Initiation and Single Dispensing in Cardiovascular and Insulin Medications: Prevalence and Explanatory Factors
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Design
2.3. Variables
2.4. Analysis
2.5. Availability of Data
3. Results
3.1. Prevalence of Non-initiation and Single Dispensing
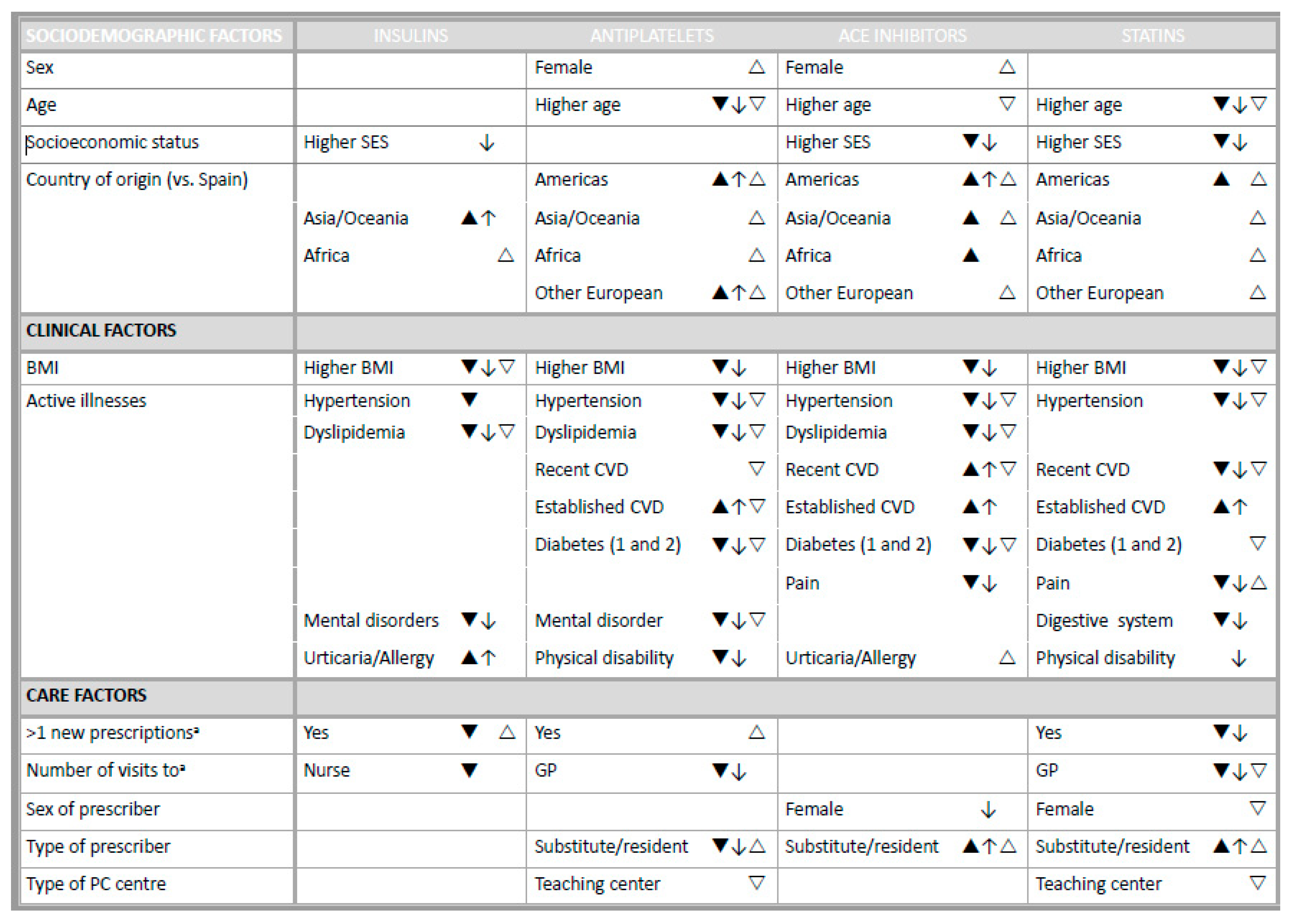
3.2. Non-initiation Explanatory Factors
3.3. Single Dispensing Explanatory Factors
4. Discussion
4.1. Strengths and Limitations
4.2. Practical Implications
4.3. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gheorghe, A.; Griffiths, U.; Murphy, A.; Legido-Quigley, H.; Lamptey, P.; Perel, P. The economic burden of cardiovascular disease and hypertension in low-and middle-income countries: A systematic review. BMC Public Health 2018, 18, 975. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Health Statistics and Information Systems. Estimates for 2000–2016. Available online: who.int/healthinfo/global_burden_disease/estimates/en/index1.html (accessed on 3 February 2020).
