Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China
Abstract
1. Introduction
2. Site Description
2.1. Geography and Climate
2.2. Geomorphological Setting
2.3. Geologic and Hydrogeological Setting
3. Materials and Methods
3.1. Sample Collection and Analysis
3.2. Irrigation Water Quality Evaluation
4. Results and Discussion
4.1. Groundwater Chemistry
4.1.1. Physiochemical Parameters
4.1.2. Hydrochemical Facies
4.1.3. Groundwater Natural Formative Mechanisms
4.1.4. Sources of Major Ions
4.2. Groundwater Suitability for Irrigation
4.2.1. Salinity Hazard (SH)
4.2.2. Sodium Percentage (Na%)
4.2.3. Sodium Adsorption Ratio (SAR)
4.2.4. Residual Sodium Carbonate (RSC)
4.2.5. Magnesium Hazard (MH)
4.2.6. Permeability Index (PI)
4.2.7. Kelley’s Ratio (KR)
4.2.8. Potential Salinity (PS)
4.2.9. Synthetic Harmful Coefficient (K)
4.2.10. Irrigation Coefficient (Ka)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Sun, Z. Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo. Health 2016, 8, 311–329. [Google Scholar] [CrossRef]
- Li, P.; He, S.; He, X.; Tian, R. Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a paper wastewater irrigation area, northwest China. Expo. Health 2018, 10, 241–258. [Google Scholar] [CrossRef]
- Liang, C.-P.; Jang, C.-S.; Chen, C.-F.; Chen, J.-S. Zonal management of multi-purposes groundwater utilization based on water quality and impact on the aquifer. Environ. Monit. Assess. 2016, 188, 431. [Google Scholar] [CrossRef]
- Li, P.; Tian, R.; Xue, C.; Wu, J. Progress, opportunities, and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ. Sci. Pollut. Res. 2017, 24, 13224–13234. [Google Scholar] [CrossRef]
- Li, P.Y.; Qian, H.; WU, J.H. Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. J. Chem. 2010, 7, S209–S216. [Google Scholar]
- Howard, K.W. Sustainable cities and the groundwater governance challenge. Environ. Earth Sci. 2015, 73, 2543–2554. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Li, X. Challenges and prospects of sustainable groundwater management in an agricultural plain along the Silk Road Economic Belt, north-west China. Int. J. Water Resour. Dev. 2018, 34, 354–368. [Google Scholar] [CrossRef]
