Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona itama) Honey Collected across Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Isolation of Bacterial Strains from Stingless Bee Honey
2.3. Bacterial Strains and Growth Conditions
2.4. Identification of Bacteria Using Molecular Technique
Genomic DNA Extraction, 16S rRNA Amplification, and Gene Analysis
2.5. Antimicrobial Activity Assessment
2.6. Screening for Probiotic Properties
2.6.1. Acid and Bile Tolerance
2.6.2. Hydrophobicity
2.6.3. Autoaggregation
2.7. Safety Assessment
2.7.1. Antibiotic Susceptibility
2.7.2. Blood Hemolysis
3. Results
3.1. Isolation and Preliminary Detection of Bacillus Isolates
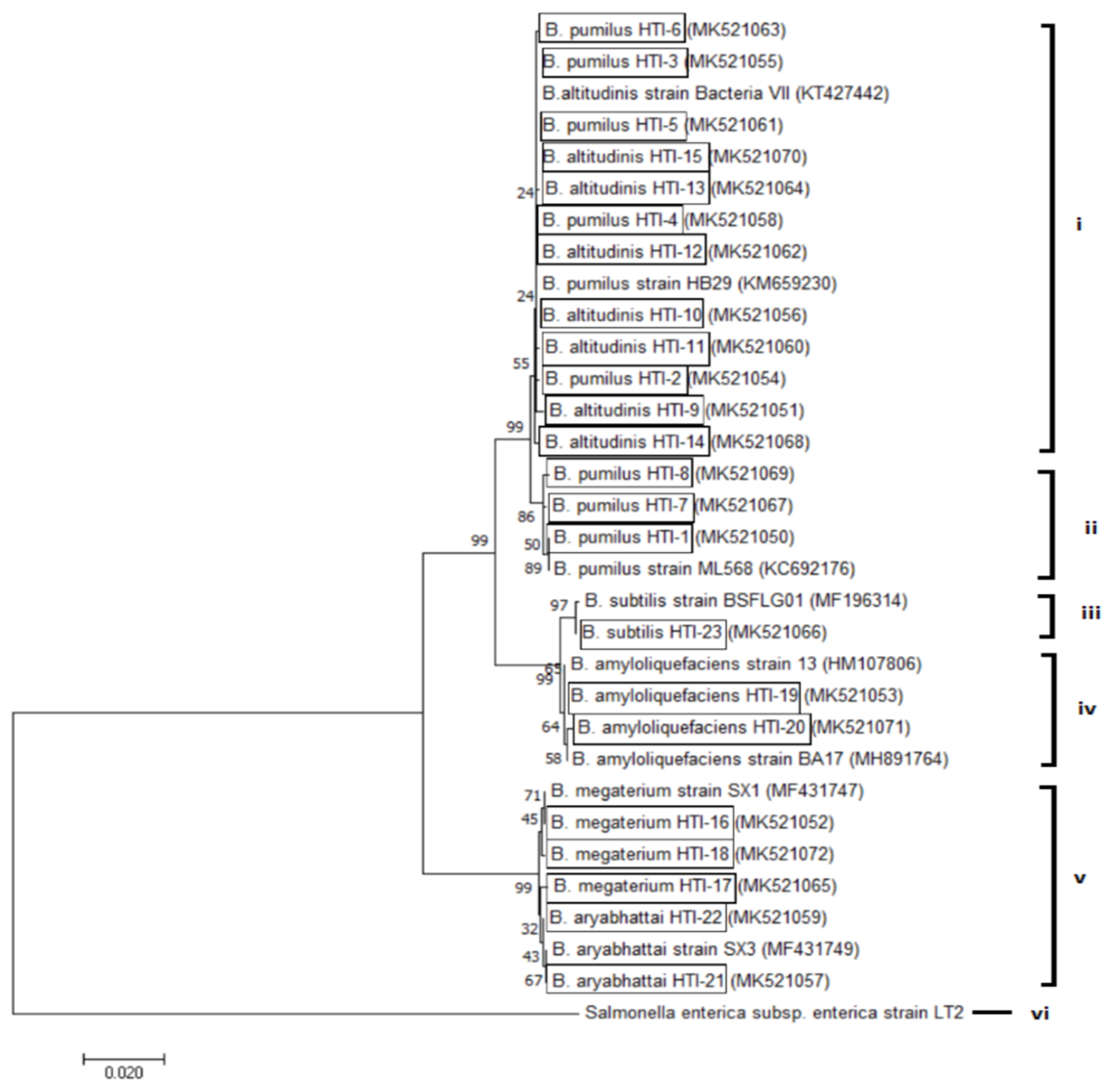
3.2. Molecular Identification through 16S rRNA Gene Sequence Analysis
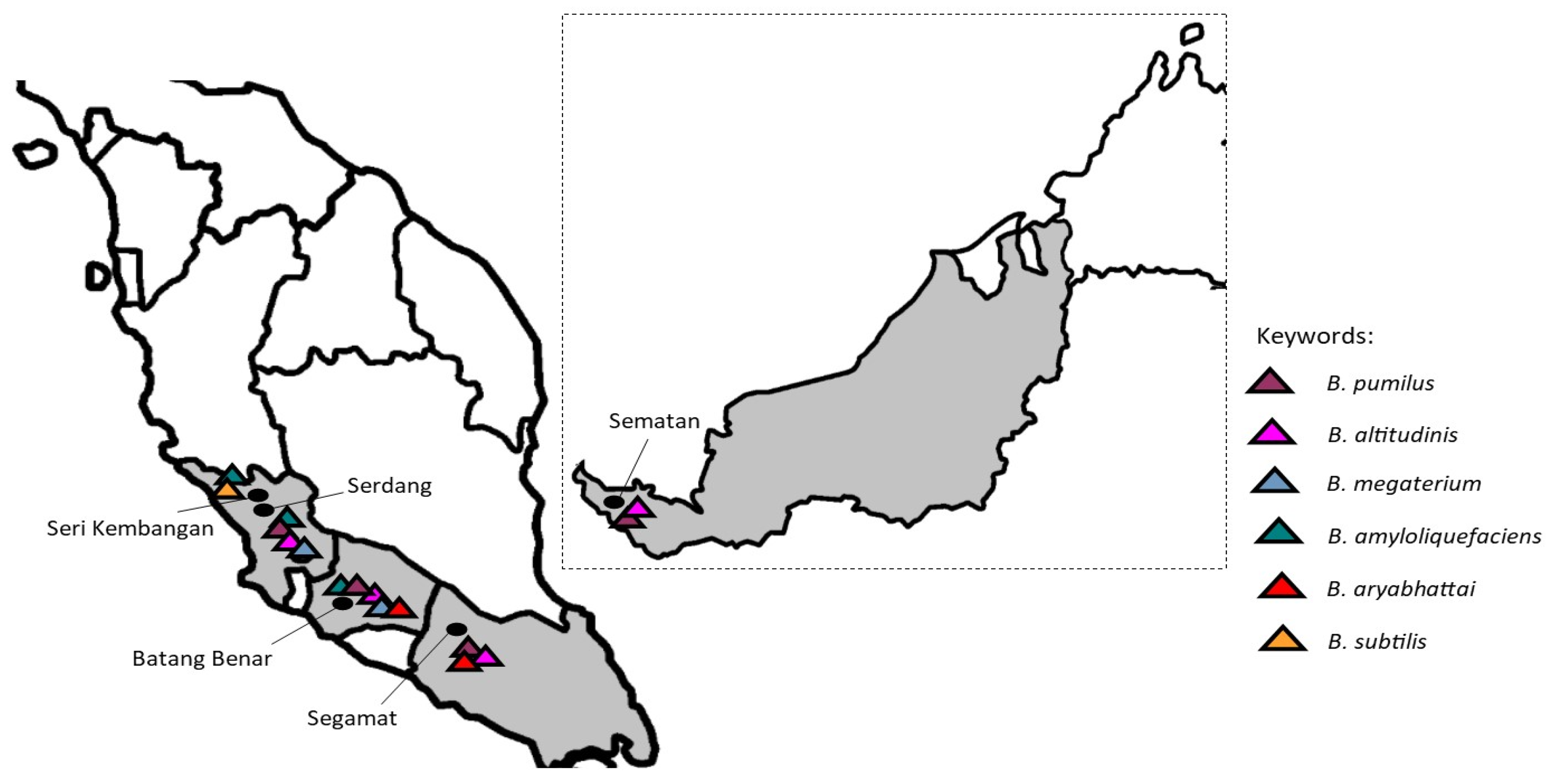
3.3. Distribution of the Bacillus Species Isolated from Stingless Bee Honey from Different Geographical Locations
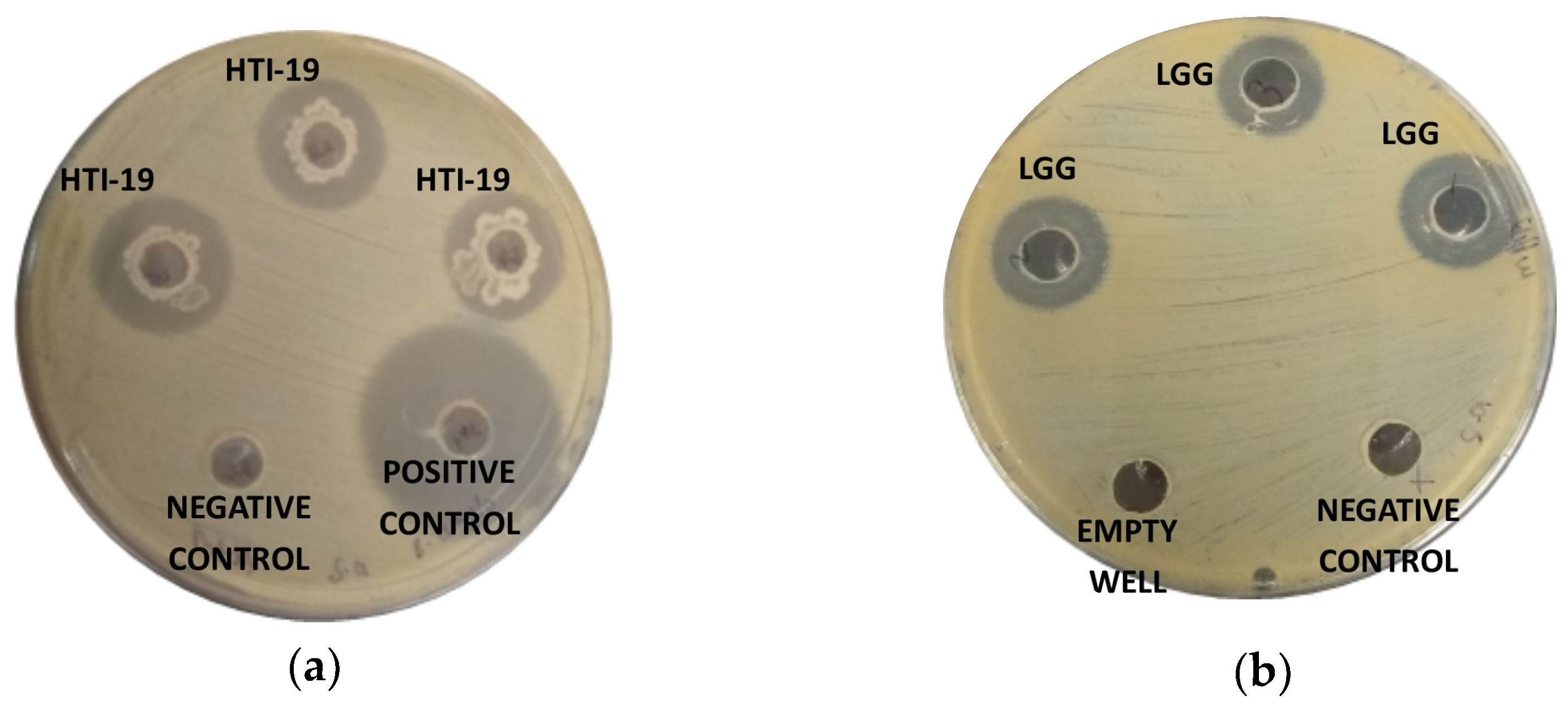
3.4. Antimicrobial Test against Pathogenic Bacteria