- Chowdhury, R.; Khan, H.; Heydon, E.; Shroufi, A.; Fahimi, S.; Moore, C.; Stricker, B.; Mendis, S.; Hofman, A.; Mant, J.; et al. Adherence to cardiovascular therapy: A meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013, 34, 2940–2948. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Xu, T.; Yu, X.; Ou, S.; Liu, X.; Yuan, J.; Tan, X.; Chen, Y. Adherence to Antihypertensive Medications and Stroke Risk: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2017, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.H.; Dragomir, A.; Blais, L.; Bérard, A.; Pilon, D.; Perreault, S. Impact of adherence to statins on coronary artery disease in primary prevention. Br. J. Clin. Pharmacol. 2007, 63, 698–708. [Google Scholar] [CrossRef]
- Cutler, R.L.; Fernandez-Llimos, F.; Frommer, M.; Benrimoj, C.; Garcia-Cardenas, V. Economic impact of medication non-adherence by disease groups: A systematic review. BMJ Open 2018, 8, 16982. [Google Scholar] [CrossRef]
- Hutchins, D.S.D.S.; Zeber, J.E.; Roberts, C.S.; Williams, A.F.; Manias, E.; Peterson, A.M.; Raofi, S.; Schappert, S.M.; Blaschke, T.F.; Osterberg, L.; et al. Initial Medication Adherence—Review and Recommendations for Good Practices in Outcomes Research: An ISPOR Medication Adherence and Persistence Special Interest Group Report. Value Health 2015, 18, 690–699. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- Evans, C.D.; Eurich, D.T.; Remillard, A.J.; Shevchuk, Y.M.; Blackburn, D. First-fill medication discontinuations and nonadherence to antihypertensive therapy: An observational study. Am. J. Hypertens. 2012, 25, 195–203. [Google Scholar] [CrossRef]
- Lemstra, M.; Blackburn, D. Nonadherence to Statin Therapy: Discontinuation After a Single Fill. Can. J. Cardiol. 2012, 28, 567–573. [Google Scholar] [CrossRef]
- Pedan, A.; Varasteh, L.; Schneeweiss, S. Analysis of factors associated with statin adherence in a hierarchical model considering physician, pharmacy, patient, and prescription characteristics. J. Manag. Care Ph. 2007, 13, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A.; Ellis, J.L.; Carroll, N.M.; Bayliss, E.A.; Mcginnis, B.; Schroeder, E.B.; Shetterly, S.; Xu, S.; Steiner, J.F. Characteristics of Patients with Primary Non-adherence to Medications for Hypertension, Diabetes, and Lipid Disorders. J. Gen. Intern. Med. 2011, 27, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Lou, I.; Fernández, A.; Gil-Girbau, M.; Fajó-Pascual, M.; Moreno-Peral, P.; Peñarrubia-María, M.T.; Serrano-Blanco, A.; Sánchez-Niubó, A.; March-Pujol, M.A.; Jové, A.M.; et al. Initial medication non-adherence: Prevalence and predictive factors in a cohort of 1.6 million primary care patients. Br. J. Clin. Pharmacol. 2017, 83, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Christensen, R.P.; Houji, A.; Christiansen, C.B.; Paulsen, M.S.; Thomsen, J.L.; Hallas, J. Primary non-adherence in general practice: A Danish register study. Eur. J. Clin. Pharmacol. 2014, 70, 757–763. [Google Scholar] [CrossRef]
- Lee, S.-Q.; Raamkumar, A.S.; Li, J.; Cao, Y.; Witedwittayanusat, K.; Chen, L.; Theng, Y.-L. Reasons for Primary Medication Nonadherence: A Systematic Review and Metric Analysis. J. Manag. Care Spec. Pharm. 2018, 24, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Gil-Girbau, M.; Aznar-Lou, I.; Peñarrubia-Maria, M.T.; Moreno-Peral, P.; Fernández, A.; Bellón, J.Á.; Jové, A.M.; Mendive, J.; Fernández-Vergel, R.; Figueiras, A.; et al. Reasons for medication non-initiation: A qualitative exploration of the patients’ perspective. Res. Soc. Adm. Pharm. 2020, 16, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Polinski, J.M.; Kesselheim, A.S.; Frolkis, J.P.; Wescott, P.; Allen-Coleman, C.; Fischer, M.A. A matter of trust: Patient barriers to primary medication adherence. Health Educ. Res. 2014, 29, 755–763. [Google Scholar] [CrossRef]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP medication adherence reporting guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef]
