The Effect of Acute Erythromycin Exposure on the Swimming Ability of Zebrafish (Danio rerio) and Medaka (Oryzias latipes)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
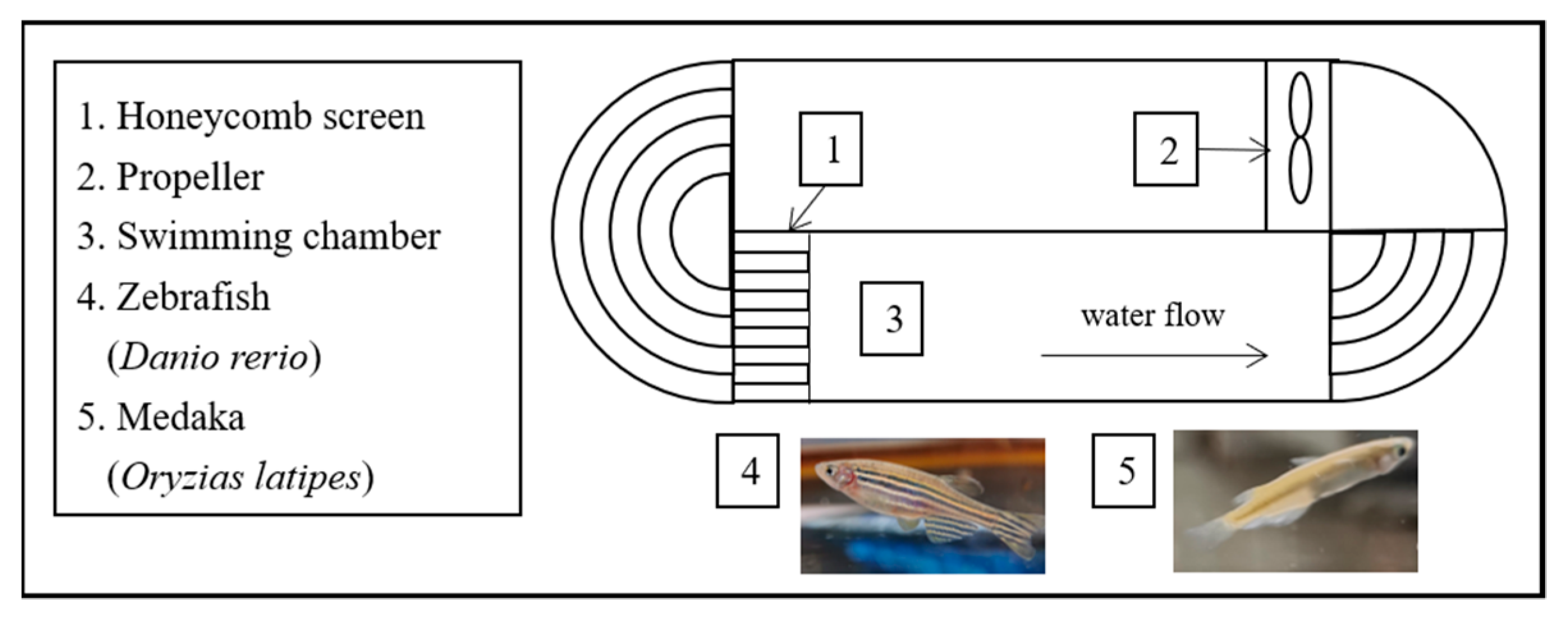
2.2. Experimental Design
2.3. Measurement of Ucrit and Uburst
2.4. RNA Sequencing
2.5. Quantitative Real-Time PCR (qRT-PCR) Validation
2.6. Statistical Analyses
2.7. Ethics Statement
3. Results
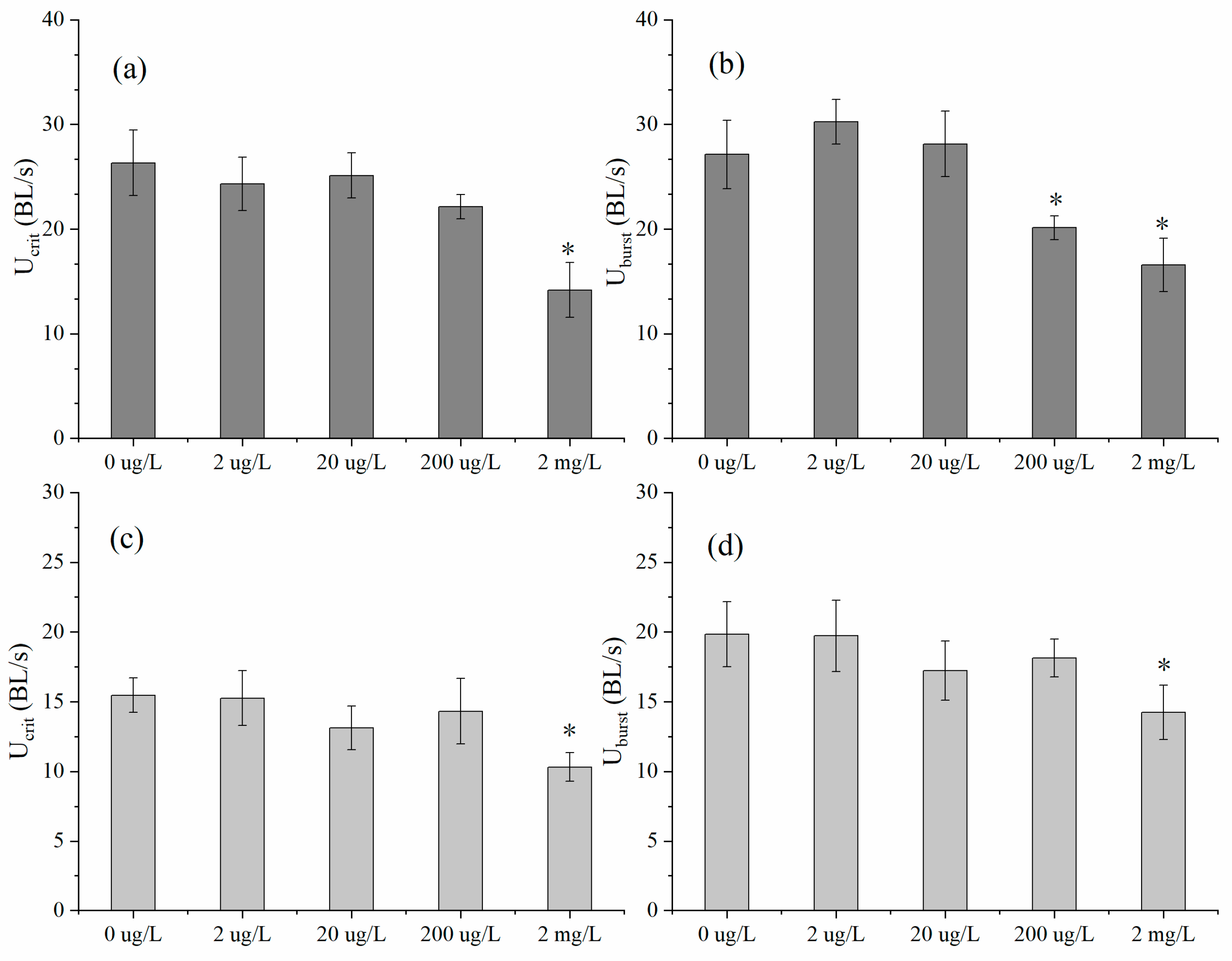
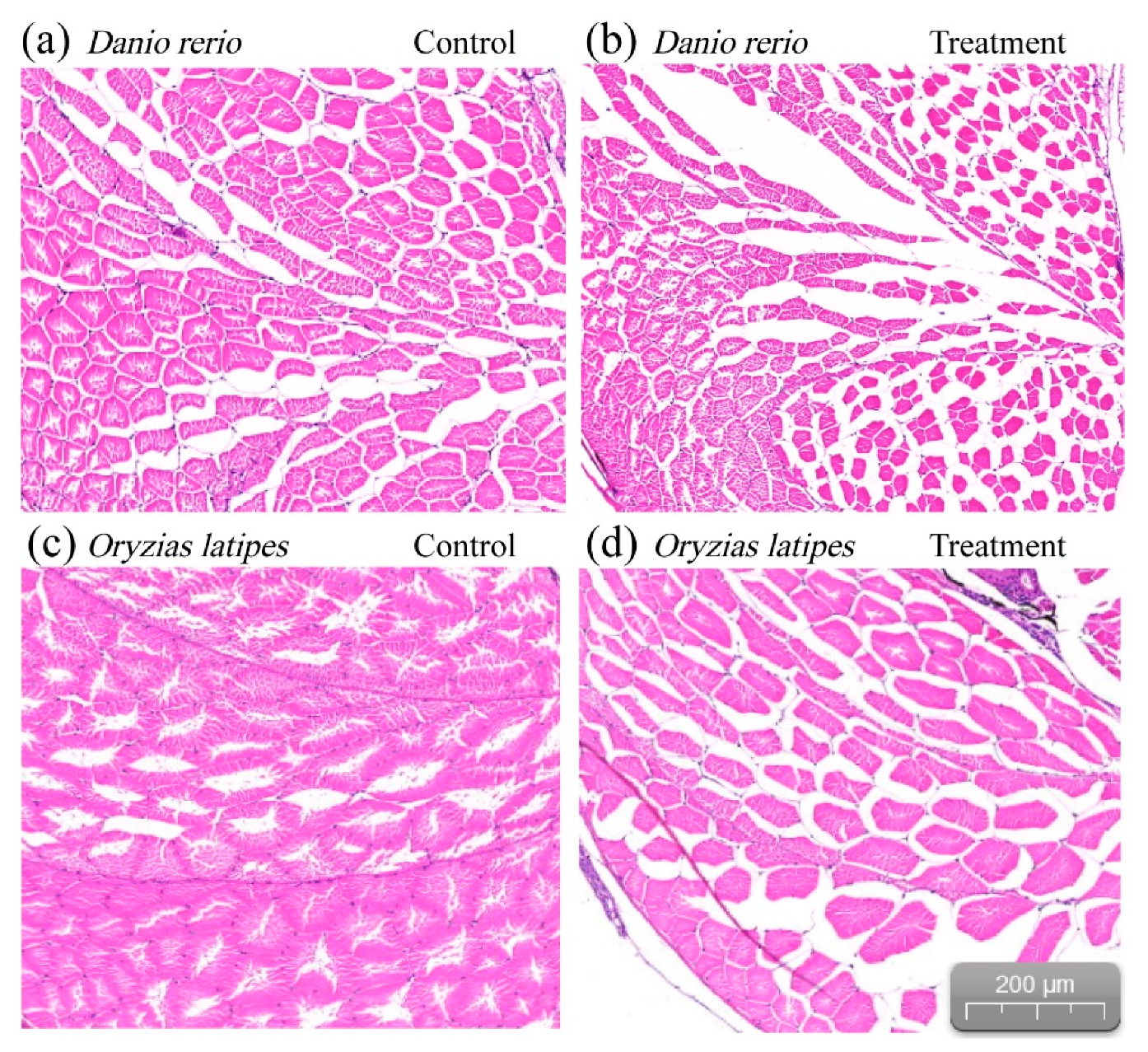
3.1. Swimming Performance and Muscle Fibers
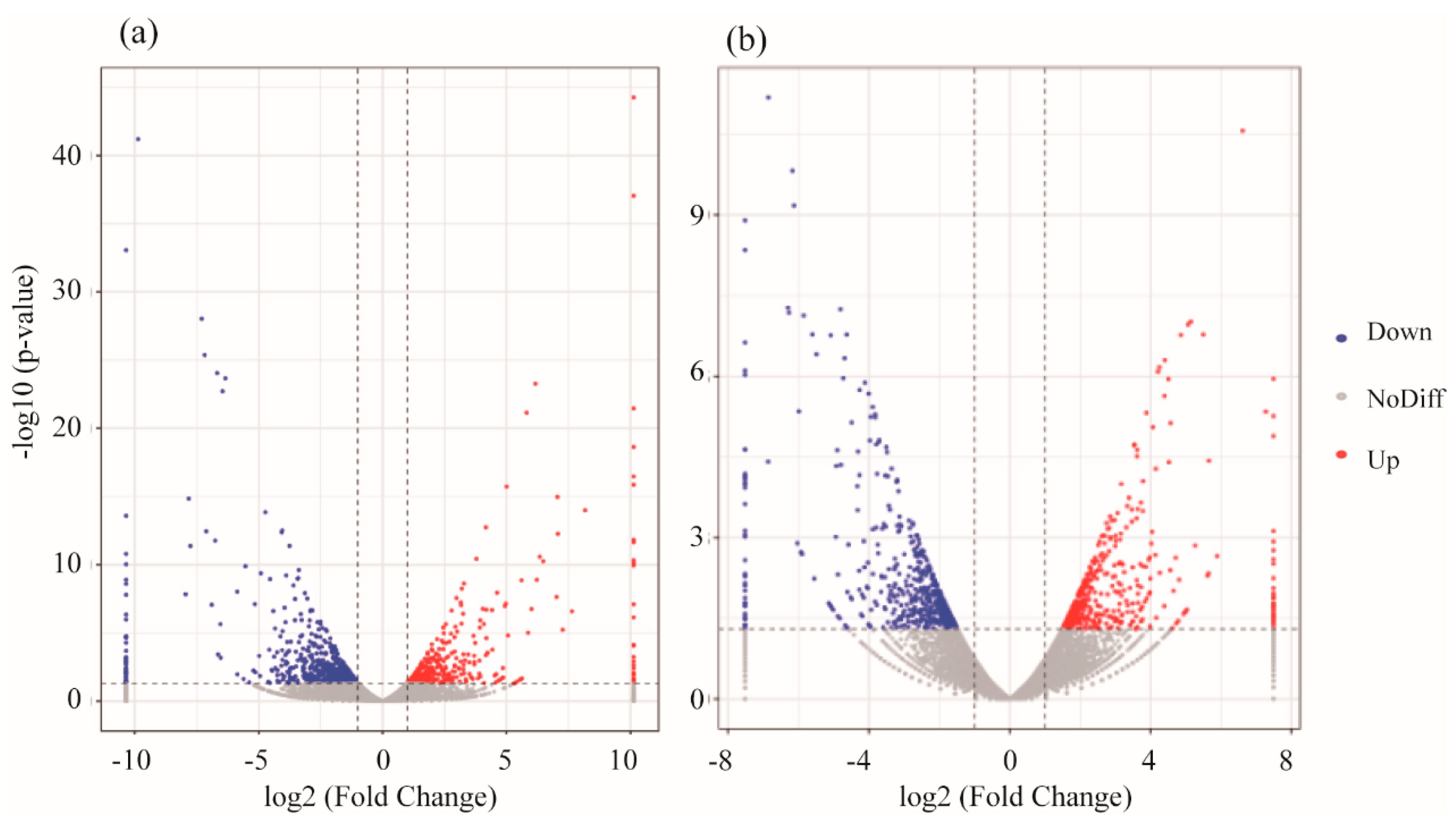
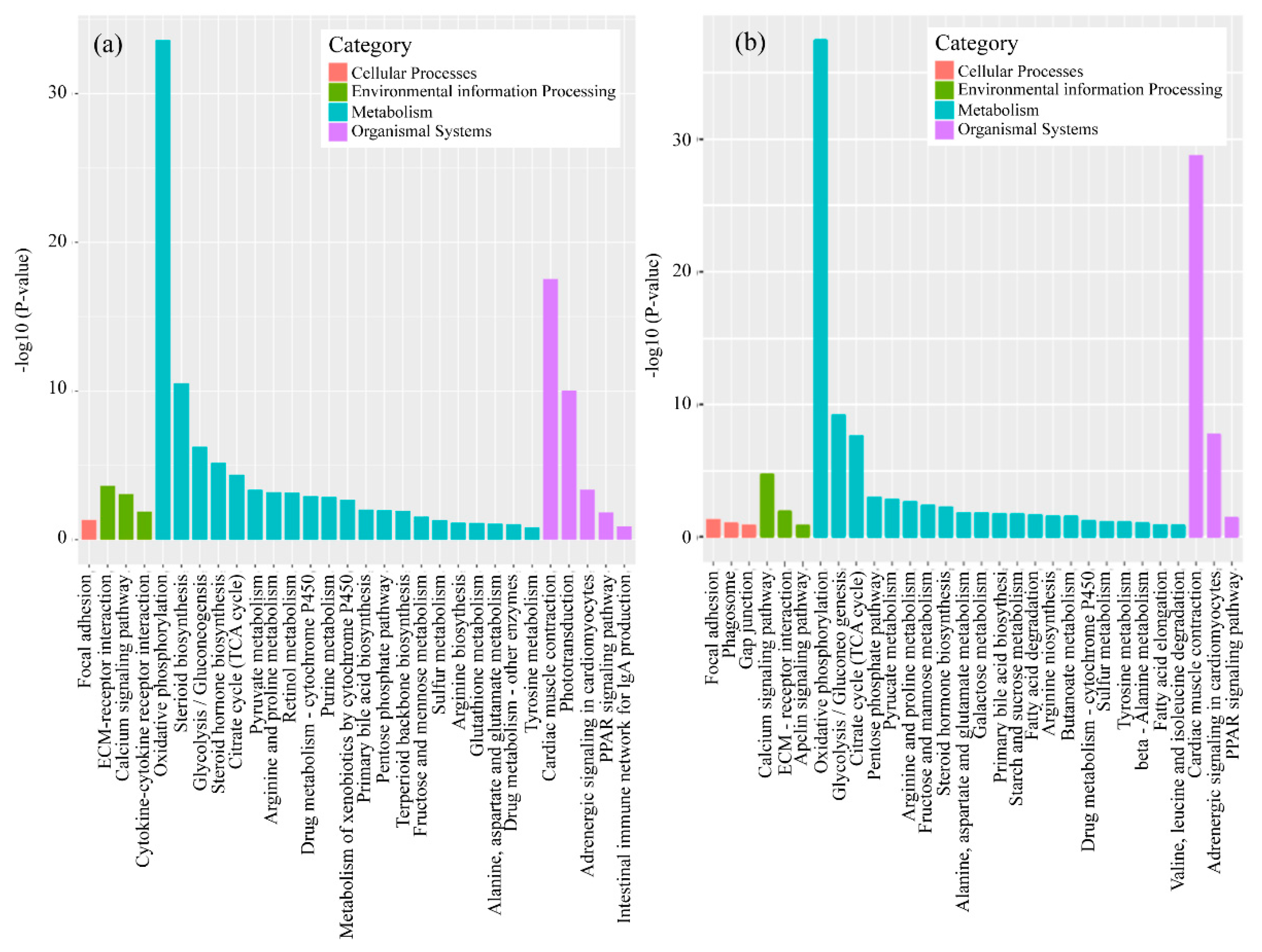
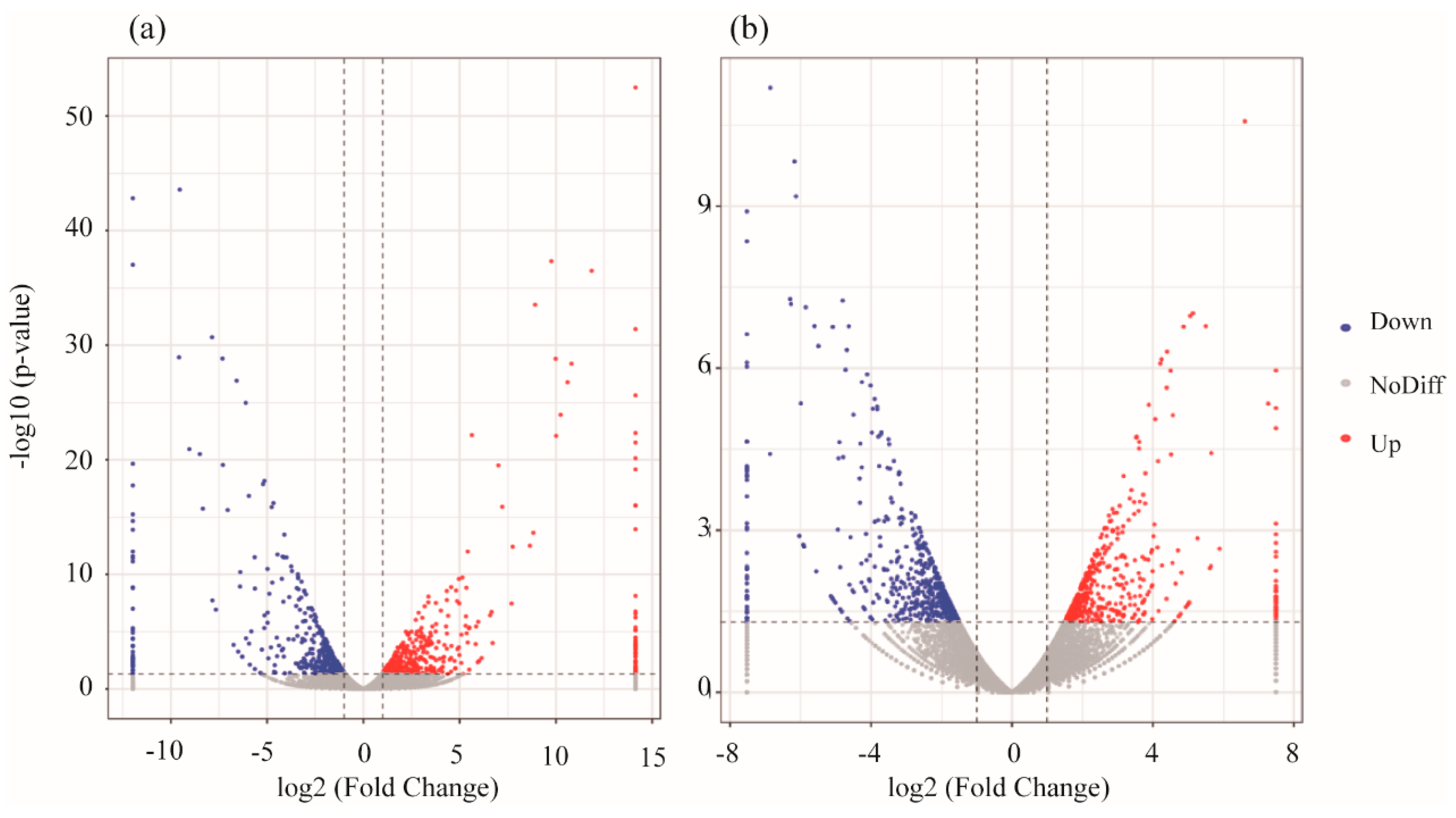
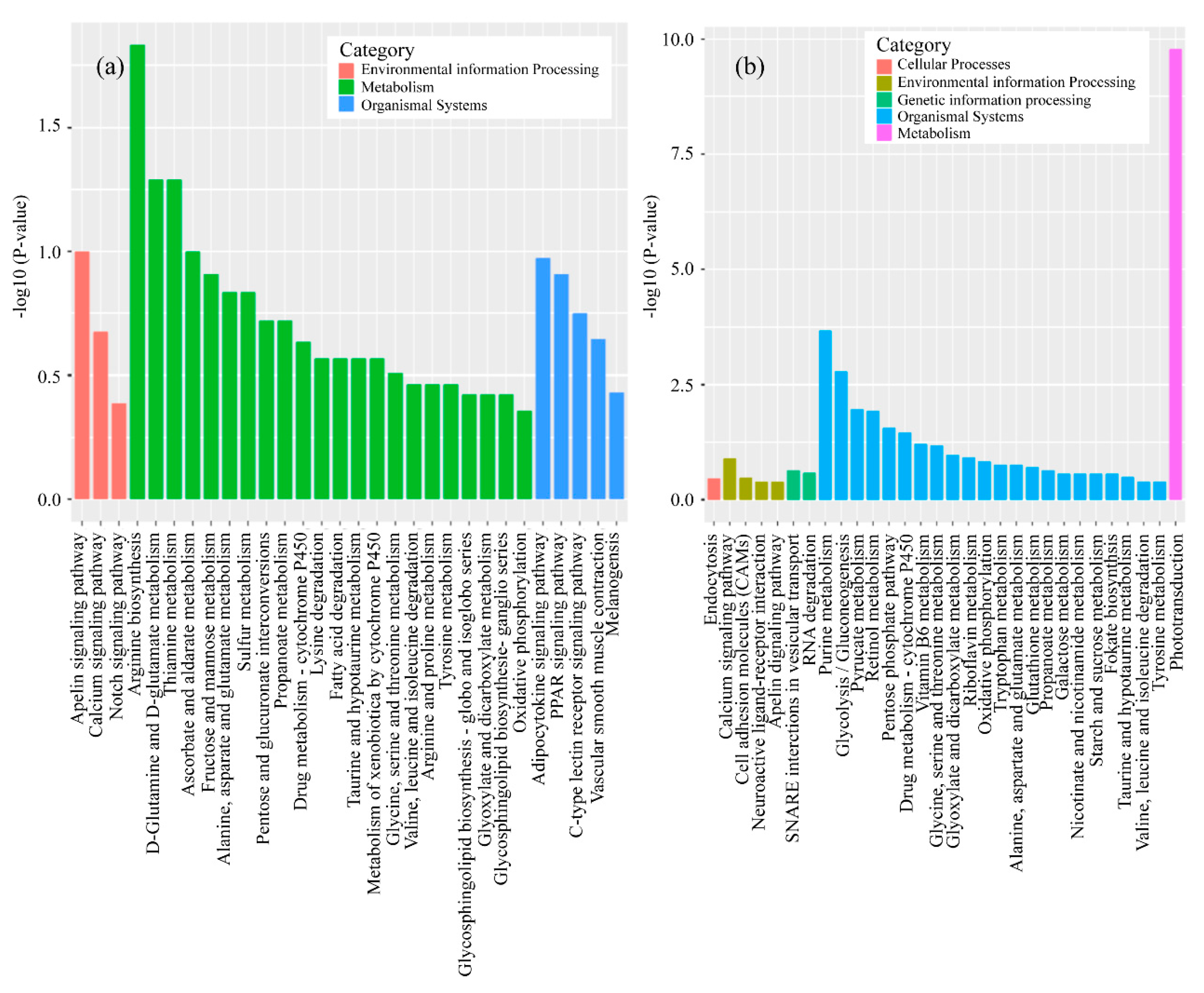
3.2. mRNA Expression Levels of Genes
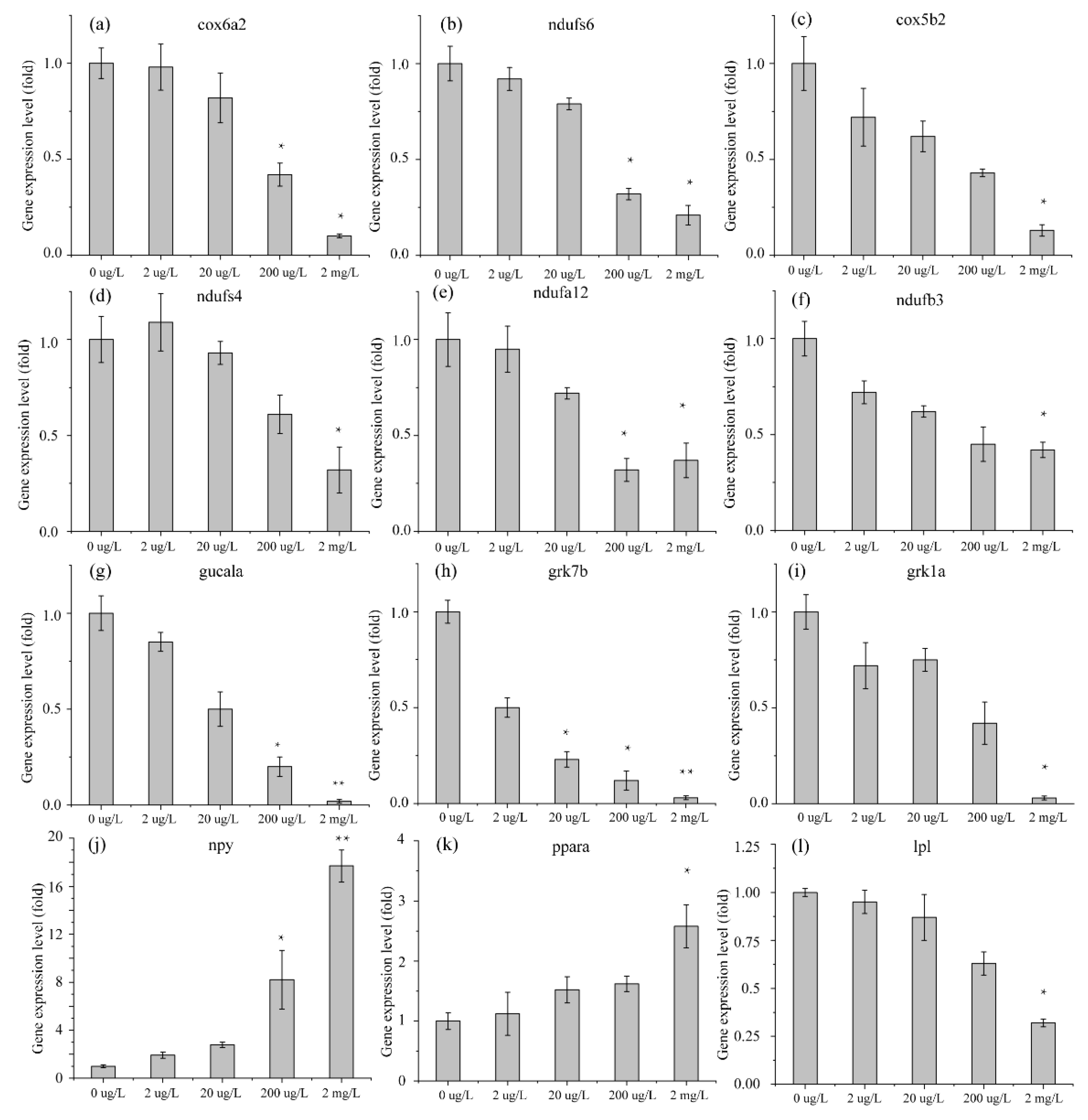
3.3. Validation of RNA-Seq DEG Expression Profiles in Danio rerio and Oryzias latipes by qRT-PCR
4. Discussion
4.1. Swimming Performance
4.2. Analysis of mRNA in Muscle
4.3. Analysis of mRNA in Head
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodgers, C.J.; Furones, M.D. Antimicrobial agents in aquaculture: Practice, needs and issues. Options Méditerrané 2009, 86, 41–59. [Google Scholar]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Qiang, Z.; Adams, C.; Zhang, H.; Chen, L. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography—Mass spectrometry. J. Chromatogr. A 2008, 1202, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Hargrave, B.T.; Haya, K. Antibiotic Use in Finfish Aquaculture: Modes of Action, Environmental Fate, and Microbial Resistance; Springer: Berlin/Heidelberg, Germany, 2005; pp. 341–357. [Google Scholar]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, J.; Xin, Q. