Hard Water, More Elastic Arteries: A Case Study from Krupina District, Slovakia
Abstract
1. Introduction
2. Materials and Methods
2.1. Area Description
2.2. Selection of Respondents
2.3. Measurement of Arterial Stiffness
2.4. Determination of Total Dissolved Solids and Hardness in Drinking Water
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeLoach, S.S.; Townsend, R.R. Vascular stiffness: Its measurements and significance for epidemiologic and outcome studies. Clin. J. Am. Soc. Nephrol. 2008, 3, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Bonarjee, V.V.S. Arterial stiffness: A prognostic marker in coronary heart disease. Available methods and clinical application. Front. Cardiovasc. Med. 2018, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organisation: Geneva, Switzerland, 2011; Available online: https://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ (accessed on 20 December 2018).
- Bhatnagar, A. Environmental cardiology: Studying mechanistic links between pollution and heart disease. Circ. Res. 2006, 99, 692–705. [Google Scholar] [CrossRef]
- Mitchell, E.; Frisbie, S.H.; Sarkar, B. Exposure to multiple metals from groundwater—A global crisis: Geology, climate change, health effects, testing, and mitigation. Metallomics 2011, 3, 874–908. [Google Scholar] [CrossRef] [PubMed]
- Sturchio, E.; Zanellato, M.; Minoia, C.; Bemporad, E. Arsenic: Environmental Contamination and Exposure. In Arsenic: Sources, Environmental Impact, Toxicity and Human Health—A Medical Geology Perspective, 1st ed.; Masotti, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 3–38. [Google Scholar]
- Chang, K.Y.; Wu, I.W.; Huang, B.R.; Juang, J.G.; Wu, J.C.; Chang, S.W.; Chang, C.C. Associations between water quality measures and chronic kidney disease prevalence in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2726. [Google Scholar] [CrossRef]
- Deng, Q.; Chen, L.; Wei, Y.; Li, Y.; Han, X.; Liang, W.; Zhao, Y.; Wang, X.; Yin, J. Understanding the association between environmental factors and longevity in Hechi, China: A drinking water and soil quality perspective. Int. J. Environ. Res. Public Health 2018, 15, 2272. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Ramond, A.; O’Keeffe, L.M.; Shahzad, S.; Kunutsor, S.K.; Muka, T.; Gregson, J.; Willeit, P.; Warmakula, S.; Khan, H.; et al. Environmental toxic metal contaminants and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2018, 362, k3310. [Google Scholar] [CrossRef] [PubMed]
- Bencko, V.; Novák, J.; Suk, M. Health and Natural Conditions (Medicine and geology); Dolin, s.r.o.: Praha, Czech Republic, 2011; p. 398. (In Czech) [Google Scholar]
- NRC. Geochemistry of water in relation to cardiovascular disease. National Research Council, National Academy of Sciences: Washington, DC, USA, 1979; Available online: https://www.nap.edu/read/19844/chapter/1 (accessed on 3 January 2019).
- Kožíšek, F. Health risks from drinking demineralised water. In Nutrients in drinking water; Water, Sanitation and Health Protection and the Human Environment, World Health Organization: Geneva, Switzerland, 2005; pp. 148–163. Available online: https://www.who.int/water_sanitation_health/dwq/nutrientsindw.pdf (accessed on 1 February 2019).
- Rosanoff, A. The high heart health value of drinking-water magnesium. Med. Hypotheses 2013, 81, 1063–1065. [Google Scholar] [CrossRef]
- Davies, B.E. The UK geochemical environment and cardiovascular diseases: Magnesium in food and water. Environ. Geochem. Health 2015, 37, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Rapant, S.; Fajčíková, K.; Cvečková, V.; Ďurža, A.; Stehlíková, B.; Sedláková, D.; Ženišová, Z. Chemical composition of groundwater and relative mortality for cardiovascular diseases in the Slovak Republic. Environ. Geochem. Health 2015, 37, 745–756. [Google Scholar] [CrossRef]
- McLeod, L.; Bharadwaj, L.; Epp, T.Y.; Waldner, C.L. Ecological analysis of associations between groundwater quality and hypertension and cardiovascular disease in rural Saskatchewan, Canada using Bayesian hierarchical models and administrative health data. Environ. Res. 2018, 167, 329–340. [Google Scholar] [CrossRef]
- Wodschow, K.; Hansen, B.; Schullehner, J.; Ersbøll, A.K. Stability of major geogenic cations in drinking water—an issue of public health importance: A Danish study, 1980–2017. Int. J. Environ. Res. Public Health 2018, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Shlezinger, M.; Amital, Y.; Goldenberg, I.; Shechter, M. Desalinated seawater supply and all-cause mortality in hospitalized acute myocardial infarction patients from the Acute Coronary Syndrome Israeli Survey 2002-2013. Int. J. Cardiol. 2016, 220, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Rosborg, I.; Kozisek, F. Macrominerals at optimum concentrations – protective against diseases. In Drinking Water Minerals and Mineral Balance—Importance, Health Significance, Safety Precautions, 1st ed.; Rosborg, I., Ed.; Springer International Publishing Switzerland, Springer Verlag: Berlin, Germany, 2015; pp. 33–52. [Google Scholar]
- WHO. Guidelines for Drinking Water Quality, 4th ed.World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf?sequence=1 (accessed on 20 December 2018).
