Risk Factors and Prediction of Leptospiral Seropositivity Among Dogs and Dog Handlers in Malaysia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Collection
2.3. Microscopic Agglutination Test (MAT)
2.4. Questionnaires
2.5. Definition of Risk Factors
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levett, P.N. Leptospirosis: A forgotten zoonosis? Clin. Appl. Immunol. Rev. 2004, 4, 435–448. [Google Scholar] [CrossRef]
- Pappas, G.; Papadimitriou, P.; Siozopoulou, V.; Christou, L.; Akritidis, N. The globalization of leptospirosis: Worldwide incidence trends. Int. J. Infect. Dis. 2008, 12, 351–357. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef]
- Abdul Wahab, Z. Epidemiology and current situation of Leptospirosis in Malaysia. In Proceedings of the Local Authorities Environmental Health Conference, Labuan, Malaysia, 8–9 September 2015; Available online: http://jkt.kpkt.gov.my/jkt/resources/PDF/Persidangan_2015/persidangan%20kesihatan/Leptospirosis_in_Malaysia.pdf (accessed on 27 November 2018).
- Garba, B.; Bahaman, A.R.; Khairani-Bejo, S.; Zakaria, Z.; Mutalib, A.R. Retrospective Study of Leptospirosis in Malaysia. Ecohealth 2017, 14, 389–398. [Google Scholar] [CrossRef]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Thayaparan, S.; Robertson, I.D.; Fairuz, A.; Suut, L.; Abdullah, M. Leptospirosis, an Emerging Zoonotic Disease in Malaysia. Available online: https://www.academia.edu/5449490/Leptospirosis_an_emerging_zoonotic_disease_in_Malaysia._2013 (accessed on 27 November 2018).
- Al-orry, W.; Arahou, M.; Hassikou, R.; Quasmaoui, A.; Charof, R.; Mennane, Z. Leptospirosis: Transmission, Diagnosis and Prevention. Int. J. Innov. Appl. Stud. 2016, 15, 457–467. [Google Scholar]
- Lau, C.; Smythe, L.; Weinstein, P. Leptospirosis: An emerging disease in travellers. Travel Med. Infect. Dis. 2010, 8, 33–39. [Google Scholar] [CrossRef]
- Mwachui, M.A.; Crump, L.; Hartskeerl, R.; Zinsstag, J.; Hattendorf, J. Environmental and Behavioural Determinants of Leptospirosis Transmission: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef]
- Ridzuan, J.M.; Aziah, D.; Zahiruddin, W.M. Work Environment- Related Risk Factors for Leptospirosis among Plantation Workers in Tropical Countries: Evidence from Malaysia. Int. J. Occup. Environ. Med. 2016, 77, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Shafei, M.N.; Sulong, M.R.; Yaacob, N.A.; Hassan, H.; Wan Mohamad, W.M.Z.; Daud, A.; Ismail, Z.; Abdullah, M.R. Seroprevalence of Leptospirosis among Town Service Workers in Northeastern State of Malaysia. Int. J. Collab. Res. Intern. Med. Public Heal. 2012, 4, 395–403. [Google Scholar]