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology 2015, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.; McDonough, S.; Ladewig, J.C.; Soares, A.M.; Nogueira, A.J.; Domingues, I. Effects of oxytetracycline and amoxicillin on development and biomarkers activities of zebrafish (Danio rerio). Environ. Toxicol. Pharm. 2013, 36, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.P.; Alloy, M.M.; Oris, J.T. Review of the photo-induced toxicity of environmental contaminants. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2017, 191, 160–167. [Google Scholar] [CrossRef]
- Nakano, T.; Hayashi, S.; Nagamine, N. Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environ. Sci. Pollut. R. 2018, 25, 7121–7128. [Google Scholar] [CrossRef]
- Almeida, A.R.; Tacão, M.; Machado, A.L.; Golovko, O.; Zlabek, V.; Domingues, I.; Henriques, I. Long-term effects of oxytetracycline exposure in zebrafish: A multi-level perspective. Chemosphere 2019, 222, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ying, G.; Pan, C.; Liu, Y.; Zhao, J. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Yu, X.; Wu, Y.; Deng, M.; Liu, Y.; Wang, S.; He, X.; Allaire-Leung, M.; Wan, J.; Zou, Y.; Yang, C. Tetracycline antibiotics as PI3K inhibitors in the Nrf2-mediated regulation of antioxidative stress in zebrafish larvae. Chemosphere 2019, 226, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kim, D.; Jin, S.; Choi, Y.; Lee, S.; Huh, H.; Chae, S.; Lee, A. A case of fixed drug eruption due to doxycycline and erythromycin present in food. Allergy Asthma Immunol. Res. 2013, 5, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shi, Y.; Li, W.; Niu, H.; Liu, J.; Cai, Y. Occurrence of antibiotics in eight sewage treatment plants in Beijing, China. Chemosphere 2012, 86, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Acute and chronic effects of erythromycin exposure on oxidative stress and genotoxicity parameters of Oncorhynchus mykiss. Sci. Total Environ. 