- Geohealth. Available online: http://www.geology.sk/geohealth/?lang=en (accessed on 23 November 2018).
- Rapant, S.; Cvečková, V.; Dietzová, Z.; Fajčíková, K.; Hiller, E.; Finkelman, R.B.; Škultétyová, S. The potential impact of geological environment on health status of residents of the Slovak Republic. Environ. Geochem. Health 2014, 36, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Rapant, S.; Cvečková, V.; Fajčíková, K.; Sedláková, D.; Stehlíková, B. Impact of calcium and magnesium in groundwater and drinking water on the health of inhabitants of the Slovak Republic. Int. J. Environ. Res. Public Health 2017, 14, 278. [Google Scholar] [CrossRef]
- Rapant, S.; Salminen, R.; Tarvainen, T.; Krčmová, K.; Cvečková, V. Application of a risk assessment method on European wide geochemical baseline data. Geochem. Explor. Environ. Anal. 2008, 8, 291–299. [Google Scholar] [CrossRef]
- International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10)-WHO. Available online: https://icd.who.int/browse10/2016/en#/IX (accessed on 11 April 2019).
- Life for Krupina. Available online: www.geology.sk/lifeforkrupina (accessed on 23 November 2018).
- Shirwany, N.A.; Zou, M.H. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef]
- Illyes, M. A new and fast rapid screening method for measuring complex hemodynamical parameters and arterial stiffness non–invasively with a simple arm cuff. Am. J. Hypertens. 2005, 18, A15. [Google Scholar] [CrossRef][Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Duprez, D.; Starmans-Kool, M.J.; Safar, M.E.; Giannattasio, C.; Cockcroft, J.; Kaiser, D.R.; Thuillez, C. Clinical applications of arterial stiffness, Task Force III: Recommendations for user procedures. Am. J. Hypertens. 2002, 15, 445–452. [Google Scholar] [CrossRef]
- Laurent, S. Hypertension and macrovascular disease. Eur. Soc. Hypertens. Sci. Newslett. 2007, 8. Available online: https://pdfs.semanticscholar.org/23f9/bbf4eab9f1a74ea75501979afd895bcaf1d1.pdf (accessed on 28 April 2019).
- Lenkey, Z.; Illyés, M.; Böcskei, R.; Husznai, R.; Sárszegi, Z.; Meiszterics, Z.; Molnár, F.T.; Hild, G.; Szabadosi, S.; Cziráki, A.; et al. Comparison of arterial stiffness parameters in patients with coronary artery disease and diabetes mellitus using arteriography. Physiol. Res. 2014, 63, 429–437. [Google Scholar]
- TensioMed, The Arteriograph Company. Available online: https://youtu.be/JgLklpdzZ0g (accessed on 28 February 2019).
- The Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar] [CrossRef]
- USEPA. Methods for chemical analysis of water and wastes. United States Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 20460; 1983; Available online: https://www.wbdg.org/FFC/EPA/EPACRIT/epa600_4_79_020.pdf (accessed on 8 April 2019).