- Pijnacker, R.; Goris, M.G.; te Wierik, M.J.; Broens, E.M.; van der Giessen, J.W.; de Rosa, M.; Wagenaar, J.A.; Hartskeerl, R.A.; Notermans, D.W.; Maassen, K.; et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance 2016, 21, 1–7. [Google Scholar] [CrossRef]
- Awosanya, E.J.; Nguku, P.; Oyemakinde, A.; Omobowale, O. Factors associated with probable cluster of Leptospirosis among kennel workers in Abuja, Nigeria. Pan Afr. Med. J. 2013, 16, 1–6. [Google Scholar] [CrossRef]
- Vojinović, D.; Bogićević, N.; Vasić, A.; Manić, M.; Elezović Radovanović, M.; Rogožarski, D.; Marić, J.; Valčić, M. Seroepidemiological survey of leptospiral infection in stray dogs in Serbia. Turkish J. Vet. Anim. Sci. 2015, 39, 719–723. [Google Scholar] [CrossRef]
- Ambily, R.; Mini, M.; Joseph, S.; Krishna, S.V.; Abhinay, G. Canine leptospirosis—A seroprevalence study from kerala, india. Vet. World 2013, 6, 42–44. [Google Scholar] [CrossRef]
- OIE. Leptospirosis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 7th ed.; World Organisation for Animal Health (OIE): Paris, France, 2012; pp. 209–222. ISBN 978-92-9044-879-2. [Google Scholar]
- Monahan, A.M.; Miller, I.S.; Nally, J.E. Leptospirosis: Risks during recreational activities. J. Appl. Microbiol. 2009, 107, 707–716. [Google Scholar] [CrossRef]
- Sejvar, J.; Bancroft, E.; Winthrop, K.; Bettinger, J.; Bajani, M.; Bragg, S.; Shutt, K.; Kaiser, R.; Marano, N.; Popovic, T.; et al. Leptospirosis in “Eco-Challenge” athletes, Malaysian Borneo, 2000. Emerg. Infect. Dis. 2003, 9, 702–707. [Google Scholar] [CrossRef]
- Sykes, J.E.; Hartmann, K.; Lunn, K.F.; Moore, G.E.; Stoddard, R.A.; Goldstein, R.E. 2010 ACVIM Small Animal Consensus Statement on Leptospirosis: Diagnosis, Epidemiology, Treatment, and Prevention. J. Vet. Intern. Med. 2011, 25, 1–13. [Google Scholar] [CrossRef]
- Feigin, R.D.; Lobes, L.A.; Anderson, D.; Pickering, L. Human Leptospirosis from Immunized Dogs. Ann. Intern. Med. 1973, 79, 777–785. [Google Scholar] [CrossRef]
- Pui, C.F.; Bilung, L.M.; Apun, K.; Su’ut, L. Diversity of Leptospira spp. in Rats and Environment from Urban Areas of Sarawak, Malaysia. J. Trop. Med. 2017. [Google Scholar] [CrossRef]
- Prabhakaran, S.G.; Shanmughapriya, S.; Dhanapaul, S.; James, A.; Natarajaseenivasan, K. Risk factors associated with rural and urban epidemics of leptospirosis in Tiruchirappalli district of Tamilnadu, India. J. Public Heal. 2014, 22, 323–333. [Google Scholar] [CrossRef]