- García-Armesto, S.; Abadía-Taira, M.; Durán, A.; Hernández-Quevedo, C.; Bernal-Delgado, E. Spain Health system review. Health Syst. Transit. 2010, 12, 1–295. [Google Scholar]
- Aznar-Lou, I.; Pottegård, A.; Fernández, A.; Peñarrubia-María, M.T.; Serrano-Blanco, A.; Sabés-Figuera, R.; Fajó-Pascual, M. Effect of copayment policies on initial medication non-adherence according to income: A population- based study. BMJ Qual. Saf. 2018, 27, 878–891. [Google Scholar] [CrossRef]
- Bolíbar, B.; Avilés, F.; Morros, R.; Garcia-Gil, M.M.; Hermosilla, E.; Ramos, R.; Rosell, M.; Rodríguez, J.; Medina, M.; Calero, S.; et al. SIDIAP database: Electronic clinical records in primary care as a source of information for epidemiologic research. Med. Clin. 2012, 138, 617–621. [Google Scholar] [CrossRef]
- Ramos, R.; Balló, E.; Marrugat, J.; Elosua, R.; Sala, J.; Grau, M.; Vila, J.; Bolíbar, B.; García-Gil, M.; Martí, R.; et al. Validity for Use in Research on Vascular Diseases of the SIDIAP (Information System for the Development of Research in Primary Care): The EMMA Study. Revista Española de Cardiología 2011, 65, 29–37. [Google Scholar] [CrossRef]
- Dominguez-Berjon, M.F.; Borrell, C.; Cano-Serral, G.; Esnaola, S.; Nolasco, A.; Pasarin, M.I.; Ramis, R.; Saurina, C.; Escolar-Pujolar, A. Constructing a deprivation index based on census data in large Spanish cities(the MEDEA project). Gac. Sanit. 2008, 22, 179–187. [Google Scholar]
- Aznar-Lou, I.; Iglesias-González, M.; Gil-Girbau, M.; Serrano-Blanco, A.; Fernández, A.; Peñarrubia-María, M.T.; Sabés-Figuera, R.; Murrugarra-Centurión, A.G.; March-Pujol, M.; Bolívar-Prados, M.; et al. Impact of initial medication non-adherence to SSRIs on medical visits and sick leaves. J. Affect. Disord. 2018, 226, 282–286. [Google Scholar] [CrossRef]
- Aznar-Lou, I.; Fernández, A.; Gil-Girbau, M.; Sabés-Figuera, R.; Fajó-Pascual, M.; Peñarrubia-María, M.T.; Serrano-Blanco, A.; Moreno-Peral, P.; Sánchez-Niubó, A.; March-Pujol, M.; et al. Impact of initial medication non-adherence on use of healthcare services and sick leave: A longitudinal study in a large primary care cohort in Spain. Br. J. Gen. Pract. 2017, 67, e614–e622. [Google Scholar] [CrossRef] [PubMed]
- Mickey, R.M.; Greenland, S. The Impact Of Confounder Selection Criteria On Effect Estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Thengilsdóttir, G.; Pottegard, A.; Linnet, K.; Halldórsson, M.; Almarsdóttir, A.B.; Gardarsdóttir, H. Do patients initiate therapy? Primary non-adherence to statins and antidepressants in Iceland. Int. J. Clin. Pract. 2015, 69, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, R.; Eguale, T.; Huang, A.; Winslade, N.; Doran, P. The incidence and determinants of primary nonadherence with prescribed medication in primary care: A cohort study. Ann. Intern. Med. 2014, 160, 441–450. [Google Scholar] [CrossRef]
- Hertz, R.P.; Unger, A.N.; Lustik, M.B. Adherence with pharmacotherapy for type 2 diabetes: A retrospective cohort study of adults with employer-sponsored health insurance. Clin. Ther. 2005, 27, 1064–1073. [Google Scholar] [CrossRef]