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Nakamoto, M. Association between dietary calcium intake and arterial stiffness according to dietary vitamin D intake in men. Br. J. Nutr. 2014, 112, 1333–1340. [Google Scholar] [CrossRef]
- Anderson, J.J.B.; Kruszka, B.; Delaney, J.A.C.; He, K.; Burke, G.L.; Alonso, A.; Bild, D.E.; Budoff, M.; Michos, E.D. Calcium intake from diet and supplements and the risk of coronary artery calcification and its progression among older adults: 10-year follow-up of the multi-ethnic study of atherosclerosis (MESA). J. Am. Heart Assoc. 2016, 5, e003815. [Google Scholar] [CrossRef]
- Hruby, A.; O’Donnell, C.J.; Jacques, P.F.; Meigs, J.B.; Hoffmann, U.; McKeown, N.M. Magnesium intake is inversely associated with coronary artery calcification. The Framingham Heart Study. Jacc Cardiovasc. Imaging 2014, 7, 59–69. [Google Scholar] [CrossRef]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Long-term magnesium supplementation improves arterial stiffness in overweight and obese adults: Results of a randomized, double-blind, placebo-controlled intervention trial. Am. J. Clin. Nutr. 2016, 103, 1260–1266. [Google Scholar] [CrossRef]
- Kostov, K.; Halacheva, L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef]
- Catling, L.A.; Abubakar, I.; Lake, I.R.; Swift, L.; Hunter, P.R. A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness. J. Water Health 2008, 6, 433–442. [Google Scholar] [CrossRef]
- Gianfredi, V.; Bragazzi, N.L.; Nucci, D.; Villarini, M.; Moretti, M. Cardiovascular diseases and hard drinking waters: Implications from a systematic review with meta-analysis of case-control studies. J. Water Health 2016, 15, 31–40. [Google Scholar] [CrossRef]
- Yousefi, M.; Saleh, H.N.; Yaseri, M.; Jalizadeh, M.; Mohammadi, A.A. Association of consumption of excess hard water, body mass index and wait circumference with risk of hypertension in individuals living in hard and soft water areas. Environ. Geochem. Health 2018. [Google Scholar] [CrossRef]