3.5. Tolerance to Acidic Conditions and Bile Salts
3.6. Cell Adhesion Activity of Bacillus Species
3.7. Antibiotic Susceptibility and Hemolytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, S.; Duwal, R.K.; Lee, W. Diversity of stingless bees (Hymenoptera, Apidae, Meliponini) from Cambodia and Laos. J. Asia-Pac. Entomol. 2016, 19, 947–961. [Google Scholar] [CrossRef]
- Codex. Codex Alimentarius Commission Standards; CODEX Stand. HONEY: Rome, Italy, 2001. [Google Scholar]
- Amin, F.A.Z.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar]
- Lage, L.G.; Coelho, L.L.; Resende, H.C.; Tavares, M.G.; Campos, L.A.; Fernandes-Salomão, T.M. Honey physicochemical properties of three species of the Brazilian Melipona. Anais Academia Brasileira Ciências 2012, 84, 605–608. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Z.S.Z.; Ismail, M.; Chan, K.W.; Ooi, D.J.; Ismail, N.; Zawawi, N.; Mohd Esa, N. Comparison of Sugar Content, Mineral Elements and Antioxidant Properties of Heterotrigona Itama Honey from Suburban and Forest in Malaysia. Malays. J. Med. Health Sci. 2019, 15, 104–112. [Google Scholar]