- Raebel, M.A.; Carroll, N.M.; Ellis, J.L.; Schroeder, E.B.; Bayliss, E.A. Importance of Including Early Nonadherence in Estimations of Medication Adherence. Ann. Pharmacother. 2011, 45, 1053–1060. [Google Scholar] [CrossRef]
- Karter, A.J.; Subramanian, U.; Saha, C.; Crosson, J.C.; Parker, M.M.; Swain, B.E.; Moffet, H.H.; Marrero, D.G. Barriers to Insulin Initiation. Diabetes Care 2010, 33, 733–735. [Google Scholar] [CrossRef]
- Catalan, V.S.; Lelorier, J. Predictors of long-term persistence on statins in a subsidized clinical population. Value Health 2000, 3, 417–426. [Google Scholar] [CrossRef]
- Halava, H.; Korhonen, M.J.; Huupponen, R.; Setoguchi, S.; Pentti, J.; Kivimäki, M.; Vahtera, J. Lifestyle factors as predictors of nonadherence to statin therapy among patients with and without cardiovascular comorbidities. Can. Med. Assoc. J. 2014, 186, E449–E456. [Google Scholar] [CrossRef][Green Version]
- Pound, P.; Britten, N.; Morgan, M.; Yardley, L.; Pope, C.; Daker-White, G.; Campbell, R. Resisting medicines: A synthesis of qualitative studies of medicine taking. Soc. Sci. Med. 2005, 61, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jamal, S.Z.; Qadir, F. Medication Adherence In Post Myocardial Infarction Patients. J. Ayub Med. Coll. Abbottabad 2018, 30, 552–557. [Google Scholar] [PubMed]
- Huber, C.A.; Meyer, M.R.; Steffel, J.; Blozik, E.; Reich, O.; Rosemann, T. Post-myocardial Infarction (MI) Care: Medication Adherence for Secondary Prevention After MI in a Large Real-world Population. Clin. Ther. 2019, 41, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, B.; Van Der Weijden, T.; Timmermans, D.; Vaes, J.; Stoffers, J.; Grol, R. Patients’ ideas, fears and expectations of their coronary risk: Barriers for primary prevention. Patient Educ. Couns. 2004, 55, 301–307. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef]
- Van Geffen, E.C.G.; Heerdink, E.R.; Hugtenburg, J.G.; Siero, F.W.; Egberts, A.C.G.; Van Hulten, R. Patients’ perceptions and illness severity at start of antidepressant treatment in general practice. Int. J. Pharm. Pract. 2010, 18, 217–225. [Google Scholar] [CrossRef]
- Luciano, J.V.; Fernández, A.; Pinto-Meza, A.; Luján, L.; Bellón, J.A.; Garcia-Campayo, J.; Peñarrubia, M.T.; Fernández, R.; Sanavia, M.; Blanco, M.E.; et al. Frequent attendance in primary care: Comparison and implications of different definitions. Br. J. Gen. Pract. 2010, 60, 95–100. [Google Scholar] [CrossRef]
- Velasco, C.; Vinasco, A.M.; Trilla, A. Immigrant perceptions of the Spanish National Healthcare System and its services. Aten. Primaria 2016, 48, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Agència de Salut Pública de Barcelona. La Salut de la Població Immigrant a Barcelona (The Health of the Immigrant Population in Barcelona); Agència de Salut Pública de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- Leslie, K.H.; McCowan, C.; Pell, J.P. Adherence to cardiovascular medication: A review of systematic reviews. J. Public Health 2019, 41, e84–e94. [Google Scholar] [CrossRef] [PubMed]
- Derose, S.F.; Green, K.; Marrett, E.; Tunceli, K.; Craig Cheetham, T.; Chiu, V.Y.; Harrison, T.N.; Reynolds, K.; Vansomphone, S.S.; Scott, R.D. Automated Outreach to Increase Primary Adherence to Cholesterol-Lowering Medications. JAMA Intern. Med. 2013, 173, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kerner, D.E.; Knezevich, E.L. Use of communication tool within electronic medical record to improve primary nonadherence. J. Am. Pharm. Assoc. 2017, 57, S270–S273. [Google Scholar] [CrossRef]