- Zabala, M.; Martínez, S.; Manzano, M.; Vives, L. Groundwater chemical baseline values to assess the Recovery Plan in the Matanza-Riachuelo River basin, Argentina. Sci. Total Environ. 2016, 541, 1516–1530. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Elango, L. Groundwater chemistry and quality in an intensively cultivated river delta. Water Qual. Expo. Health 2015, 7, 125–141. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Tian, R.; He, S.; He, X.; Xue, C.; Zhang, K. Geochemistry, Hydraulic Connectivity and Quality Appraisal of Multilayered Groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ. 2018, 37, 222–237. [Google Scholar] [CrossRef]
- Jang, C.-S.; Chen, C.-F.; Liang, C.-P.; Chen, J.-S. Combining groundwater quality analysis and a numerical flow simulation for spatially establishing utilization strategies for groundwater and surface water in the Pingtung Plain. J. Hydrol. 2016, 533, 541–556. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Howard, K.W.; Wu, J. Building a new and sustainable “Silk Road economic belt”. Environ. Earth Sci. 2015, 74, 7267–7270. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo. Health 2016, 8, 331–348. [Google Scholar] [CrossRef]
- Sun, Y.B.; Wang, W.K.; Duan, L.; Zhang, C.C.; Wang, Y.H. Geochemical evolution mechanisms of shallow Groundwater in Guanzhong basin, China. Hydrogeol. Eng. Geol. 2014, 41, 29–35. (In Chinese) [Google Scholar]
- Duan, L.; Wang, W.K.; Sun, Y.Q.; Yang, X.T. Health risk assessment of “ Three Nitrogen” in shallow groundwater in the Guanzhong Basin. Hydrogeol. Eng. Geol. 2011, 38, 92–97. (In Chinese) [Google Scholar]
- Tao, Y.; Li, J.; Feng, K.W. Analysis of the guarantee degree of irrigation water resources and its spatial difference in Shaanxi Province. Chin. J. Agric. Resour. Reg. Plan. 2018, 39, 97–104. (In Chinese) [Google Scholar]
- Tahmasebi, P.; Mahmudy-Gharaie, M.H.; Ghassemzadeh, F.; Karouyeh, A.K. Assessment of groundwater suitability for irrigation in a gold mine surrounding area, NE Iran. Environ. Earth Sci. 2018, 77, 766. [Google Scholar] [CrossRef]
- Saxena, V.; Ahmed, S. Dissolution of fluoride in groundwater: A water-rock interaction study. Environ. Geol. 2001, 40, 1084–1087. [Google Scholar]