- Nascimento, A.; Marchini, L.; Carvalho, C.; Araújo, D.; Olinda, R.; Silveira, T. Physical-Chemical Parameters of Honey of Stingless Bee (Hymenoptera: Apidae). Am. Chem. Sci. J. 2015, 7, 139–149. [Google Scholar] [CrossRef]
- Esawy, M.A.; Awad, G.E.A.; Ahmed, E.F.; Danial, E.N.; Mansour, N.M. Evaluation of Honey as a New Reservoir for Probiotic Bacteria. Adv. Food Sci. 2012, 34, 72–81. [Google Scholar]
- Hasali, N.H.M.; Zamri, A.I.; Lani, M.N.; Mubarak, A.; Suhaili, Z. Identification of Lactic Acid Bacteria from Meliponine Honey and Their Antimicrobial Activity against Pathogenic Bacteria. Am. J. Sustain. Agric. 2015, 9, 1–6. [Google Scholar]
- Begum, S.B.; Roobia, R.R.; Karthikeyan, M.; Murugappan, R. Validation of nutraceutical properties of honey and probiotic potential of its innate microflora. LWT 2015, 60, 743–750. [Google Scholar] [CrossRef]
- Sabaté, D.C.; Carrillo, L.; Audisio, M.C. Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res. Microbiol. 2009, 160, 193–199. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Rahman, R.N.Z.R.A.; Yusof, M.T.; Syahir, A.; Sabri, S. Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis. PeerJ 2019, 7, e7478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajor, M.; Worobo, R.W.; Milewski, S.; Szweda, P. The Antimicrobial Potential of Bacteria Isolated from Honey Samples Produced in the Apiaries Located in Pomeranian Voivodeship in Northern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutting, S.M. Bacillus Probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Agbagwa, O.; Otokunefor, T.; Frank-Peterside, N. Preliminary Detection of Bacillus species in Commercial Honey. Br. Microbiol. Res. J. 2014, 4, 1370–1380. [Google Scholar] [CrossRef]
- Isolauri, E.; Salminen, S.; Ouwehand, A. Microbial-Gut Interactions in Health and Disease. Probiotics. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 299–313. [Google Scholar] [CrossRef]
- Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO/WHO: Ontario, Canada; London, UK, 2002.