- Colt, S.; Pavlin, B.I.; Kool, J.L.; Johnson, E.; McCool, J.P.; Woodward, A.J. Human leptospirosis in the federated states of micronesia: A hospital-based febrile illness survey. BMC Infect. Dis. 2014, 14, 1–6. [Google Scholar] [CrossRef]
- Himsworth, C.G.; Parsons, K.L.; Jardine, C.; Patrick, D.M. Rats, Cities, People, and Pathogens: A Systematic Review and Narrative Synthesis of Literature Regarding the Ecology of Rat-Associated Zoonoses in Urban Centers. Vector-Borne Zoonotic Dis. 2013, 13, 349–359. [Google Scholar] [CrossRef]
- Ullmann, L.; Langoni, H. Interactions between environment, wild animals and human leptospirosis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 119–129. [Google Scholar] [CrossRef]
- Cutler, S.J.; Fooks, A.R.; Van Der Poel, W.H.M. Public Health Threat of New, Zoonoses in the Industrialized World Reemerging, and Neglected. Emerg. Infect. Dis. 2010, 16, 1–7. [Google Scholar] [CrossRef]
- Feresu, S.B.; Bolin, C.A.; Korver, H.; Terpstra, W.J. Classification of Leptospires of the Pyrogenes Serogroup Isolated from Cattle in Zimbabwe by Cross-Agglutinin Absorption and Restriction Fragment Length Polymorphism Analysis. Int. J. Syst. Bacteriol. 1994, 44, 541–546. [Google Scholar] [CrossRef][Green Version]
- Lai, C.-J.; Pan, M.-J.; Liu, E.C.C.; Ho, D. Seroprevalence of leptospiral infection among attended dogs in Taiwan. Taiwan Vet. J. 2006, 32, 1–6. [Google Scholar]
- Jimenez-coello, M.; Ortega-pacheco, A.; Guzman-marin, E.; Guiris-andrade, D.M.; Martinez-figueroa, L.; Acosta-viana, K.Y. Stray Dogs as Reservoirs of the Zoonotic Agents. Vector-Borne Zoonotic Dis. 2010, 10, 135–141. [Google Scholar] [CrossRef]
- Goldstein, R.E. Canine Leptospirosis. Vet. Clin. North Am. Small Anim. Pract. 2010, 40, 1091–1101. [Google Scholar] [CrossRef]
- Azócar-Aedo, L.; Monti, G. Meta-Analyses of Factors Associated with Leptospirosis in Domestic Dogs. Zoonoses Public Health 2016, 63, 328–336. [Google Scholar] [CrossRef]
- Raghavan, R.; Brenner, K.; Higgins, J.; Van der Merwe, D.; Harkin, K.R. Evaluations of land cover risk factors for canine leptospirosis: 94 cases (2002–2009). Prev. Vet. Med. 2011, 101, 241–249. [Google Scholar] [CrossRef][Green Version]
- Alton, G.D.; Berke, O.; Reid-Smith, R.; Ojkic, D.; Prescott, J.F. Increase in seroprevalence of canine leptospirosis and its risk factors, Ontario 1998–2006. Can. J. Vet. Res. 2009, 73, 167–175. [Google Scholar]
- Major, A.; Schweighauser, A.; Francey, T. Increasing incidence of canine leptospirosis in Switzerland. Int. J. Environ. Res. Public Health 2014, 11, 7242–7260. [Google Scholar] [CrossRef]