- Khalid, S. An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. J. Geochem. Explor. 2019, 197, 14–26. [Google Scholar]
- Taheri, M.; Gharaie, M.H.M.; Mehrzad, J.; Afshari, R.; Datta, S. Hydrogeochemical and isotopic evaluation of arsenic contaminated waters in an argillic alteration zone. J. Geochem. Explor. 2017, 175, 1–10. [Google Scholar] [CrossRef]
- Iqbal, H.H.; Shahid, N.; Qadir, A.; Ahmad, S.R.; Sarwar, S.; Ashraf, M.R.; Arshad, H.M.; Masood, N. Hydrological and Ichthyological Impact Assessment of Rasul Barrage, River Jhelum, Pakistan. Pol. J. Environ. Stud. 2017, 26. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Q.; Lin, Y.; Fang, Y.; Qian, H.; Liu, R.; Ma, H. Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated Region, Northwest China. Water 2019, 11, 96. [Google Scholar] [CrossRef]
- Mostaza-Colado, D.; Carreño-Conde, F.; Rasines-Ladero, R.; Iepure, S. Hydrogeochemical characterization of a shallow alluvial aquifer: 1 baseline for groundwater quality assessment and resource management. Sci. Total Environ. 2018, 639, 1110–1125. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.-S.; Chen, J.-S. Probabilistic assessment of groundwater mixing with surface water for agricultural utilization. J. Hydrol. 2009, 376, 188–199. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo. Health 2016, 8, 239–252. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H.; Fang, Y. Assessment of soil salinization based on a low-cost method and its influencing factors in a semi-arid agricultural area, northwest China. Environ. Earth Sci. 2014, 71, 3465–3475. [Google Scholar] [CrossRef]
- Ravikumar, P.; Somashekar, R.; Angami, M. Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State, India. Environ. Monit. Assess. 2011, 173, 459–487. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shetty, B.K.; Guddattu, V.; Chourasia, M.K.; Pundir, P. High prevalence of dental fluorosis among adolescents is a growing concern: A school based cross-sectional study from Southern India. Environ. Health Prev. Med. 2017, 22, 17. [Google Scholar] [CrossRef]
- Kawo, N.S.; Karuppannan, S. Groundwater quality assessment using water quality index and GIS technique in Modjo River Basin, central Ethiopia. J. Afr. Earth Sci. 2018, 147, 300–311. [Google Scholar] [CrossRef]