- Zhou, S.; Song, D.; Zhou, X.; Mao, X.; Zhou, X.; Wang, S.; Wei, J.; Huang, Y.; Wang, W.; Xiao, S.M.; et al. Characterization of Bacillus subtilis from Gastrointestinal Tract of Hybrid Hulong Grouper (Epinephelus Fuscoguttatus × E. Lanceolatus) and Its Effects as Probiotic Additives. Fish Shellfish Immunol. 2019, 84, 1115–1124. [Google Scholar] [CrossRef]
- Jeon, H.-L.; Yang, S.-J.; Son, S.-H.; Kim, W.-S.; Lee, N.-K.; Paik, H.-D. Evaluation of probiotic Bacillus subtilis P229 isolated from cheonggukjang and its application in soybean fermentation. LWT 2018, 97, 94–99. [Google Scholar] [CrossRef]
- Quigley, E.M. Prebiotics and probiotics; modifying and mining the microbiota. Pharmacol. Res. 2010, 61, 213–218. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. Gut Microbiota: Mining for Therapeutic Potential. Clin. Gastroenterol. Hepatol. 2007, 5, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Li, Y.-Q.; Zuo, X.-L.; Zhen, Y.-B.; Yang, J.; Liu, C.-H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Ahire, J.J.; Pawar, S.P.; Chaudhari, B.L.; Chincholkar, S.B. Comparative Accounts of Probiotic Characteristics of Bacillus spp. Isolated from Food Wastes. Food Res. Int. 2009, 42, 505–510. [Google Scholar] [CrossRef]
- Tajabadi, N.; Mardan, M.; Manap, M.Y.A.; Shuhaimi, M.; Meimandipour, A.; Nateghi, L. Detection and identification of Lactobacillus bacteria found in the honey stomach of the giant honeybee Apis dorsata. Apidologie 2011, 42, 642–649. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Soran, H.; Beyatli, Y. Antimicrobial Activities of Some Bacillus spp. Strains Isolated from the Soil. Microbiol. Res. 2006, 161, 127–131. [Google Scholar] [CrossRef]
- Klingberg, T.D.; Axelsson, L.; Naterstad, K.; Elsser, D.; Budde, B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005, 105, 419–431. [Google Scholar] [CrossRef]
- Kos, B.; Šušković, J.; Vuković, S.; Sǐmpraga, M.; Frece, J.; Matošić, S. Adhesion and Aggregation Ability of Probiotic Strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef]
- Anand, C.; Gordon, R.; Shaw, H.; Fonseca, K.; Olsen, M. Pig and Goat Blood as Substitutes for Sheep Blood in Blood-Supplemented Agar Media. J. Clin. Microbiol. 2000, 38, 591–594. [Google Scholar]
- Iurlina, M.O.; Fritz, R. Characterization of microorganisms in Argentinean honeys from different sources. Int. J. Food Microbiol. 2005, 105, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wang, L.; Jin, Y.; Zhang, J.; Su, L.; Zhang, X.; Zhou, J.; Li, Y. The Microbial Community Dynamics during the Vitex Honey Ripening Process in the Honeycomb. Front. Microbiol. 2017, 8, 1649. [Google Scholar] [CrossRef]
- Sakandar, H.A.; Kubow, S.; Sadiq, F.A. Isolation and in-vitro probiotic characterization of fructophilic lactic acid bacteria from Chinese fruits and flowers. LWT 2019, 104, 70–75. [Google Scholar] [CrossRef]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Combey, R. Microbial and Qualitative Analyses of Stingless Bee Bread Using Dry Preservation Methods. Eur. J. Zool. Res. 2017, 5, 45–50. [Google Scholar]