- Schreier, S.; Doungchawee, G.; Chadsuthi, S.; Triampo, D.; Triampo, W. Leptospirosis: Current situation and trends of specific laboratory tests. Expert Rev. Clin. Immunol. 2013, 9, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Calderón, A.; Rodríguez, V.; Máttar, S.; Arrieta, G. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop. Anim. Health Prod. 2014, 46, 427–432. [Google Scholar] [CrossRef]
- Pui, C.F.; Bilung, L.M.; Su’ut, L.; Apun, K.; Fung Pui, C.; Bilung, L.M.; Su ’ut, L.; Apun, K. Prevalence of Leptospira Species in Environmental Soil and Water from National Parks in Sarawak, Malaysia. Borneo J. Resour. Sci. Technol. 2015, 5, 49–57. [Google Scholar] [CrossRef]
- Garba, B.; Bahaman, A.R.; Bejo, S.K.; Zakaria, Z.; Mutalib, A.R.; Bande, F. Major epidemiological factors associated with leptospirosis in Malaysia. Acta Trop. 2018, 178, 242–247. [Google Scholar] [CrossRef] [PubMed]
| Risk Factors | Frequency | % |
|---|---|---|
| Dogs’ factor (n = 266) | ||
| Had small mammal contact | 219 | 82.3 |
| Had rat contact | 242 | 91.0 |
| Shared common area | 200 | 75.2 |
| Dirty kennel | 227 | 85.3 |
| Urban location | 126 | 47.4 |
| Dog handlers’ factor (n = 161) | ||
| Occupational: | ||
| Had small mammal contact | 57 | 35.4 |
| Had rat contact | 45 | 28.0 |
| Contact time with dog > 6 hours/day | 80 | 49.7 |
| Urban location | 111 | 68.9 |
| Non-occupational: | ||
| Had recreational activity in last 3 months | 69 | 42.9 |
| Rat infestation at home | 85 | 52.5 |
| Small mammal around the housing area | 105 | 65.2 |
| Had flash flood at the housing area in last 3 months | 15 | 9.3 |
| Monsoon drain within 15m radius from the house | 63 | 39.1 |
| MAT Seropositivity | ||
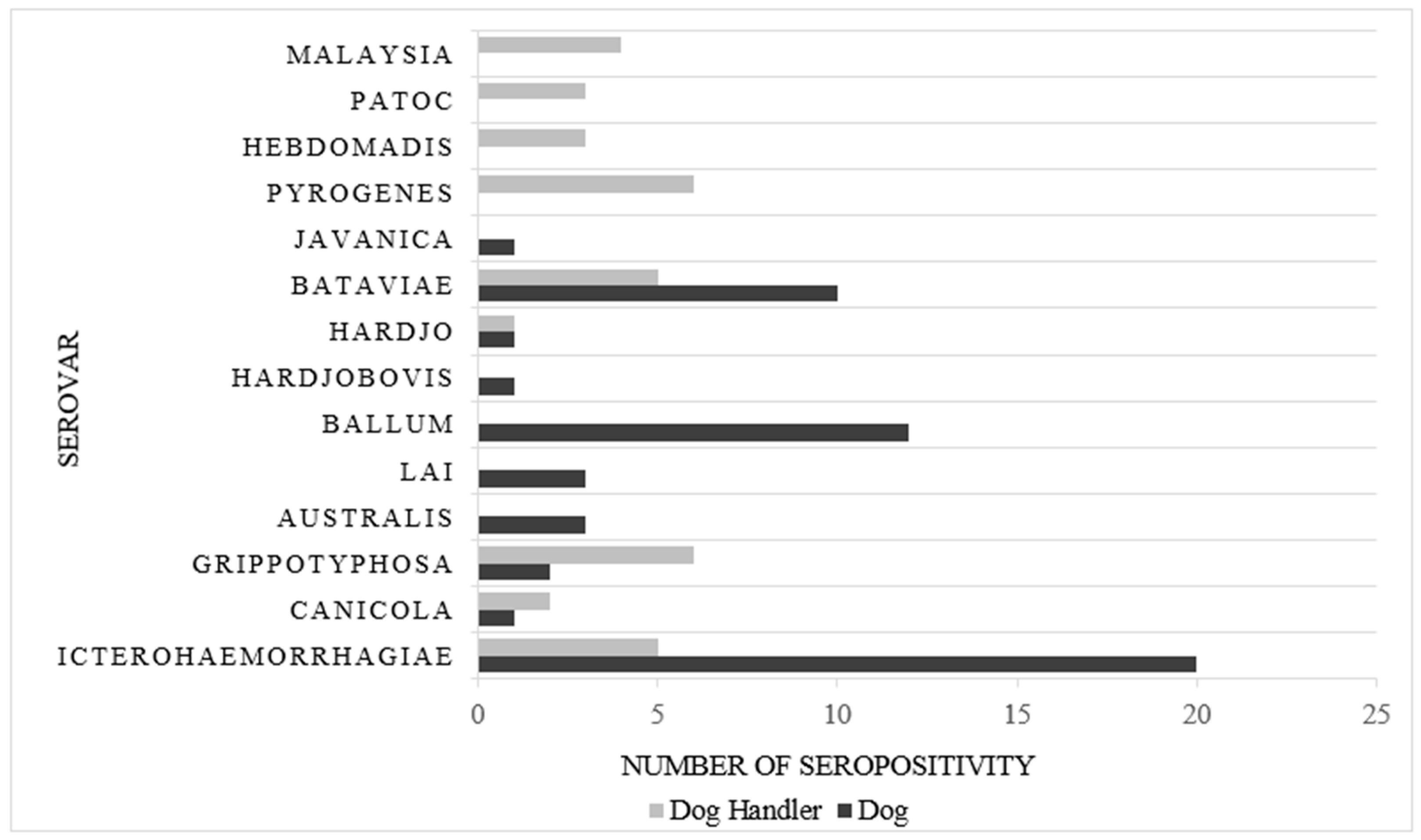
| Dogs (n = 266) | 59 | 22.2 |
| Working Dog (n = 73) | 17 | 6.4 |
| Shelter Dog (n = 193) | 42 | 15.8 |
| Dogs handlers (n = 161) | 35 | 21.7 |
| Working Dog (n = 128) | 15 | 9.3 |
| Shelter Dog (n = 33) | 20 | 12.4 |
| Variables | Simple Logistic Regression | Multiple Logistic Regression a | ||||
|---|---|---|---|---|---|---|
| b | Crude OR (95% CI) | p-Value | b | Adjusted OR (95% CI) | p-Value | |
| Had small mammal contact | −0.42 | 0.66 (0.29, 1.52) | 0.331 | |||
| Had rat contact | 1.22 | 3.39 (0.77, 14.85) | 0.105 | 1.53 | 4.61 (1.05, 20.33) | 0.043 |
| Share common area | 1.51 | 4.51 (1.72, 11.83) | 0.002 | 1.63 | 5.12 (1.94, 13.46) | 0.001 |
| Kennel cleanliness | 0.23 | 1.25 (0.57, 2.75) | 0.574 | |||
| Urban location | 0.80 | 2.23 (1.23, 4.04) | 0.008 | |||
| Risk Factors | Simple Logistic Regression | Multiple Logistic Regression a | |||||