- Momeni, M.; Gharedaghi, Z.; Amin, M.M.; Poursafa, P.; Mansourian, M. Does water hardness have preventive effect on cardiovascular disease? Int. J. Prev. Med. 2014, 5, 159–163. [Google Scholar]
- Rosenlund, M.; Berglina, N.; Hallqvist, J.; Bellander, T.; Bluhm, G. Daily intake of magnesium and calcium from drinking water in relation to myocardial infarction. Epidemiology 2005, 16, 570–576. [Google Scholar] [CrossRef][Green Version]
- Morris, R.W.; Walker, M.; Lennon, L.T.; Shaper, A.G.; Whincup, P.H. Hard drinking water does not protect against cardiovascular disease: New evidence from the British Regional Heart Study. Eur. J. Prev. Cardiol. 2008, 15, 185–189. [Google Scholar] [CrossRef]


| Municipality | Number of Males | Number of Females | All | Ca (mg·L−1) | Mg (mg·L−1) | Ca + Mg (mmol·L−1) |
|---|---|---|---|---|---|---|
| The First Phase of Measurements | ||||||
| Krupina 1 | 6 | 16 | 22 | 23.6 | 9.2 | 0.97 |
| Dudince 2 | 9 | 13 | 22 | 96.8 | 21.3 | 3.29 |
| The Second Phase of Measurements | ||||||
| Krupina 1 | 9 | 16 | 25 | 23.6 | 9.2 | 0.97 |
| Horné Mladonice 1 | 6 | 2 | 8 | 22.9 | 7.2 | 0.87 |
| Litava 1 | 5 | 4 | 9 | 21.5 | 7.4 | 0.84 |
| Senohrad 1 | 4 | 4 | 8 | 22.9 | 5.1 | 0.78 |
| Dudince 2 | 2 | 23 | 25 | 96.8 | 21.3 | 3.29 |
| Hontianske Moravce 2 | 2 | 11 | 13 | 86.5 | 26.8 | 3.34 |
| Hontianske Tesáre 2 | 3 | 9 | 12 | 88.2 | 28.0 | 3.35 |
| Statistical Parameter | Total | Dudince—Hard Drinking Water | Krupina—Soft Drinking Water | p-Value |
|---|---|---|---|---|
| Actual Age | ||||
| Average ± SD 1 | 46.3 ± 7.66 | 45.3 ± 7.58 | 47.4 ± 7.65 | 0.105 2 |
| Median | 45.0 | 44.0 | 48.0 | |
| Range | 32–60 | 32–59 | 35–60 | |
| Body Mass Index (BMI) | ||||
| Average ± SD | 25.5 ± 2.51 | 25.2 ± 2.29 | 25.8 ± 2.70 | 0.191 2 |
| Median | 25.4 | 25.3 | 25.9 | |
| Range | 18.4–32.3 | 19.5–30.6 | 18.4–32.3 | |