- Różańska, H. Microbiological Quality of Polish Honey. Bull. Vet. Inst. Pulawy 2011, 55, 443–445. [Google Scholar]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, W.-Z.; Xu, H.; Wang, Z.-W.; He, S.-Y. Bacillus in the guts of honey bees (Apis mellifera; Hymenoptera: Apidae) mediate changes in amylase values. Eur. J. Entomol. 2015, 112, 619–624. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Lv, X.-C.; Jia, R.-B.; Li, Y.; Chen, F.; Chen, Z.-C.; Liu, B.; Chen, S.-J.; Rao, P.-F.; Ni, L. Characterization of the dominant bacterial communities of traditional fermentation starters for Hong Qu glutinous rice wine by means of MALDI-TOF mass spectrometry fingerprinting, 16S rRNA gene sequencing and species-specific PCRs. Food Control 2016, 67, 292–302. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Jin, Y.-I.; Jeong, J.-C.; Chang, Y.H.; Lee, Y.; Jeong, Y.; Kim, M. Probiotic characteristics of Bacillus strains isolated from Korean traditional soy sauce. LWT 2017, 79, 518–524. [Google Scholar] [CrossRef]
- Kpikpi, E.N.; Thorsen, L.; Glover, R.; Dzogbefia, V.P.; Jespersen, L. Identification of Bacillus species occurring in Kantong, an acid fermented seed condiment produced in Ghana. Int. J. Food Microbiol. 2014, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sinacori, M.; Settanni, L.; Sannino, C.; Francesca, N.; Moschetti, G.; Cruciata, M.; Alfonzo, A. Cultivable Microorganisms Associated with Honeys of Different Geographical and Botanical Origin. Food Microbiol. 2013, 38, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashim, N.A.; Bahri, A.R.S.; Basari, N.; Sharudin, N.H. Mass Infestation of Black Soldier Fly Hermetia Illucens (Diptera: Stratiomyidae) on Colonies of the Indo-Malayan Stingless Bees Geniotrigona thoracica and Heterotrigona itama. J. Biodivers. Environ. Sci. 2017, 11, 9–15. [Google Scholar]
- Aizenberg-Gershtein, Y.; Izhaki, I.; Halpern, M. Do Honeybees Shape the Bacterial Community Composition in Floral Nectar? PLoS ONE 2013, 8, e67556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borriss, R.; Chen, X.H.; Rueckert, C.; Blom, J.; Becker, A.; Baumgarth, B.; Fan, B.; Pukall, R.; Schumann, P.; Spröer, C.; et al. Relationship of Bacillus amyloliquefaciens Clades Associated with Strains DSM 7 T and FZB42 T: A Proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. Nov. and Bacillus amyloliquefaciens subsp. Plantarum subsp. Nov. Based on Complete Gen. Int. J. Syst. Evol. Microbiol. 2011, 61, 1786–1801. [Google Scholar]
- Syed Yaacob, S.N.; Huyop, F.; Kamarulzaman Raja Ibrahim, R.; Wahab, R.A. Identification of Lactobacillus spp. and Fructobacillus spp. Isolated from Fresh Heterotrigona Itama Honey and Their Antagonistic Activities against Clinical Pathogenic Bacteria. J. Apic Res. 2018, 57, 395–405. [Google Scholar] [CrossRef]
- Manhar, A.K.; Saikia, D.; Bashir, Y.; Mech, R.K.; Nath, D.; Konwar, B.K.; Mandal, M. In Vitro Evaluation of Celluloytic Bacillus amyloliquefaciens AMS1 Isolated from Traditional Fermented Soybean (Churpi) as an Animal Probiotic. Res. Vet. Sci. 2015, 99, 149–156. [Google Scholar] [CrossRef]