2016, 545, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, X.; Cheng, J.; He, Y.; Wang, X.; Wang, Z.; Qi, J.; Yu, H.; Zhang, Q. Transcriptome profiling provides gene resources for understanding gill immune responses in Japanese flounder (Paralichthys olivaceus) challenged with Edwardsiella tarda. Fish Shellfish. Immun. 2018, 72, 593–603. [Google Scholar] [CrossRef]
- Ji, K.; Kim, S.; Han, S.; Seo, J.; Lee, S.; Park, Y.; Choi, K.; Kho, Y.; Kim, P.; Park, J. Risk assessment of chlortetracycline, oxytetracycline, sulfamethazine, sulfathiazole, and erythromycin in aquatic environment: Are the current environmental concentrations safe? Ecotoxicology 2012, 21, 2031–2050. [Google Scholar] [CrossRef]
- Klüver, N.; König, M.; Ortmann, J.; Massei, R.; Paschke, A.; Kühne, R.; Scholz, S. Fish embryo toxicity test: Identification of compounds with weak toxicity and analysis of behavioral effects to improve prediction of acute toxicity for neurotoxic compounds. Environ. Sci. Technol. 2015, 49, 7002–7011. [Google Scholar] [CrossRef]
- Reidy, S.P.; Kerr, S.R.; Nelson, J.A. Aerobic and anaerobic swimming performance of individual Atlantic cod. J. Exp. Biol. 2000, 203, 347–357. [Google Scholar]
- Huey, R.B. Sprint velocity of tadpoles (Bufoboreas) through metamorphosis. Copeia 1980, 1980, 537–540. [Google Scholar] [CrossRef]