- Zhou, J.L.; Wu, B.; Wang, Y.P.; Guo, X.J. Distribution and Quality Assessment of Medium Salinity Groundwater in Plain Areas in Tarim Basin, Xinjiang. China Rural Water Hydropower 2009, 9, 32–36. (In Chinese) [Google Scholar]
- Bouderbala, A. Assessment of groundwater quality and its suitability for agricultural uses in the Nador Plain, north of Algeria. Water Qual. Expo. Health 2015, 7, 445–457. [Google Scholar] [CrossRef]
- Abdulhussein, F.M. Hydrochemical Assessment of Groundwater of Dibdibba Aquifer in Al-Zubair Area, Basra, South of Iraq and its Suitability for IrrigationPurposes. Iraqi J. Sci. 2018, 59, 135–143. [Google Scholar]
- Zhang, C.C.; Wei, Z.; Sun, Y.B.; Li, H.; Wang, W.K.; Duan, L. Hydrogeochemical Evolution of Groundwater in Guanzhong Basin. South- -North Water Transf. Water Sci. Technol. 2013, 11, 48–52. (In Chinese) [Google Scholar]
- Jiang, G.H.; Wang, W.K.; Qiao, X.Y.; Yang, X.T. Groundwater Special Vulnerability and its Assessment in Guanzhong Basin. J. Jilin Univ. 2009, 39, 1106–1112. (In Chinese) [Google Scholar]
- Xie, F.; Cai, H.J.; Zhao, C.X.; Wang, Z.S.; Wang, J.; Xu, J.T. Chemical Properties of the Groundwater in Jing Huiqu Irrigation District and Their Response to Irrigation UsingWater from Different Sources. J. Irrig. Drain. 2017, 36, 77–82. (In Chinese) [Google Scholar]
- Duan, L.; Wang, W.K.; Sun, Y.B.; Zhang, C.C. Iodine in groundwater of the Guanzhong Basin, China: Sources and hydrogeochemical controls on its distribution. Environ. Earth Sci. 2016, 75, 970. [Google Scholar] [CrossRef]
- Xu, P.P.; Li, M.N.; Qian, H.; Zhang, Q.Y.; Liu, F.X.; Hou, K. Hydrochemistry and geothermometry of geothermal water in the central Guanzhong Basin, China: A case study in Xi’an. Environ. Earth Sci. 2019, 78, 87. [Google Scholar] [CrossRef]
- Dou, X.Y.; Song, J.X.; Wang, L.P.; Tang, B.; Xu, S.F.; Kong, F.H.; Jiang, X.H. Flood risk assessment and mapping based on a modified multi-parameter flood hazard index model in the Guanzhong Urban Area, China. Stoch. Environ. Res. Risk Assess. 2018, 32, 1131–1146. [Google Scholar] [CrossRef]