- Athukorala, S.N.; Rashid, K.Y.; Fernando, W.G.D. Identification of antifungal antibiotics of Bacillus species isolated from different microhabitats using polymerase chain reaction and MALDI-TOF mass spectrometry. Can. J. Microbiol. 2009, 55, 1021–1032. [Google Scholar] [CrossRef]
- Ajilogba, C.F. Antagonistic Effects of Bacillus Species in Biocontrol of Tomato Fusarium Wilt. Stud. Ethno-Med. 2013, 7, 205–216. [Google Scholar] [CrossRef]
- El-Mabrok, A.S.W.; Hassan, Z.; Mokhtar, A.M.; Hussain, K.M.A.; Kahar, F.K.S.B.A. Screening of Lactic Acid Bacteria as Biocontrol against (Colletotrichum Capsici) on Chilli Bangi. Res. J. Appl. Sci. 2012, 7, 446–473. [Google Scholar]
- Grover, M.; Nain, L.; Saxena, A.K. Comparision between Bacillus subtilis RP24 and its antibiotic-defective mutants. World J. Microbiol. Biotechnol. 2009, 25, 1329–1335. [Google Scholar] [CrossRef]
- Jena, P.K.; Trivedi, D.; Thakore, K.; Chaudhary, H.; Giri, S.S.; Seshadri, S. Isolation and Characterization of Probiotic Properties of Lactobacilli Isolated from Rat Fecal Microbiota. Microbiol. Immunol. 2013, 57, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Zhang, L.; Liu, W.; Zhang, Y.; Zhang, X.; Sun, T. In vitro assessment of probiotic properties of Bacillus isolated from naturally fermented congee from Inner Mongolia of China. World J. Microbiol. Biotechnol. 2010, 26, 1369–1377. [Google Scholar] [CrossRef]
- Botes, M.; Loos, B.; Van Reenen, C.A.; Dicks, L.M.T.; Reenen, C.A. Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch. Microbiol. 2008, 190, 573–584. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and Aggregation Properties of Probiotic and Pathogen Strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Teuber, M.; Meile, L.; Schwarz, F. Acquired antibiotic resistance in lactic acid bacteria from food. Antonie Leeuwenhoek 1999, 76, 115–137. [Google Scholar] [CrossRef]
- Shin, H.-J.; Choi, H.-J.; Kim, D.-W.; Ahn, C.-S.; Lee, Y.-G.; Jeong, Y.-K.; Joo, W.-H. Probiotic Potential of Pediococcus pentosaceus BCNU 9070. J. Life Sci. 2012, 22, 1194–1200. [Google Scholar] [CrossRef]
- Sorokulova, I.B.; Pinchuk, I.V.; Denayrolles, M.; Osipova, I.G.; Huang, J.M.; Cutting, S.M.; Urdaci, M.C. The Safety of Two Bacillus Probiotic Strains for Human Use. Dig. Dis. Sci. 2008, 53, 954–963. [Google Scholar] [CrossRef]
| Geographical Location | CFU/g | No. of Selected Isolates | Gram-Staining | Catalase Test | Tolerance to 7% NaCl | ||||
|---|---|---|---|---|---|---|---|---|---|
| Gram + ve | Gram − ve | Bacilli | Cocci | +ve | −ve | ||||
| Batang Benar, Negeri Sembilan | 2.2 × 102 | 17 | 17 | - | 17 | - | All isolates exhibited positive results for catalase test | 6 | |
| Segamat, Johor | 2.4 × 102 | 23 | 21 | 2 | 23 | - | 9 | ||
| Seri Kembangan, Selangor | 1.1 × 101 | 3 | 3 | - | 3 | - | 2 | ||
| Sematan, Sarawak | 9.7 × 100 | 4 | 3 | 1 | 3 | 1 | 3 | 2 | 2 |
| Serdang, Selangor | 3.7 × 102 | 11 | 11 | - | 11 | - | 6 | 5 | 4 |
| TOTAL | 23 | ||||||||
| Bacterial Isolates | Inhibition Zones against Pathogenic Bacteria, mm | |||||
|---|---|---|---|---|---|---|
| Staphlococcus aureus | Bacillus cereus | Salmonella thyphimurium | Escheria coli | Klebsiella pneumonia | Pseudomonas aeruginosa | |