- Fischer, M.A.; Jones, J.B.; Wright, E.; Van Loan, R.P.; Xie, J.; Gallagher, L.; Wurst, A.M.; Shrank, W.H. A Randomized Telephone Intervention Trial to Reduce Primary Medication Nonadherence. J. Manag. Care Spec. Pharm. 2015, 21, 124–131. [Google Scholar] [CrossRef]
- Vinagre, I.; Mata-Cases, M.; Hermosilla, E.; Morros, R.; Fina, F.; Rosell, M.; Castell, C.; Franch-Nadal, J.; Bolíbar, B.; Mauricio, D. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care 2012, 35, 774–779. [Google Scholar] [CrossRef]
- Massimi, A.; De Vito, C.; Brufola, I.; Corsaro, A.; Marzuillo, C.; Migliara, G.; Rega, M.L.; Ricciardi, W.; Villari, P.; Damiani, G. Are community-based nurse-led selfmanagement support interventions effective in chronic patients? Results of a systematic review and meta-analysis. PLoS ONE 2017, 12, e0173617. [Google Scholar] [CrossRef] [PubMed]
| PATIENTS; n = 169,143 | Insulin n = 8223 | Antiplatelet n = 33,921 | ACEI n = 73,741 | Statin n = 69,043 |
| Sex, Female, n (%) | 3848 (46.80) | 17,087 (50.37) | 36,583 (49.61) | 36,334 (52.63) |
| Age, mean ± SD | 67.50 ± 15.04 | 70.47 ± 13.94 | 65.65 ± 14.28 | 62.84 ± 12.72 |
| Socioeconomic status, n (%) | ||||
| Urban 1 (lowest SES) | 1160 (14.11) | 6101 (17.99) | 11,717 (15.89) | 11,945 (17.30) |
| Urban 2 | 1271 (15.46) | 5552 (16.37) | 12,019 (16.30) | 11,415 (16.53) |
| Urban 3 | 1352 (16.44) | 5459 (16.09) | 12,360 (16.76) | 11,378 (16.48) |
| Urban 4 | 1395 (16.96) | 5491 (16.19) | 12,231 (16.59) | 11,041 (15.99) |
| Urban 5 (highest SES) | 1509 (18.35) | 4982 (14.69) | 11,470 (15.55) | 10,040 (14.54) |
| Rural | 1536 (18.68) | 6336 (18.68) | 13,944 (18.91) | 13,224 (19.15) |
| Place of origin, n (%) | ||||
| Spain | 7446 (90.55) | 31,555 (93.02) | 66,075 (89.60) | 62,315 (90.26) |
| Americas | 281 (3.42) | 995 (2.93) | 3022 (4.10) | 2915 (4.22) |
| Asia/Oceania | 114 (1.39) | 269 (0.79) | 963 (1.31) | 758 (1.10) |
| European outside Spain | 117 (1.42) | 561 (1.65) | 1621 (2.20) | 1555 (2.25) |
| Africa | 265 (3.22) | 541 (1.59) | 2060 (2.79) | 1500 (2.17) |
| Body mass index, mean ± SD | 29.53 ± 5.56 | 28.68 ± 5.09 | 29.31 ± 5.11 | 28.70 ± 4.87 |
| New prescriptions a, n (%) | ||||
| ≥1 medications | 1487 (18.08) | 6251 (18.43) | 14,225 (19.29) | 13,218 (19.14) |
| ≥1 cardiovascular/diabetes medications | 289 (3.51) | 1030 (3.04) | 1606 (2.18) | 2095 (3.03) |
| Number of visits a, mean ± SD | ||||
| Visits to GP | 8.34 ± 6.68 | 7.05 ± 5.92 | 6.52 ± 5.54 | 5.96 ± 5.01 |
| Visits to nurse | 7.89 ± 9.31 | 4.62 ± 7.73 | 4.43 ± 6.84 | 3.43 ± 6.38 |
| PRESCRIPTIONS; n= 186,357 | Insulin n = 8270 | Antiplatelet n = 34,139 | ACEI n = 74,346 | Statin n = 69,602 |
| Illnesses at the moment of prescription, n (%) | ||||
| Pain | 3658 (44.23) | 16,919 (49.56) | 34,696 (46.67) | 31,389 (45.10) |
| Respiratory | 1145 (13.85) | 4530 (13.27) | 8174 (10.99) | 6331 (9.10) |
| Physical disability b | 3015 (36.46) | 12,261 (35.91) | 21,526 (28.95) | 16,410 (23.58) |
| Cardiovascular | ||||
| Hypertension | 5477 (66.23) | 20,996 (61.50) | 55,916 (75.21) | 32,799 (47.12) |
| Dyslipidemia | 3206 (38.77) | 10,922 (31.99) | 21,998 (29.59) | 31,451 (45.19) |
| Recent CVD (≤6 months) | 196 (2.37) | 2697 (7.90) | 1949 (2.62) | 1948 (2.80) |
| Established CVD (>6 months) | 2098 (25.37) | 6372 (18.66) | 9333 (12.55) | 6685 (9.60) |
| Alcohol and tobacco use c | 2272 (27.47) | 8456 (24.77) | 18,912 (25.44) | 19,613 (28.18) |
| Neurological | 1057 (12.78) | 3529 (10.34) | 7317 (9.84) | 7024 (10.09) |