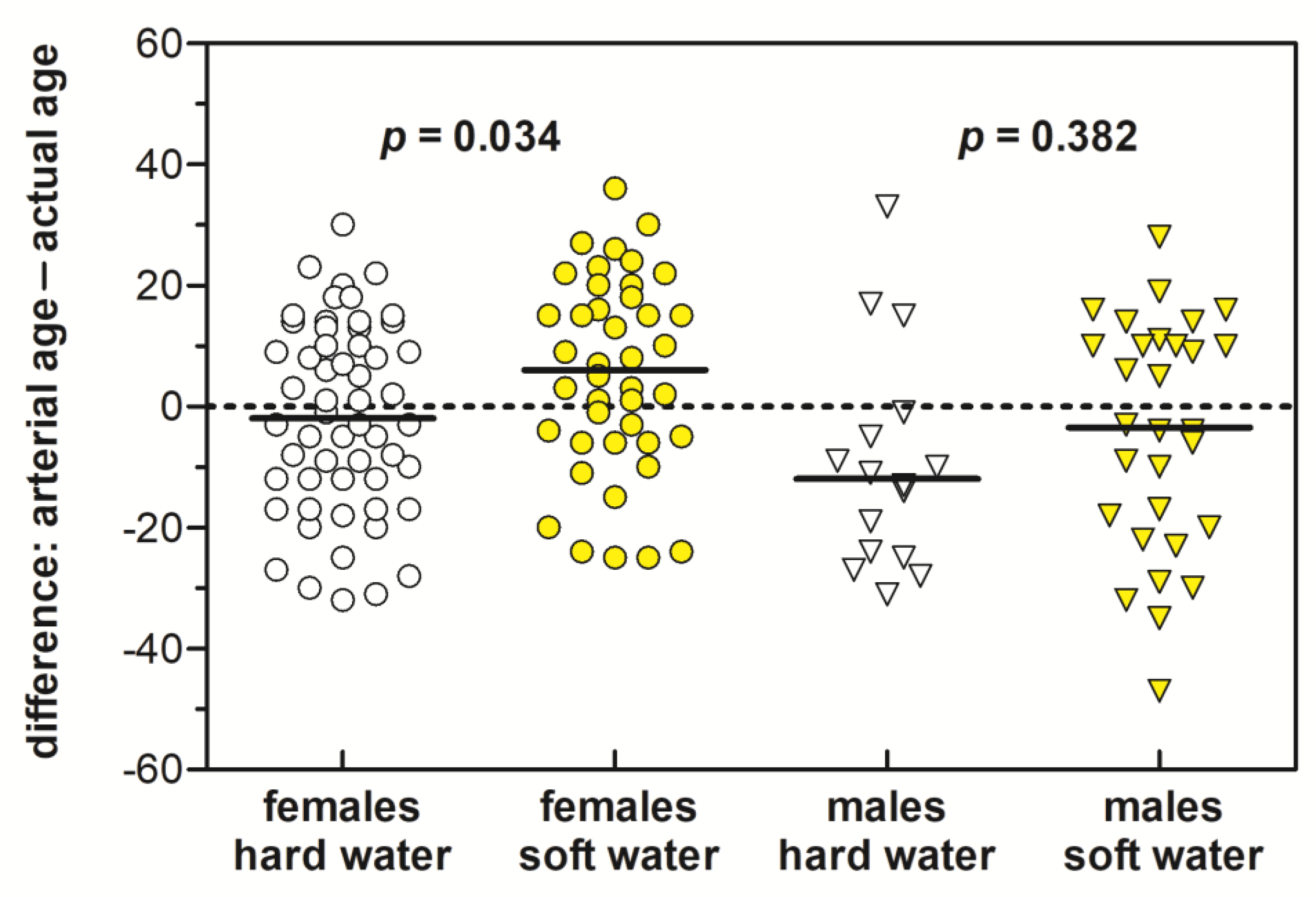
| Sex | ||||
| Male | 46 (31.9%) | 16 (22.2%) | 30 (41.7%) | 0.018 (0.015) 3 |
| Female | 98 (68.1%) | 56 (77.8%) | 42 (58.3%) | 0.018 (0.015) |
| Smoking | ||||
| Smokers | 35 (24.3%) | 20 (27.8%) | 15 (20.8%) | 0.436 (0.287) 3 |
| Non-smokers | 109 (75.7%) | 52 (72.2%) | 57 (79.2%) | 0.436 (0.287) |
| Age (years) | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PWVao (m·s−1) | 5.35 | 6.00 | 6.60 | 6.80 | 7.00 | 7.30 | 7.70 | 8.30 | 8.60 | 8.80 | 9.00 |
| Statistical Parameter | Dudince—Hard Drinking Water | Krupina—Soft Drinking Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Actual Age (years) | Arterial Age (years) | Difference 1 | PWVao 2 (m·s−1) | Actual Age (years) | Arterial Age (years) | Difference | PWVao (m·s−1) | |
| The First Phase (n = 44) 3 | ||||||||
| Range | 32–59 | 13–71 | −31 to +30 | 5.41–11.1 | 35–59 | 13–75 | −25 to +36 | 5.63–12.5 |
| Average | 44.6 | 41.0 | −3.55 | 7.97 | 45.4 | 48.3 | +1.91 | 8.70 |
| Median | 44.0 | 43.5 | −4.00 | 7.79 | 45.0 | 46.5 | 0.00 | 8.29 |
| SD4 | 8.03 | 18.3 | 16.5 | 1.45 | 8.53 | 19.4 | 14.2 | 2.02 |
| PWVao5 > 10 m·s−1 | 2 | 5 | ||||||
| p-value6 | 0.482 | 0.211 | 0.246 | 0.186 | ||||
| The Second Phase (n = 100) | ||||||||
| Range | 35–59 | 13–75 | −32 to +33 | 5.56–12.1 | 36–60 | 11–75 | −47 to +30 | 5.38–14.0 |
| Average | 45.6 | 42.3 | −3.36 | 8.14 | 47.8 | 48.8 | +0.96 | 8.95 |
| Median | 44.0 | 39.5 | −5.00 | 7.67 | 48.0 | 47.0 | +7.00 | 8.35 |
| SD | 7.44 | 19.4 | 16.5 | 1.74 | 7.28 | 22.0 | 19.5 | 2.33 |
| PWVao > 10 m·s−1 | 9 | 16 | ||||||
| p-value | 0.173 | 0.119 | 0.293 | 0.050 | ||||
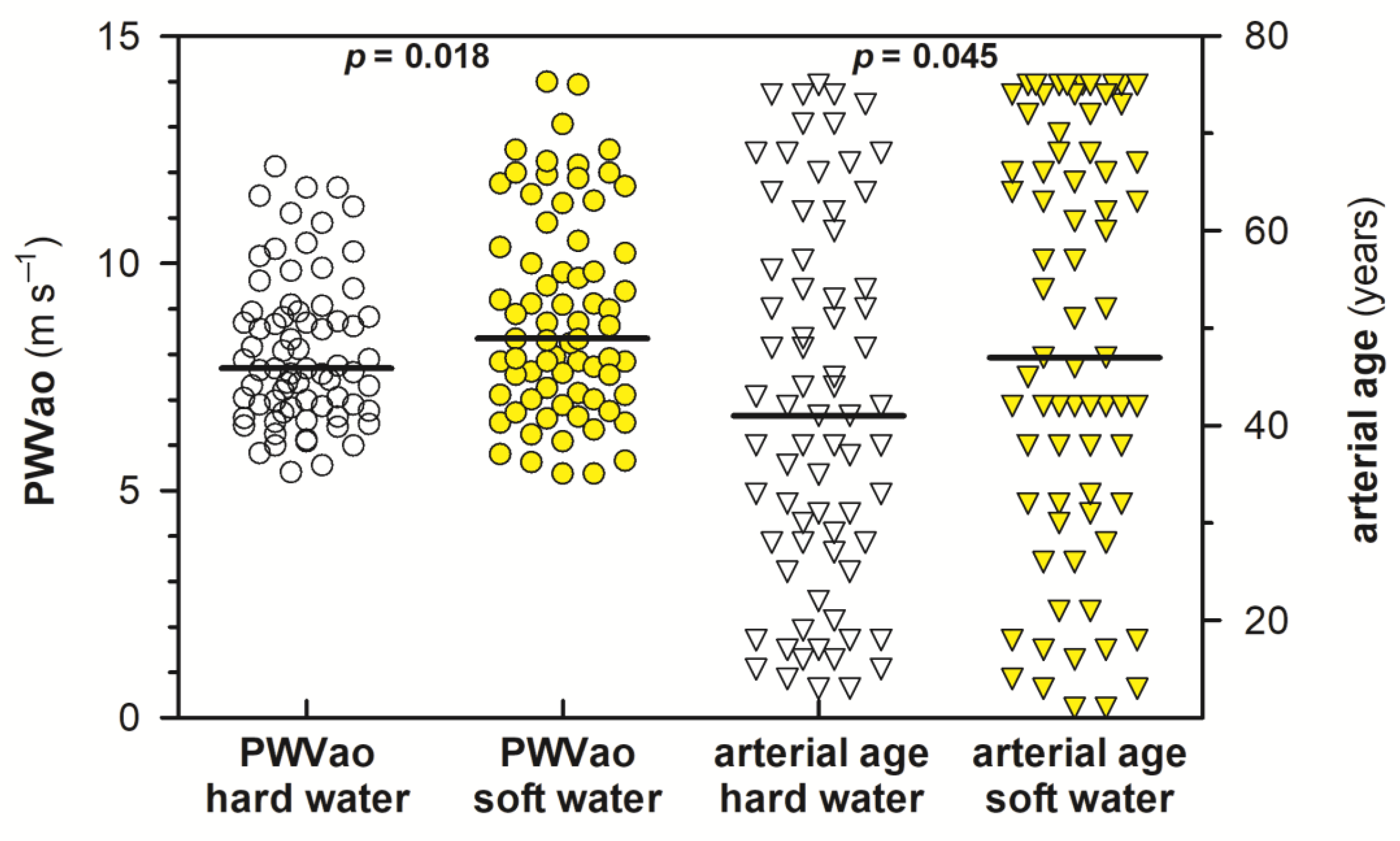
| All Measurements (n = 144) | ||||||||
| Range | 32–59 | 13–75 | −32 to +33 | 5.41–12.1 | 35–60 | 11–75 | −47 to +36 | 5.38–14.0 |
| Average | 45.3 | 41.9 | −3.42 | 8.09 | 47.4 | 48.6 | +1.25 | 8.87 |
| Median | 44.0 | 41.0 | −5.00 | 7.69 | 48.0 | 47.0 | +4.00 | 8.35 |