|---|---|---|---|---|---|---|---|
| b | Crude OR (95% CI) | p-Value | B | Adjusted OR (95% CI) | p-Value | ||
| Occupational | Small mammal contact | 1.48 | 4.40 (1.99, 9.68) | <0.001 | 0.79 | 2.21 (0.91, 5.40) | 0.082 |
| Had rat contact | 1.85 | 6.38 (2.84, 14.32) | <0.001 | - | - | - | |
| Contact time with dog > 6 hours daily | 1.53 | 4.65 (1.96, 11.04) | <0.001 | 1.19 | 3.28 (1.28, 8.40) | 0.013 | |
| Urban area | −1.14 | 0.32 (0.15, 0.70) | 0.004 | −0.98 | 0.38 (0.16, 0.91) | 0.029 | |
| Non-occupational | Had recreational activity | −0.46 | 0.63 (0.29, 1.38) | 0.249 | - | - | - |
| Rat infestation surrounding home | 1.01 | 2.75 (1.22, 6.20) | 0.015 | 0.91 | 2.44 (0.95, 6.52) | 0.065 | |
| Small mammals around the house | 0.70 | 2.01 (0.84, 4.78) | 0.115 | - | - | - | |
| Had flash flood at home in last 3 months | 1.45 | 4.25 (1.38, 3.10) | 0.012 | 1.40 | 4.04 (1.08, 15.16) | 0.038 | |
| Had monsoon drain 15m from house | −0.27 | 0.77 (0.35, 1.68) | 0.507 | - | - | - | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, S.H.; Ismail, R.; Lau, S.F.; Megat Abdul Rani, P.A.; Mohd Mohidin, T.B.; Daud, F.; Bahaman, A.R.; Khairani-Bejo, S.; Radzi, R.; Khor, K.H. Risk Factors and Prediction of Leptospiral Seropositivity Among Dogs and Dog Handlers in Malaysia. Int. J. Environ. Res. Public Health 2019, 16, 1499. https://doi.org/10.3390/ijerph16091499
Goh SH, Ismail R, Lau SF, Megat Abdul Rani PA, Mohd Mohidin TB, Daud F, Bahaman AR, Khairani-Bejo S, Radzi R, Khor KH. Risk Factors and Prediction of Leptospiral Seropositivity Among Dogs and Dog Handlers in Malaysia. International Journal of Environmental Research and Public Health. 2019; 16(9):1499. https://doi.org/10.3390/ijerph16091499
Chicago/Turabian StyleGoh, Soon Heng, Rosnah Ismail, Seng Fong Lau, Puteri Azaziah Megat Abdul Rani, Taznim Begam Mohd Mohidin, Faiz Daud, Abdul Rani Bahaman, Siti Khairani-Bejo, Rozanaliza Radzi, and Kuan Hua Khor. 2019. "Risk Factors and Prediction of Leptospiral Seropositivity Among Dogs and Dog Handlers in Malaysia" International Journal of Environmental Research and Public Health 16, no. 9: 1499. https://doi.org/10.3390/ijerph16091499
APA StyleGoh, S. H., Ismail, R., Lau, S. F., Megat Abdul Rani, P. A., Mohd Mohidin, T. B., Daud, F., Bahaman, A. R., Khairani-Bejo, S., Radzi, R., & Khor, K. H. (2019). Risk Factors and Prediction of Leptospiral Seropositivity Among Dogs and Dog Handlers in Malaysia. International Journal of Environmental Research and Public Health, 16(9), 1499. https://doi.org/10.3390/ijerph16091499