| Mental disorders | 2192 (26.51) | 9456 (27.70) | 19,589 (26.35) | 19,724 (28.34) |
| Diabetes (1 and 2) | 7698 (93.08) | 8967 (26.27) | 15,220 (20.47) | 14,755 (21.20) |
| Digestive system disorder | 1671 (20.21) | 7638 (22.37) | 14,329 (19.27) | 12,498 (17.96) |
| Urticaria/allergy | 145 (1.75) | 786 (2.30) | 1705 (2.29) | 1652 (2.37) |
| Hyper/hypothyroidism | 745 (9.01) | 2970 (8.70) | 6034 (8.12) | 6392 (9.18) |
| Number of comorbidities d, mean ± SD | 3.43 ± 1.47 | 2.79 ± 1.48 | 2.61 ± 1.43 | 2.47 ± 1.42 |
| PESCRIBER; n = 4995 | Insulin n = 3125 | Antiplatelet n = 4272 | ACEI n = 4612 | Statin n = 4541 |
| Sex, female, n (%) | 2025 (64.80) | 2867 (67.11) | 3118 (67.61) | 3043 (67.01) |
| Age, mean ± SD | 48.17 ± 9.98 | 47.06 ± 10.53 | 46.51 ± 10.68 | 46.64 ± 10.68 |
| Type of prescriber, n (%) | ||||
| Assigned GP | 2902 (92.86) | 3716 (86.99) | 3884 (84.22) | 3858 (84.96) |
| Substitute/resident GP | 223 (7.14) | 556 (13.01) | 728 (15.78) | 683 (15.04) |
| CENTER; n = 287 | Insulin n = 283 | Antiplatelet n = 285 | ACEI n = 285 | Statin n = 286 |
| Teaching centers, n (%) | 72 (25.44) | 72 (25.26) | 72 (25.26) | 72 (25.17) |
| Pharmacological Subgroup (ATC Code) | Non-Initiation after 3 Months | Non-Initiation after 6 Months | Single Dispensing |
|---|---|---|---|
| Insulin (A10AE) | 643 (7.78) | 507 (6.13) | 1207 (14.59) |
| Antiplatelet (B01AC) | 3111 (9.11) | 2685 (7.86) | 4639 (13.59) |
| ACEI (C09AA) | 4210 (5.66) | 3678 (4.95) | 13,691 (18.42) |
| Statin (C10AA) | 4693 (6.74) | 4056 (5.83) | 7362 (10.58) |
| Insulina n = 8223 | Antiplateletb n = 33,921 | ACEIc n = 73,741 | Statind n = 69,043 | |||||
|---|---|---|---|---|---|---|---|---|
| OR 95% CI | p-Value | OR 95% CI | p-Value | OR 95% CI | p-Value | OR 95% CI | p-Value | |
| Constant | 0.458 (0.25;0.84) | 0.012 | 0.531 (0.39;0.72) | 0.001 | 0.145 (0.12;0.18) | 0.001 | 0.326 (0.25;0.42) | 0.001 |
| Female patient (vs. male) | 0.994 (0.84;1.18) | 0.950 | — | — | — | — | 0.994 (0.93;1.06) | 0.845 |
| Patient’s age (cont.) | 0.998 (0.99;1.00) | 0.492 | 0.987 (0.98;0.99) | 0.001 | — | — | 0.988 (0.98;0.99) | 0.001 |
| Patient’s SESa | ||||||||
| Urban 1 (lowest SES) | Ref. | Ref. | Ref. | Ref. | ||||
| Urban 2 | 0.842 (0.63;1.13) | 0.255 | 1.024 (0.89;1.17) | 0.730 | 0.812 (0.73;0.91) | 0.001 | 0.807 (0.73;0.89) | 0.001 |
| Urban 3 | 0.741 (0.55;1.00) | 0.052 | 0.982 (0.85;1.13) | 0.803 | 0.762 (0.68;0.85) | 0.001 | 0.768 (0.69;0.85) | 0.001 |
| Urban 4 | 0.763 (0.56;1.03) | 0.079 | 0.898 (0.78;1.04) | 0.150 | 0.683 (0.61;0.77) | 0.001 | 0.703 (0.63;0.78) | 0.001 |
| Urban 5 (highest SES) | 0.830 (0.62;1.12) | 0.216 | 0.931 (0.80;1.08) | 0.361 | 0.698 (0.62;0.79) | 0.001 | 0.696 (0.62;0.78) | 0.001 |
| Rural | 0.887 (0.66;1.19) | 0.422 | 1.151 (1.00;1.32) | 0.049 | 0.798 (0.71;0.89) | 0.001 | 0.798 (0.72;0.89) | 0.001 |
| Patient’s place of origin | ||||||||
| Spain | Ref. | Ref. | Ref. | Ref. | ||||
| Americas | 1.148 (0.76;1.74) | 0.516 | 1.335 (1.10;1.63) | 0.004 | 1.265 (1.09;1.47) | 0.002 | 1.225 (1.07;1.40) | 0.003 |
| Asia/Oceania | 1.864 (1.08;3.21) | 0.024 | 1.173 (0.81;1.70) | 0.398 | 1.354 (1.05;1.74) | 0.019 | 1.180 (0.91;1.53) | 0.209 |
| Europe outside Spain | 1.238 (0.68;2.26) | 0.488 | 1.632 (1.30;2.06) | 0.001 | 1.192 (0.97;1.46) | 0.087 | 1.134 (0.94;1.36) | 0.178 |
| Africa | 1.267 (0.84;1.91) | 0.261 | 1.143 (0.87;1.51) | 0.345 | 1.234 (1.04;1.48) | 0.025 | 0.910 (0.74;1.12) | 0.375 |
| BMI (cont.) | 0.971 (0.95;0.99) | 0.001 | 0.979 (0.97;0.99) | 0.001 | 0.981 (0.98;0.99) | 0.001 | 0.991 (0.99;1.00) | 0.009 |