- Driedzic, W.R.; Hochachka, P.W. Control of energy metabolism in fish white muscle. Am. J. Physiol. Content 1976, 230, 579–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, N.; Yoshida, M.; Matsumoto, N.; Uematsu, K. Artificial control of swimming in goldfish by brain stimulation: Confirmation of the midbrain nuclei as the swimming center. Neurosci. Lett. 2009, 452, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Baltz, D.M.; Chesney, E.J.; Tarr, M.A.; Kolok, A.S.; Bradley, M.J. Toxicity and sublethal effects of methanol on swimming performance of juvenile Florida pompano. Trans. Am. Fish. Soc. 2005, 134, 730–740. [Google Scholar] [CrossRef]
- Hopkins, W.A.; Snodgrass, J.W.; Staub, B.P.; Jackson, B.P.; Congdon, J.D. Altered swimming performance of a benthic fish (Erimyzon sucetta) exposed to contaminated sediments. Arch. Environ. Contam. Toxicol. 2003, 44, 0383–0389. [Google Scholar]
- Beamish, F. Swimming capacity. Fish Physiol. 1978, 7, 101–187. [Google Scholar]
- Alsop, D.; Wood, C. The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 1997, 200, 2337–2346. [Google Scholar]
- Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: A statistical review. Can. J. Fish. Aquat. Sci. 1985, 42, 630–648. [Google Scholar] [CrossRef]
- Wilson, R.W.; Bergman, H.L.; Wood, C.M. Metabolic costs and physiological consequences of acclimation to aluminum in juvenile rainbow trout (Oncorhynchus mykiss). 1: Acclimation specificity, resting physiology, feeding, and growth. Can. J. Fish. Aquat. Sci. 1994, 51, 527–535. [Google Scholar] [CrossRef]
- Randall, D.; Brauner, C. Effects of environmental factors on exercise in fish. J. Exp. Biol. 1991, 160, 113–126. [Google Scholar]
- Plaut, I. Critical swimming speed: Its ecological relevance. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Wen, C.; Wang, Y.; Chen, W.; Chen, L.; Tsay, H. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol. Teratol. 