- Wan, Z.J. Temporal and Spatial Variation of Mineral Nitrogen Pollution in Groundwater in Guanzhong Basin, Shanxi Province. Master’s Thesis, Northwest A & F University, Yangling, China, 2018. [Google Scholar]
- Dou, X.Y. Flood risk assessment based on flood hazard index model: A case study of Guanzhong Area. Master’s Thesis, Northwest University, Xi’an, China, 2018. [Google Scholar]
- Zheng, X.Y. Mechanism of groundwater response to climate change in Guanzhong basin. Master’s Thesis, Chang’an University, Xi’an, China, 2012. [Google Scholar]
- Wu, J.; Li, P.; Qian, H.; Duan, Z.; Zhang, X. Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: A case study in Laoheba phosphorite mine in Sichuan, China. Arab. J. Geosci. 2014, 7, 3973–3982. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Chen, J.; Zhang, Y.; Zhang, H. Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Env. Earth Sci. 2014, 71, 3133–3145. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, K.; Ramanathan, A.; Saxena, R. A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ. Geol. 2007, 53, 553–574. [Google Scholar] [CrossRef]
- Doneen, L.D. Notes on Water Quality in Agriculture; Department of Water Science and Engineering, University of California: Oakland, CA, USA, 1964. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Eostransactions Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Huo, C.C.; Qian, H.; Wu, H. Environmental background values of shallow groundwater in the Guanzhong Basin. South--North Water Transf. Water Sci. Technol. 2016, 14, 99–106. (In Chinese) [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Marghade, D.; Malpe, D.; Zade, A. Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ. Monit. Assess. 2012, 184, 2405–2418. [Google Scholar] [CrossRef]
- Li, P.; Qian, H.; Wu, J.; Zhang, Y.; Zhang, H. Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ. 2013, 32, 195–206. [Google Scholar] [CrossRef]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Anantha, R.V.; Chandrakanta, G. Major ion chemistry, hydro-geochemical studies and mapping of variability in ground water quality of Sitanadi basin, Southern Karnataka. Octa J. Environ. Res. 2014, 2, 178–196. [Google Scholar]