| ≥1New general prescriptions a(vs. none) | 0.756 (0.60;0.96) | 0.022 | 0.919 (0.83;1.02) | 0.119 | — | — | 0.848 (0.78;0.93) | 0.001 |
| ≥1 CV/diabetes new prescriptionsa (vs. none) | — | — | — | — | — | — | 1.028 (0.83;1.27) | 0.794 |
| Number of visitsa (cont.) | ||||||||
| Visits to GP | 0.995 (0.98;1.01) | 0.580 | 0.978 (0.97;0.99) | 0.001 | — | — | 0.982 (0.97;0.99) | 0.001 |
| Visits to nurse | 0.985 (0.97;1.00) | 0.030 | 0.993 (0.99;1.00) | 0.052 | — | — | 0.998 (0.99;1.00) | 0.543 |
| Active illnesses | ||||||||
| Pain | 0.909 (0.76;1.09) | 0.305 | 1.000 (0.92;1.08) | 0.993 | 0.850 (0.80;0.91) | 0.001 | 0.919 (0.86;0.98) | 0.011 |
| Respiratory | — | — | 0.950 (0.84;1.07) | 0.401 | — | — | 0.963 (0.86;1.08) | 0.510 |
| Physical disability b | — | — | 0.873 (0.80;0.96) | 0.003 | — | — | 0.925 (0.85;1.00) | 0.058 |
| Cardiovascular conditions | ||||||||
| Hypertension | 0.751 (0.62;0.91) | 0.004 | 0.813 (0.75;0.88) | 0.001 | 0.800 (0.75;0.86) | 0.001 | 0.767 (0.72;0.82) | 0.001 |
| Dyslipidemia | 0.723 (0.60;0.87) | 0.001 | 0.856 (0.78;0.93) | 0.001 | 0.859 (0.80;0.92) | 0.001 | — | — |
| Recent CVD (≤ 6 months) | — | — | 0.909 (0.77;1.07) | 0.256 | 1.268 (1.05;1.53) | 0.014 | 0.625 (0.49;0.79) | 0.001 |
| Established CVD (>6 months) | — | — | 3.155 (2.90;3.44) | 0.001 | 2.265 (2.09;2.45) | 0.001 | 1.504 (1.36;1.66) | 0.001 |
| Mental disorders | 0.801 (0.65;0.99) | 0.041 | 0.839 (0.77;0.92) | 0.001 | 0.969 (0.90;1.04) | 0.400 | — | — |
| Neurological | — | — | — | — | — | — | — | — |
| Diabetes (1 and 2) | — | — | 0.817 (0.74;0.90) | 0.001 | 0.871 (0.80;0.95) | 0.001 | 1.000 (0.92;1.08) | 0.994 |
| Digestive system disorder | 0.812 (0.64;1.02) | 0.078 | 0.980 (0.89;1.08) | 0.684 | — | — | 0.917 (0.84;1.00) | 0.046 |
| Urticaria/allergy | 2.110 (1.27;3.5) | 0.004 | — | — | — | — | — | — |
| Hyper/hypothyroidism | — | — | 0.939 (0.81;1.08) | 0.389 | — | — | 0.927 (0.83;1.04) | 0.182 |
| Substitute/resident GP (vs. other) | — | — | 0.805 (0.66;0.99) | 0.036 | 1.212 (1.05;1.40) | 0.009 | 1.204 (1.05;1.38) | 0.006 |
| Insulin a n = 8223 | Antiplatelet b n = 33,921 | ACEI c n = 73,741 | Statin d n = 69,043 | |||||
|---|---|---|---|---|---|---|---|---|
| OR 95% CI | p-Value | OR 95% CI | p-Value | OR 95% CI | p-Value | OR 95% CI | p-Value | |
| Constant | 0.378 (0.26;0.53) | 0.001 | 1.048 (0.82;1.34) | 0.714 | 0.512 (0.46;0.56) | 0.001 | 0.502 (0.41;0.61) | 0.001 |
| Female patient (vs. male) | — | — | 1.316 (1.23;1.41) | 0.001 | 1.195 (1.15;1.24) | 0.001 | — | — |
| Patient’s age (cont.) | — | — | 0.979 (0.98;0.98) | 0.001 | 0.991 (0.99;0.99) | 0.001 | 0.985 (0.98;0.99) | 0.001 |
| Patient’s place of origin | — | — | — | — | — | — | — | — |
| Spain | Ref. | Ref. | Ref. | Ref. | ||||
| Americas | 1.264 (0.92;1.75) | 0.155 | 1.257 (1.06;1.49) | 0.008 | 1.179 (1.08;1.29) | 0.001 | 1.441 (1.30;1.60) | 0.001 |
| Asia/Oceania | 1.570 (0.97;2.54) | 0.066 | 1.562 (1.16;2.11) | 0.004 | 1.398 (1.20;1.63) | 0.001 | 1.258 (1.02;1.55) | 0.032 |
| Europe outside Spain | 1.388 (0.86;2.24) | 0.179 | 1.321 (1.05;1.66) | 0.016 | 1.434 (1.28;1.61) | 0.001 | 1.522 (1.32;1.75) | 0.001 |
| Africa | 1.756 (1.30;2.38) | 0.001 | 1.312 (1.05;1.63) | 0.015 | 1.095 (0.98;1.22) | 0.108 | 1.218 (1.05;1.42) | 0.010 |
| BMI (cont.) | 0.980 (0.97;0.99) | 0.001 | 0.997 (0.99;1.00) | 0.286 | — | — | 0.993 (0.99;1.00) | 0.011 |
| ≥1 new general prescriptionsa(vs. none) | 1.223 (1.05;1.43) | 0.011 | 1.229 (1.14;1.33) | 0.001 | 0.895 (0.78;1.03) | 0.114 | — | — |
| Number of visits a (cont.) | ||||||||