2008, 30, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Lee, Y.; Chau, Y.; Lu, K.; Kung, H. Short-term exposure of zebrafish embryos to arecoline leads to retarded growth, motor impairment, and somite muscle fiber changes. Zebrafish 2015, 12, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; Henriques, J.F.; Almeida, A.R.; Machado, A.L.; Koba, O.; Giang, P.T.; Soares, A.M.; Domingues, I. Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat. Toxicol. 2016, 170, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Calder, W.A. Size, Function, and Life History; Courier Corporation: Chelmsford, MA, USA, 1996. [Google Scholar]
- Somero, G.N.; Childress, J.J. Scaling of ATP-supplying enzymes, myofibrillar proteins and buffering capacity in fish muscle: Relationship to locomotory habit. J. Exp. Biol. 1990, 149, 319–333. [Google Scholar]
- Milligan, C.L.; Wood, C.M. Intracellular and extracellular acid-base status and H+ exchange with the environment after exhaustive exercise in the rainbow trout. J. Exp. Biol. 1986, 123, 93–121. [Google Scholar] [PubMed]
- Dobson, G.P.; Hochachka, P.W. Role of glycolysis in adenylate depletion and repletion during work and recovery in teleost white muscle. J. Exp. Biol. 1987, 129, 125–140. [Google Scholar]
- Adachi, K.; Okuyama, T. Study on the reduced pyridine nucleotide dehydrogenase of bovine erythrocytes: I. Crystallization and properties of the reduced pyridine nucleotide dehydrogenase of bovine erythrocytes. Biochim. Biophys. Acta (BBA)-Enzymol. 1972, 268, 629–637. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Shiva, S. The Ligand Binding Battle at Cytochrome C Oxidase: How No Regulates Oxygen Gradients in Tissue; Am Heart Assoc: Dallas, TX, USA, 2009. [Google Scholar]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide Inhibition of and Metabolism by Cytochrome C Oxidase; Portland Press Ltd.: London, UK, 2013. [Google Scholar]