- Carol, E.S.; Kruse, E.E.; Laurencena, P.C.; Rojo, A.; Deluchi, M.H. Ionic exchange in groundwater hydrochemical evolution. Study case: The drainage basin of El Pescado creek (Buenos Aires province, Argentina). Environ. Earth Sci. 2012, 65, 421–428. [Google Scholar] [CrossRef]
- Kumar, M.; Ramanathan, A.; Rao, M.; Kumar, B. Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ. Geol. 2006, 50, 1025–1039. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H.; Lyu, X.; Liu, H. Origin and assessment of groundwater pollution and associated health risk: A case study in an industrial park, northwest China. Environ. Geochem. Health 2014, 36, 693–712. [Google Scholar] [CrossRef]
- Han, Y.; Wang, G.; Cravotta, C.A., III; Hu, W.; Bian, Y.; Zhang, Z.; Liu, Y. Hydrogeochemical evolution of Ordovician limestone groundwater in Yanzhou, North China. Hydrol. Process 2013, 27, 2247–2257. [Google Scholar] [CrossRef]
- Barzegar, R.; Moghaddam, A.A.; Tziritis, E.; Fakhri, M.S.; Soltani, S. Identification of hydrogeochemical processes and pollution sources of groundwater resources in the Marand plain, northwest of Iran. Environ. Earth Sci. 2017, 76, 297. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H. Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: A case study in and around Hua County, China. Arab. J. Geosci. 2016, 9, 15. [Google Scholar] [CrossRef]
- Alam, F. Evaluation of hydrogeochemical parameters of groundwater for suitability of domestic and irrigational purposes: A case study from central Ganga Plain, India. Arab. J. Geosci. 2014, 7, 4121–4131. [Google Scholar] [CrossRef]
- Naseem, S.; Hamza, S.; Bashir, E. Groundwater geochemistry of Winder agricultural farms, Balochistan, Pakistan and assessment for irrigation water quality. Eur. Water 2010, 31, 21–32. [Google Scholar]
- Singaraja, C.; Chidambaram, S.; Anandhan, P.; Prasanna, M.V.; Thivya, C.; Thilagavathi, R.; Sarathidasan, J. Hydrochemistry of groundwater in a coastal region and its repercussion on quality, a case study—Thoothukudi district, Tamil Nadu, India. Arab. J. Geosci. 2014, 7, 939–950. [Google Scholar] [CrossRef]
- Wilcox, L. Classification and Use of Irrigation Waters; Agriculture Circular: Washington, DC, USA, 1955; p. 19. [Google Scholar]
- United States Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils. Available online: https://www.ars.usda.gov/ARSUserFiles/20360500/hb60_pdf/hb60complete.pdf (accessed on 25 April 2019).