| Visits to GP | — | — | — | — | — | — | 0.992 (0.99;1.00) | 0.007 |
| Visits to nurse | — | — | — | — | — | — | 0.997 (0.99;1.00) | 0.218 |
| Active illnesses | ||||||||
| Pain | 0.897 (0.79;1.02) | 0.096 | — | — | — | — | 1.056 (1.00;1.11) | 0.040 |
| Respiratory | — | — | 0.990 (0.90;1.09) | 0.839 | — | — | 0.970 (0.89;1.06) | 0.507 |
| Physical disability b | — | — | 1.002 (0.93;1.08) | 0.955 | 1.042 (1.00;1.09) | 0.077 | 0.956 (0.90;1.02) | 0.175 |
| Cardiovascular conditions | ||||||||
| Hypertension | 0.879 (0.77;1.01) | 0.062 | 0.773 (0.72;0.83) | 0.001 | 0.751 (0.72;0.78) | 0.001 | 0.784 (0.74;0.83) | 0.001 |
| Dyslipidemia | 0.825 (0.72;0.94) | 0.004 | 0.869 (0.81;0.93) | 0.001 | 0.895 (0.86;0.94) | 0.001 | — | — |
| Recent CVD (≤6 months) | — | — | 0.558 (0.49;0.64) | 0.001 | 0.861 (0.76;0.98) | 0.022 | 0.709 (0.59;0.84) | 0.001 |
| Established CVD (>6 months) | — | — | 0.680 (0.61;0.75) | 0.001 | 1.058 (0.99;1.13) | 0.081 | 1.050 (0.96;1.15) | 0.310 |
| Mental disorders | — | — | 0.847 (0.79;0.91) | 0.001 | — | — | — | — |
| Diabetes (1 and 2) | — | — | 0.658 (0.61;0.71) | 0.001 | 0.735 (0.70;0.77) | 0.001 | 0.826 (0.77;0.89) | 0.001 |
| Digestive system disorder | — | — | 1.025 (0.95;1.11) | 0.550 | — | — | 0.973 (0.91;1.04) | 0.423 |
| Urticaria/allergy | — | — | — | — | 1.157 (1.03;1.30) | 0.017 | — | — |
| Female GP (vs. male) | — | — | — | — | — | — | 0.876 (0.83;0.92) | 0.001 |
| Substitute/resident GP (vs. other) | — | — | 1.282 (1.11;1.48) | 0.001 | 1.320 (1.21;1.44) | 0.001 | 1.312 (1.18;1.46) | 0.001 |
| Teaching PC center (vs. regular) | — | — | 0.901 (0.83;0.98) | 0.015 | — | — | 0.888 (0.83;0.95) | 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilaplana-Carnerero, C.; Aznar-Lou, I.; Peñarrubia-María, M.T.; Serrano-Blanco, A.; Fernández-Vergel, R.; Petitbò-Antúnez, D.; Gil-Girbau, M.; March-Pujol, M.; Mendive, J.M.; Sánchez-Viñas, A.; et al. Initiation and Single Dispensing in Cardiovascular and Insulin Medications: Prevalence and Explanatory Factors. Int. J. Environ. Res. Public Health 2020, 17, 3358. https://doi.org/10.3390/ijerph17103358
Vilaplana-Carnerero C, Aznar-Lou I, Peñarrubia-María MT, Serrano-Blanco A, Fernández-Vergel R, Petitbò-Antúnez D, Gil-Girbau M, March-Pujol M, Mendive JM, Sánchez-Viñas A, et al. Initiation and Single Dispensing in Cardiovascular and Insulin Medications: Prevalence and Explanatory Factors. International Journal of Environmental Research and Public Health. 2020; 17(10):3358. https://doi.org/10.3390/ijerph17103358
Chicago/Turabian StyleVilaplana-Carnerero, Carles, Ignacio Aznar-Lou, María Teresa Peñarrubia-María, Antoni Serrano-Blanco, Rita Fernández-Vergel, Dolors Petitbò-Antúnez, Montserrat Gil-Girbau, Marian March-Pujol, Juan Manuel Mendive, Alba Sánchez-Viñas, and et al. 2020. "Initiation and Single Dispensing in Cardiovascular and Insulin Medications: Prevalence and Explanatory Factors" International Journal of Environmental Research and Public Health 17, no. 10: 3358. https://doi.org/10.3390/ijerph17103358
APA StyleVilaplana-Carnerero, C., Aznar-Lou, I., Peñarrubia-María, M. T., Serrano-Blanco, A., Fernández-Vergel, R., Petitbò-Antúnez, D., Gil-Girbau, M., March-Pujol, M., Mendive, J. M., Sánchez-Viñas, A., Carbonell-Duacastella, C., & Rubio-Valera, M. (2020). Initiation and Single Dispensing in Cardiovascular and Insulin Medications: Prevalence and Explanatory Factors. International Journal of Environmental Research and Public Health, 17(10), 3358. https://doi.org/10.3390/ijerph17103358