| B. pumilus HTI-1 | +++ | NI | NI | NI | NI | NI |
| B. pumilus HTI-2 | +++ | NI | NI | NI | NI | NI |
| B. pumilus HTI-3 | NI | NI | NI | NI | NI | NI |
| B. pumilus HTI-4 | ++ | NI | NI | NI | + | NI |
| B. pumilus HTI-5 | ++ | NI | NI | NI | NI | NI |
| B. pumilus HTI-6 | +++ | NI | NI | NI | NI | NI |
| B. pumilus HTI-7 | +++ | ++ | NI | NI | ++ | NI |
| B. pumilus HTI-8 | +++ | NI | NI | NI | NI | NI |
| B. altitudinis HTI-11 | NI | NI | NI | NI | NI | ++ |
| B. altitudinis HTI-14 | +++ | NI | NI | NI | NI | NI |
| B. altitudinis HTI-15 | +++ | NI | NI | NI | NI | NI |
| B. megaterium HTI-16 | NI | NI | NI | NI | NI | NI |
| B. megaterium HTI-17 | NI | NI | NI | NI | NI | NI |
| B. megaterium HTI-18 | NI | NI | NI | NI | NI | NI |
| B. amyloliquefaciens HTI-19 | +++ | ++ | ++ | ++ | NI | + |
| B. amyloliquefaciens HTI-20 | +++ | NI | NI | +++ | NI | NI |
| B. aryabhattai HTI-21 | NI | NI | NI | NI | NI | NI |
| B. aryabhattai HTI-22 | NI | NI | NI | NI | NI | NI |
| B. subtilis HTI-23 | + | NI | +++ | NI | ++ | ++ |
| L. rhamnosus GG | +++ | +++ | +++ | +++ | ++ | +++ |
| Tetracycline(20ug/µl) | +++ | +++ | +++ | +++ | +++ | +++ |
| Isolates | Survival Rates, % | |
|---|---|---|
| Acid Tolerance | Bile Tolerance | |
| pH 2.0 | 0.3% | |
| B. amyloliquefaciens HTI-19 | 86.56 a | 129.10 a |
| B. subtilis HTI-23 | 86.72 a | 140.50 b |
| L. rhamnosus GG | 97.46 b | 106.76 c |
| Isolates | Autoaggregation (%) | Hydrophobicity (%) |
|---|---|---|
| B. amyloliquefaciens HTI-19 | 84.13 a | 53.64 a |
| B. subtilis HTI-23 | 57.51 b | 60.82 a |
| L. rhamnosus GG | 69.99 ab | 61.04 a |
| Isolates | Susceptibility to Antibiotics | Hemolytic Activity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMP10 | C30 | CIP5 | E15 | CN10 | K30 | TE30 | TEC30 | VA30 | RD30 | S10 | ||
| B. amyloliquefaciens HTI-19 | S | S | S | S | S | S | S | S | S | S | S | α-hemolytic |
| B. subtilis HTI-23 | S | S | S | S | S | S | S | S | S | S | S | γ-hemolytic |
| L. rhamnosus GG | S | S | S | S | S | S | S | R | R | S | S | γ-hemolytic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkhairi Amin, F.A.; Sabri, S.; Ismail, M.; Chan, K.W.; Ismail, N.; Mohd Esa, N.; Mohd Lila, M.A.; Zawawi, N. Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona itama) Honey Collected across Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 278. https://doi.org/10.3390/ijerph17010278
Zulkhairi Amin FA, Sabri S, Ismail M, Chan KW, Ismail N, Mohd Esa N, Mohd Lila MA, Zawawi N. Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona itama) Honey Collected across Malaysia. International Journal of Environmental Research and Public Health. 2020; 17(1):278. https://doi.org/10.3390/ijerph17010278
Chicago/Turabian StyleZulkhairi Amin, Fatin Aina, Suriana Sabri, Maznah Ismail, Kim Wei Chan, Norsharina Ismail, Norhaizan Mohd Esa, Mohd Azmi Mohd Lila, and Norhasnida Zawawi. 2020. "Probiotic Properties of Bacillus Strains Isolated from Stingless Bee (Heterotrigona itama) Honey Collected across Malaysia" International Journal of Environmental Research and Public Health 17, no. 1: 278. https://doi.org/10.3390/ijerph17010278