- Singh, A.K.; Mondal, G.; Tewary, B.; Sinha, A. Major Ion Chemistry, Solute Acquisition Processes and Quality Assessment of Mine Water in Damodar Valley Coalfields, India. In Proceedings of the International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009. [Google Scholar]
- Selvakumar, S.; Ramkumar, K.; Chandrasekar, N.; Magesh, N.; Kaliraj, S. Groundwater quality and its suitability for drinking and irrigational use in the Southern Tiruchirappalli district, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 411–420. [Google Scholar] [CrossRef]
- Joshi, D.M.; Kumar, A.; Agrawal, N. Assessment of the irrigation water quality of river Ganga in Haridwar district. Rasayan J Chem. 2009, 2, 285–292. [Google Scholar]
- Ghalib, H.B. Groundwater chemistry evaluation for drinking and irrigation utilities in east Wasit province, Central Iraq. Appl. Water Sci. 2017, 7, 3447–3467. [Google Scholar] [CrossRef]
- Hussain, Y.; Ullah, S.F.; Akhter, G.; Aslam, A.Q. Groundwater quality evaluation by electrical resistivity method for optimized tubewell site selection in an ago-stressed Thal Doab Aquifer in Pakistan. Model. Earth Syst. Environ. 2017, 3, 15. [Google Scholar] [CrossRef]
- Singh, A.K.; Mondal, G.; Kumar, S.; Singh, T.; Tewary, B.; Sinha, A. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ. Geol. 2008, 54, 745–758. [Google Scholar] [CrossRef]
- Raju, N.J. Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ. Geol. 2007, 52, 1067–1074. [Google Scholar] [CrossRef]
- Krishnakumar, P.; Lakshumanan, C.; Kishore, V.P.; Sundararajan, M.; Santhiya, G.; Chidambaram, S. Assessment of groundwater quality in and around Vedaraniyam, South India. Environ. Earth Sci. 2014, 71, 2211–2225. [Google Scholar] [CrossRef]
- Doneen, L. The Influence of Crop and Soil on Percolating Water. In Proceedings of the 1961 Biennial conference on Groundwater recharge, Berkeley, CA, USA, 26–27 June 1962; pp. 156–163. [Google Scholar]

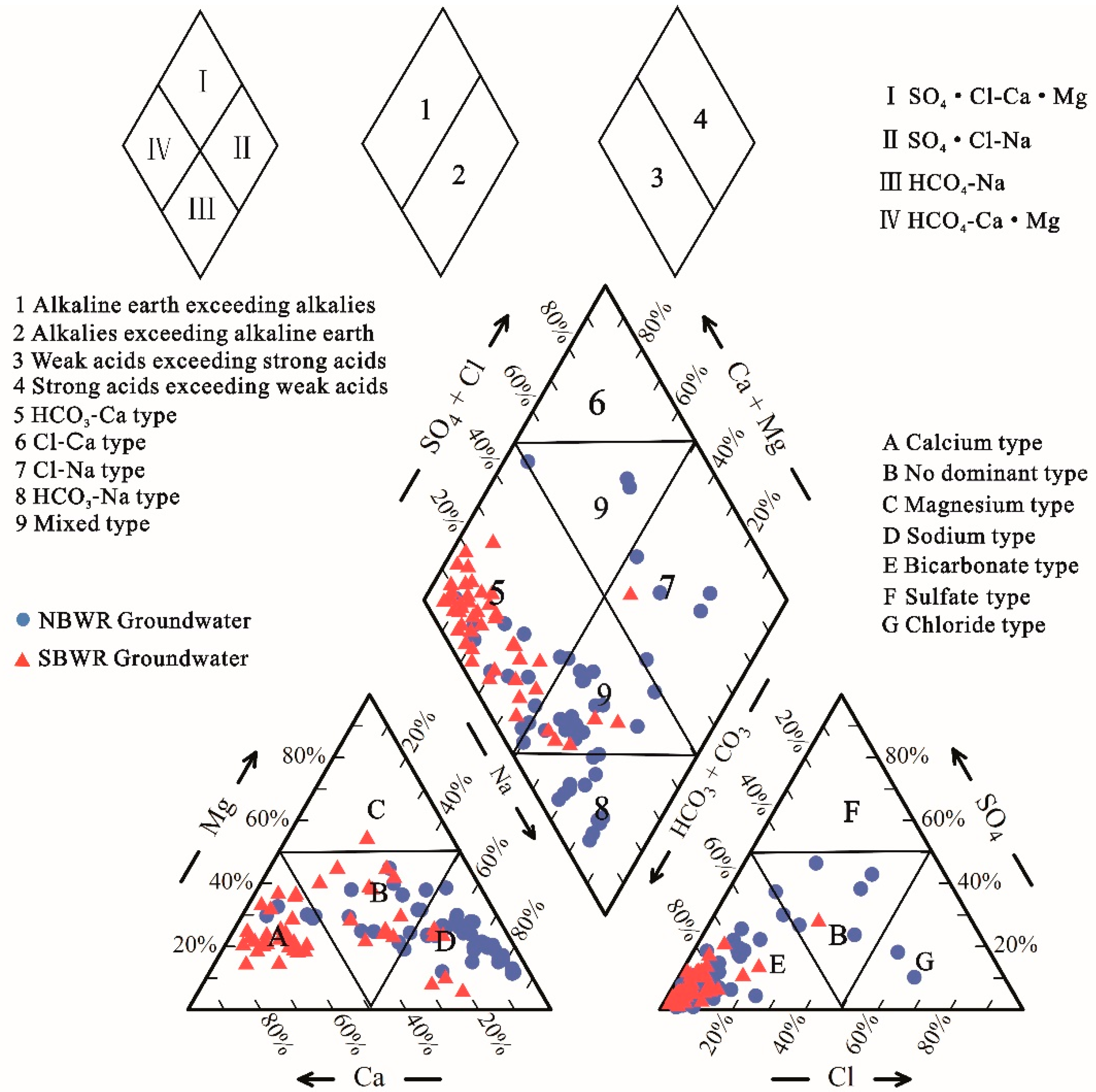
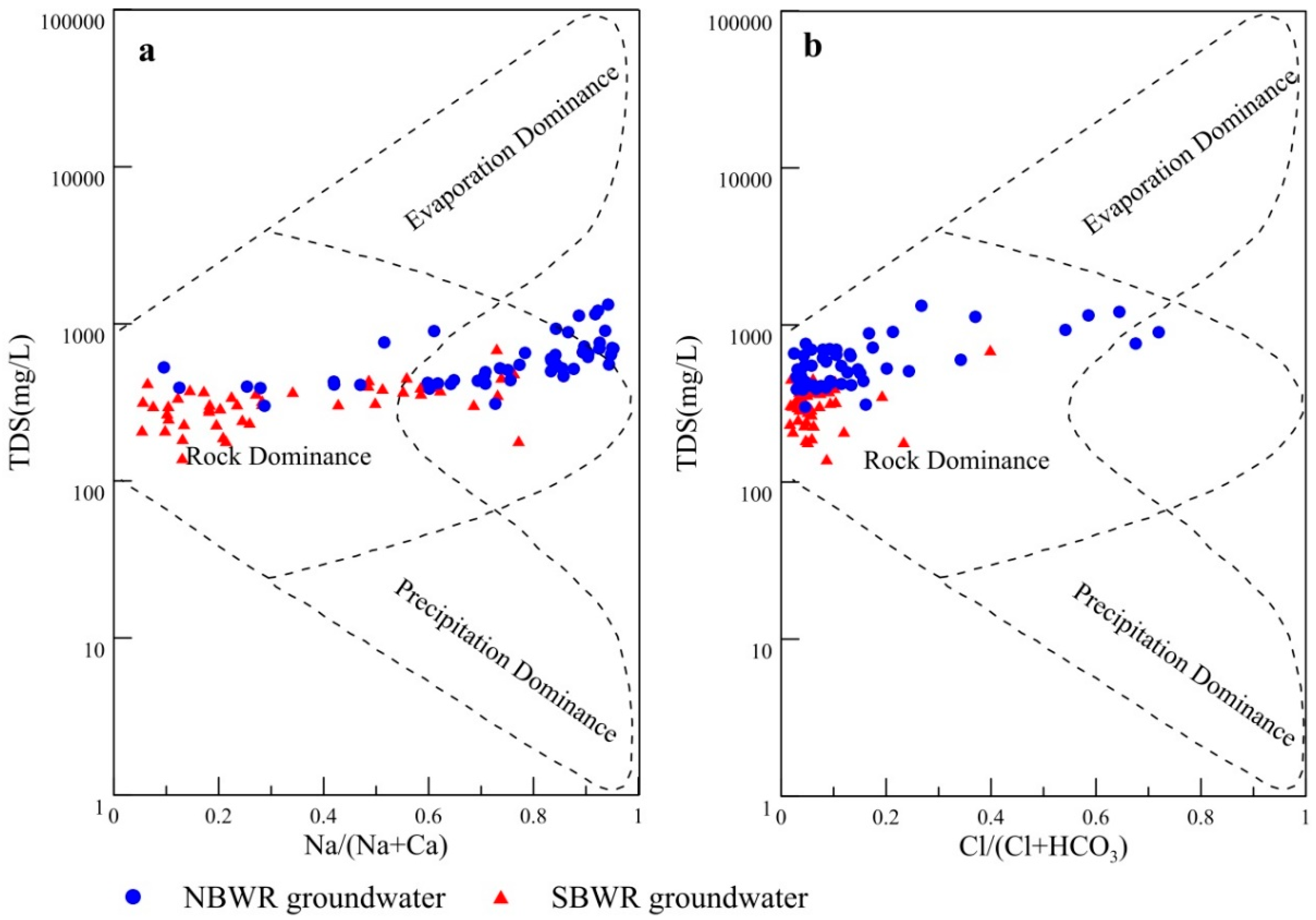
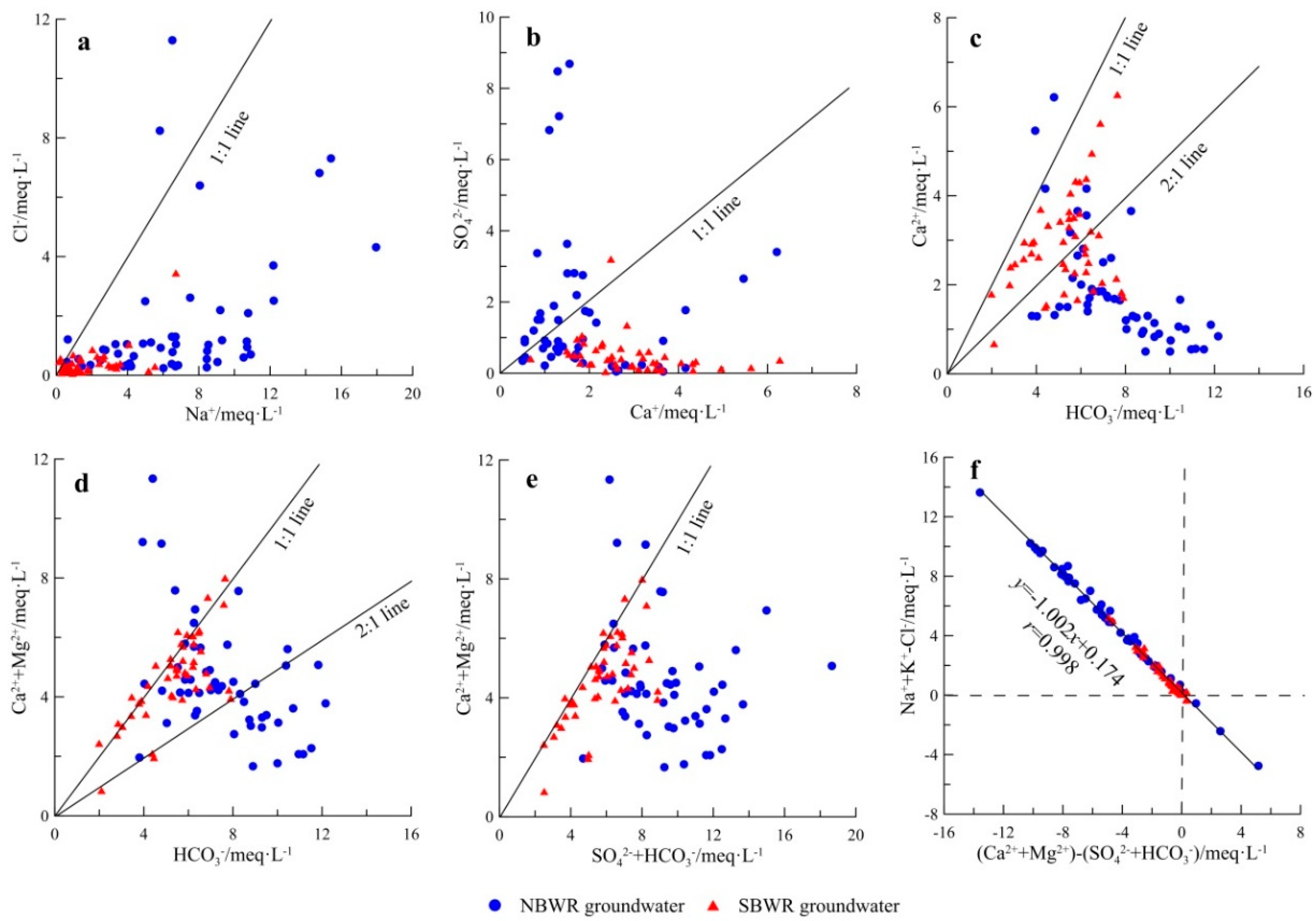
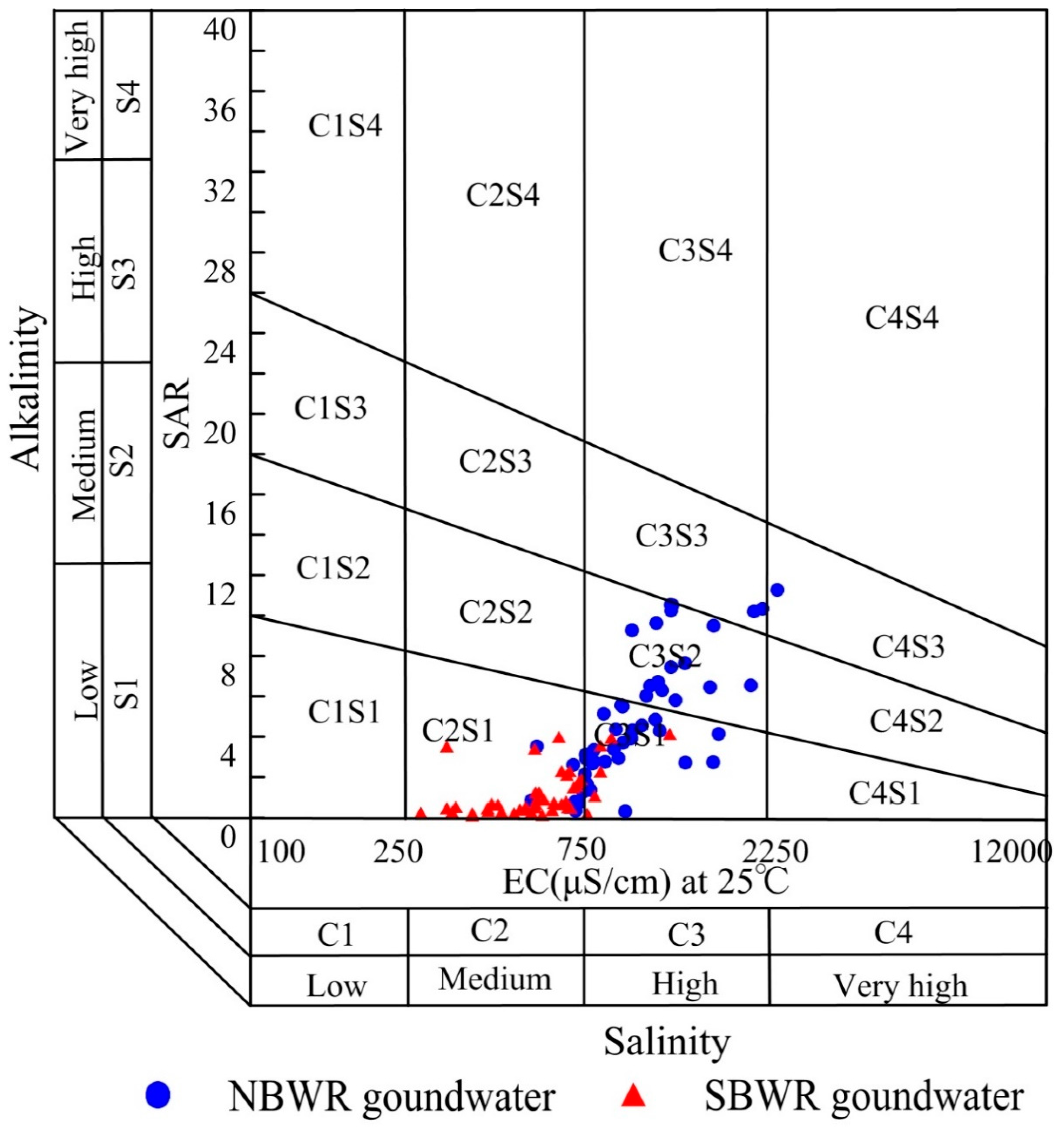
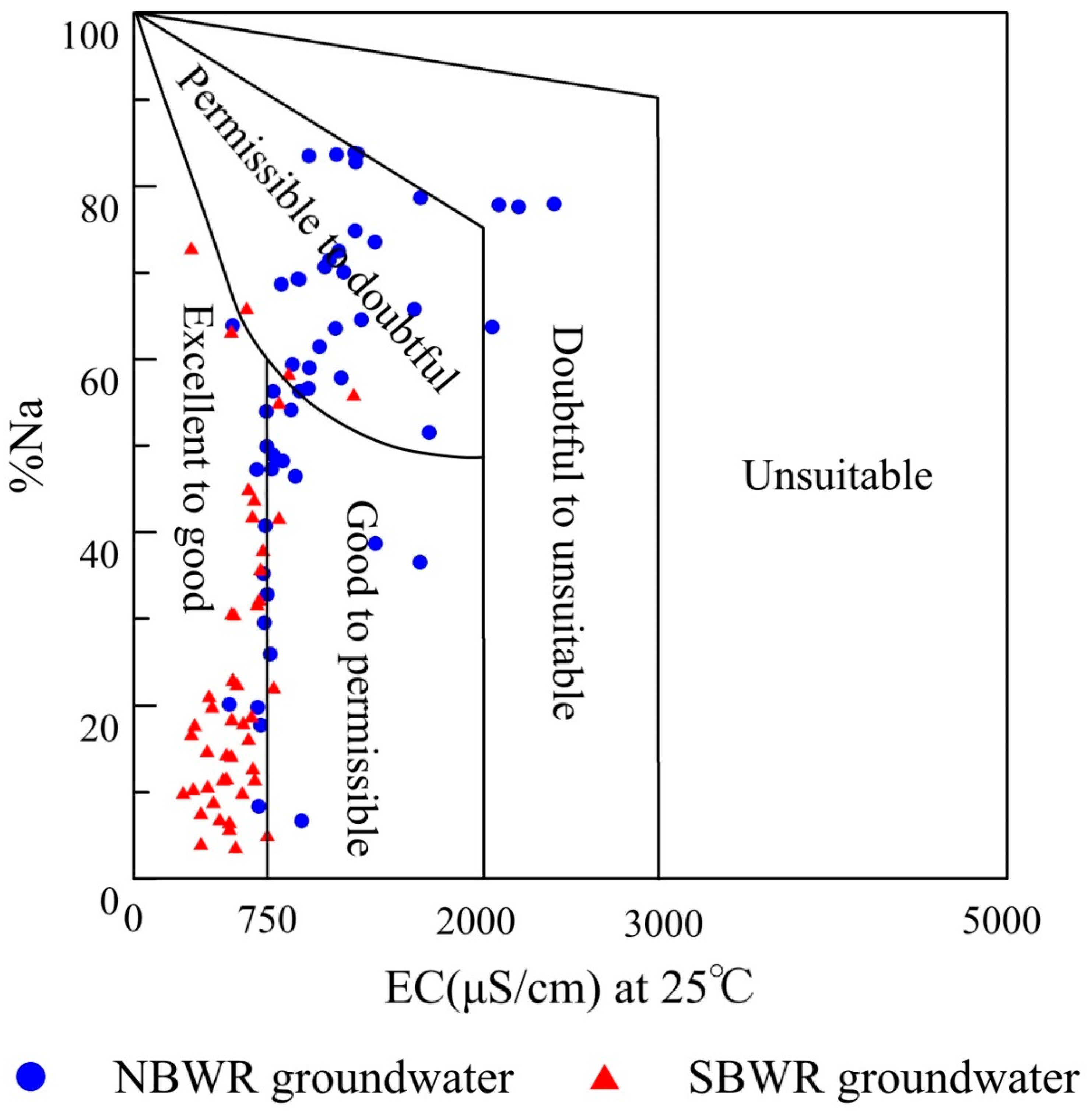
