposure.
The I
2VP technology has also been tested elsewhere. A study funded by the US Navy demonstrated the potential for the patented I
2 infusion system to reduce the rate of bio-fouling within Department of Defense shipboard heat exchangers [
23,
47]. For this project, periodic infusion of air containing elemental iodine vapor into the heat exchanger reduced the need for routine physical cleaning (which requires hazardous chemicals) while maintaining with acceptable operating parameters. Based on our findings, it is likely that this technology can be used for emergency and household drinking water treatment, sanitation of waste streams such as municipal sewage, and for managing biofilms that form within water lines that are used in food manufacturing and food animal agriculture.