| SD | 7.58 | 18.9 | 16.4 | 1.65 | 7.65 | 21.1 | 18.0 | 2.23 |
| PWVao > 10 m·s−1 | 11 | 21 | ||||||
| p-value | 0.105 | 0.045 | 0.106 | 0.018 | ||||
| Statistics | Dudince—Hard Drinking Water | Krupina—Soft Drinking Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Age | Sex | BMI 1 | Smoking | Age | Sex | BMI | Smoking | |
| Lambda | 0.767 | 0.052 | 0.169 | 0.011 | 0.365 | 0.085 | 0.043 | 0.015 |
| F (observed) | 8.435 | 1.179 | 1.859 | 0.254 | 4.013 | 1.923 | 0.475 | 0.338 |
| DF1 2 | 6 | 3 | 6 | 3 | 6 | 3 | 6 | 3 |
| DF2 2 | 132 | 68 | 132 | 68 | 132 | 68 | 132 | 68 |
| F (critical) | 2.168 | 2.740 | 2.168 | 2.740 | 2.168 | 2.740 | 2.168 | 2.740 |
| p-value | <0.0001 | 0.324 | 0.093 | 0.858 | 0.001 | 0.134 | 0.826 | 0.798 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapant, S.; Cvečková, V.; Fajčíková, K.; Hajdúk, I.; Hiller, E.; Stehlíková, B. Hard Water, More Elastic Arteries: A Case Study from Krupina District, Slovakia. Int. J. Environ. Res. Public Health 2019, 16, 1521. https://doi.org/10.3390/ijerph16091521
Rapant S, Cvečková V, Fajčíková K, Hajdúk I, Hiller E, Stehlíková B. Hard Water, More Elastic Arteries: A Case Study from Krupina District, Slovakia. International Journal of Environmental Research and Public Health. 2019; 16(9):1521. https://doi.org/10.3390/ijerph16091521
Chicago/Turabian StyleRapant, Stanislav, Veronika Cvečková, Katarína Fajčíková, Igor Hajdúk, Edgar Hiller, and Beáta Stehlíková. 2019. "Hard Water, More Elastic Arteries: A Case Study from Krupina District, Slovakia" International Journal of Environmental Research and Public Health 16, no. 9: 1521. https://doi.org/10.3390/ijerph16091521
APA StyleRapant, S., Cvečková, V., Fajčíková, K., Hajdúk, I., Hiller, E., & Stehlíková, B. (2019). Hard Water, More Elastic Arteries: A Case Study from Krupina District, Slovakia. International Journal of Environmental Research and Public Health, 16(9), 1521. https://doi.org/10.3390/ijerph16091521