- Amiard-Triquet, C. Behavioral disturbances: The missing link between sub-organismal and supra-organismal responses to stress? Prospects based on aquatic research. Hum. Ecol. Risk Assess. 2009, 15, 87–110. [Google Scholar] [CrossRef]
- Granato, M.; Van Eeden, F.J.; Schach, U.; Trowe, T.; Brand, M.; Furutani-Seiki, M.; Haffter, P.; Hammerschmidt, M.; Heisenberg, C.; Jiang, Y. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 1996, 123, 399–413. [Google Scholar]
- Nolan, P.M.; Peters, J.; Vizor, L.; Strivens, M.; Washbourne, R.; Hough, T.; Wells, C.; Glenister, P.; Thornton, C.; Martin, J. Implementation of a large-scale ENU mutagenesis program: Towards increasing the mouse mutant resource. Mamm. Genome 2000, 11, 500–506. [Google Scholar] [CrossRef]
- Easter, S.S., Jr.; Nicola, G.N. The development of vision in the zebrafish (Danio rerio). Dev. Biol. 1996, 180, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Muto, A.; Orger, M.B.; Wehman, A.M.; Smear, M.C.; Kay, J.N.; Page-McCaw, P.S.; Gahtan, E.; Xiao, T.; Nevin, L.M.; Gosse, N.J. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005, 1, e66. [Google Scholar] [CrossRef] [PubMed]
- Oren, D.A. Bilirubin, REM sleep, and phototransduction of environmental time cues. A hypothesis. Chronobiol. Int. 1997, 14, 319–329. [Google Scholar] [CrossRef] [PubMed]
- van de Pol, I.; Flik, G.; Gorissen, M. Comparative physiology of energy metabolism: Fishing for endocrine signals in the early vertebrate pool. Front. Endocrinol. 2017, 8, 36. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Anand, B.K.; Brobeck, J.R. Hypothalamic control of food intake in rats and cats. Yale J. Boil. Med. 1951, 24, 123. [Google Scholar]
- Mayer, J. Regulation of energy intake and the body weight: The glucostatic theory and the lipostatic hypothesis. Ann. N. Y. Acad. Sci. 1955, 63, 15–43. [Google Scholar] [CrossRef]
- Shimmura, T.; Nakayama, T.; Shinomiya, A.; Fukamachi, S.; Yasugi, M.; Watanabe, E.; Shimo, T.; Senga, T.; Nishimura, T.; Tanaka, M. Dynamic plasticity in phototransduction regulates seasonal changes in color perception. Nat. Commun. 2017, 8, 412. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, M.; Zhang, C.; Li, E.; Fan, M.; Huang, M. Transcriptomic analyses of tributyltin-induced sexual dimorphisms in rare minnow (Gobiocypris rarus) brains. Ecotox. Environ. Saf. 2018, 156, 18–24. [Google Scholar] [CrossRef]
- Robinson, S.L.; Thiele, T.E. The Role of Neuropeptide Y (Npy) in Alcohol and Drug Abuse Disorders; Elsevier: Amsterdam, The Netherlands, 2017; Volume 136, pp. 177–197. [Google Scholar]
- Decressac, M.; Barker, R.A. Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 2012, 238, 265–272. [Google Scholar] [CrossRef]
- Heilig, M.; Widerlöv, E. Neurobiology and clinical aspects of neuropeptide Y. Crit. Rev. Neurobiol. 1995, 9, 115–136. [Google Scholar] [PubMed]
- Tatemoto, K.; Neuropeptide, Y. Historyandoverview. In Michel, MC Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Colmers, W.F.; Bahh, B.E. Neuropeptide Y and epilepsy. Epilepsy Curr. 2003, 3, 53–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acuna-Goycolea, C.; Tamamaki, N.; Yanagawa, Y.; Obata, K.; van den Pol, A.N. Mechanisms of neuropeptide Y, peptide YY, and pancreatic polypeptide inhibition of identified green fluorescent protein-expressing GABA neurons in the hypothalamic neuroendocrine arcuate nucleus. J. Neurosci. 2005, 25, 7406–7419. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, F.; Holzer, P. Neuropeptide Y: A stressful review. Neuropeptides 2016, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Brand, S.; Yang, R. Plasma neuropeptide Y concentrations in combat exposed veterans: Relationship to trauma exposure, recovery from PTSD, and coping. Biol. Psychiat. 2006, 59, 660–663. [Google Scholar] [CrossRef]
- Heinzmann, C.; Ladias, J.; Antonarakis, S.; Kirchgessner, T.; Schotz, M.; Lusis, A.J. RFLP for the human lipoprotein lipase (LPL) gene: HindIII. Nucleic Acids Res. 1987, 15, 6763. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, J. The Effect of Acute Erythromycin Exposure on the Swimming Ability of Zebrafish (Danio rerio) and Medaka (Oryzias latipes). Int. J. Environ. Res. Public Health 2020, 17, 3389. https://doi.org/10.3390/ijerph17103389
Li Y, Zhang J. The Effect of Acute Erythromycin Exposure on the Swimming Ability of Zebrafish (Danio rerio) and Medaka (Oryzias latipes). International Journal of Environmental Research and Public Health. 2020; 17(10):3389. https://doi.org/10.3390/ijerph17103389
Chicago/Turabian StyleLi, Yanyi, and Jiabo Zhang. 2020. "The Effect of Acute Erythromycin Exposure on the Swimming Ability of Zebrafish (Danio rerio) and Medaka (Oryzias latipes)" International Journal of Environmental Research and Public Health 17, no. 10: 3389. https://doi.org/10.3390/ijerph17103389
APA StyleLi, Y., & Zhang, J. (2020). The Effect of Acute Erythromycin Exposure on the Swimming Ability of Zebrafish (Danio rerio) and Medaka (Oryzias latipes). International Journal of Environmental Research and Public Health, 17(10), 3389. https://doi.org/10.3390/ijerph17103389