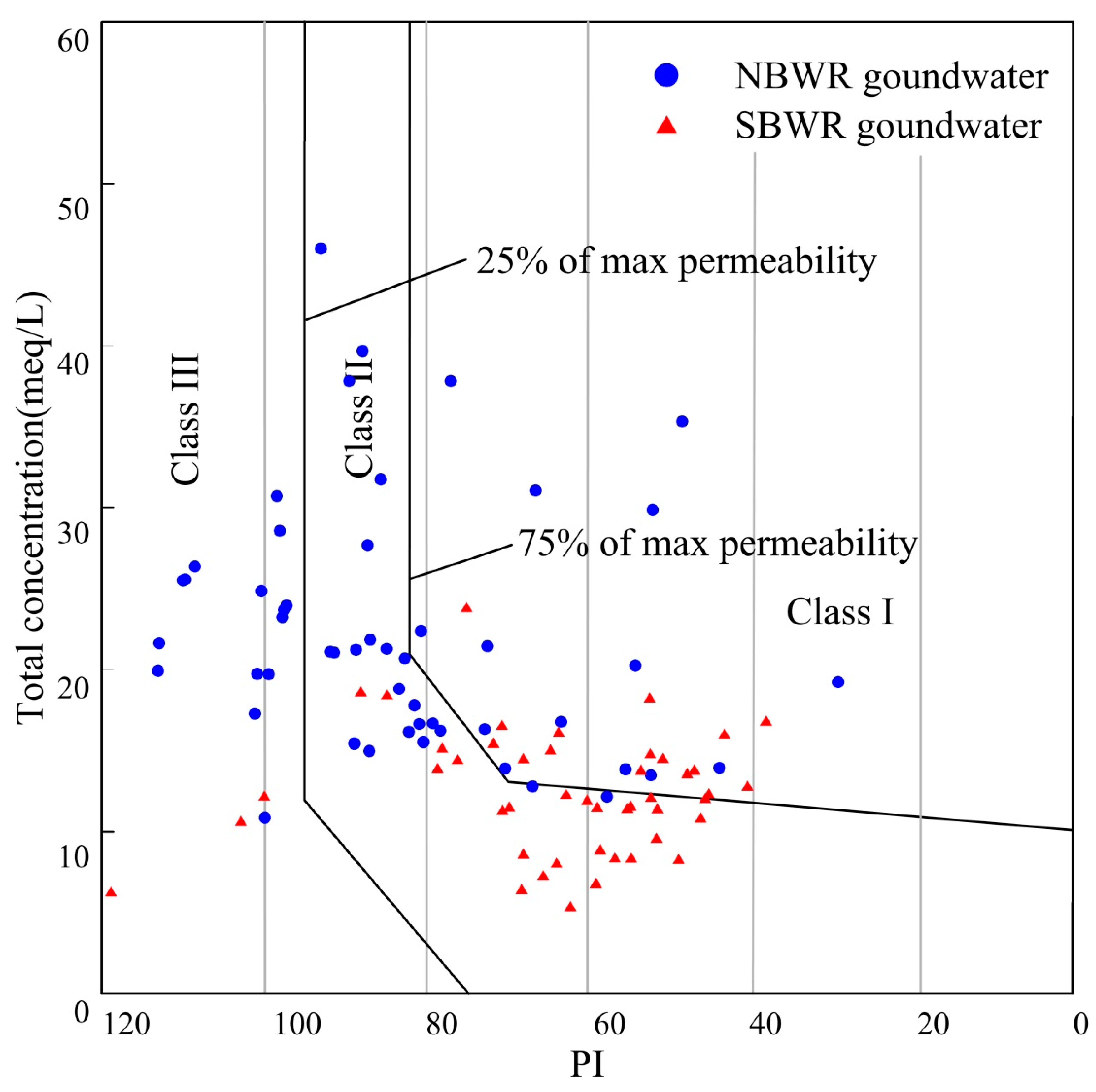
| Index | Unit | NBWR Groundwater | SBWR Groundwater | ||||
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | Maximum | Minimum | Mean | ||
| K+ + Na+ | mg/L | 412.9 | 13.7 | 153.0 | 154.6 | 3.9 | 38.7 |
| Ca2+ | mg/L | 124.2 | 10.0 | 37.1 | 125.5 | 13.6 | 58.3 |
| Mg2+ | mg/L | 86.2 | 7.9 | 32.2 | 59.7 | 2.1 | 20.4 |
| Cl− | mg/L | 400.6 | 6.0 | 60.4 | 122.3 | 2.5 | 14.0 |
| SO42+ | mg/L | 416.9 | 2.4 | 80.2 | 153.7 | 2.2 | 22.6 |
| HCO3− | mg/L | 742.0 | 231.9 | 463.3 | 482.0 | 121.4 | 323.3 |
| pH | - | 8.3 | 7.2 | 7.8 | 8.2 | 7.0 | 7.5 |
| TDS | mg/L | 1324.2 | 300.0 | 608.8 | 692.0 | 140.0 | 323.5 |
| EC | μS/cm | 2407.6 | 545.5 | 1106.8 | 1258.2 | 280.0 | 588.8 |
| Parameter | Unit | NBWR Groundwater | SBWR Groundwater | ||||
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | Maximum | Minimum | Mean | ||
| SH (EC) | μS/cm | 2407.6 | 545.5 | 1106.8 | 1258.2 | 280.0 | 588.8 |
| Na% | % | 83.8 | 6.7 | 56.0 | 72.9 | 3.7 | 24.7 |
| SAR | - | 11.3 | 0.3 | 4.9 | 4.1 | 0.1 | 1.2 |
| RSC | meq/L | 9.3 | −6.9 | 3.1 | 4.0 | −0.7 | 0.7 |
| MH | % | 80.2 | 32.1 | 60.7 | 69.9 | 15.8 | 35.5 |
| PI | % | 113.0 | 29.0 | 82.6 | 118.8 | 37.9 | 62.8 |
| KR | - | 5.2 | 0.1 | 1.8 | 2.7 | 0.1 | 0.5 |
| PS | meq/L | 12.2 | 0.3 | 2.5 | 5.0 | 0.1 | 0.6 |
| K | - | 27.7 | 4.6 | 12.4 | 12.7 | 2.0 | 5.2 |
| Ka | - | 120.4 | 3.0 | 13.0 | 422.1 | 6.4 | 94.5 |
| SH Class | EC (μS/cm) | Water Quality | No. of Samples (Percent) | |
|---|---|---|---|---|
| NBWR Groundwater | SBWR Groundwater | |||
| C1 | <250 | Excellent | - | - |
| C2 | 250–750 | Good | 8 (15.7%) | 40 (87.0%) |
| C3 | 750–2250 | Doubtful | 42 (82.4%) | 6 (13.0%) |
| C4 | >2250 | Unsuitable | 1 (1.9%) | - |
| Na% | Water Quality | No. of Samples (Percent) | |
|---|---|---|---|
| NBWR Groundwater | SBWR Groundwater | ||
| <20 | Excellent | 4 (7.8%) | 26 (56.5%) |
| 20–40 | Good | 7 (13.7%) | 10 (21.7%) |
| 40–60 | Permissible | 16 (31.4%) | 7 (15.3%) |
| 60–80 | Doubtful | 19 (37.3%) | 3 (6.5%) |
| >80 | Unsuitable | 5 (9.8%) | - |
| RSC (meq/L) | Water Quality | No. of Samples (Percent) | |
|---|---|---|---|
| NBWR Groundwater | SBWR Groundwater | ||
| <1.25 | Good | 13 (25.5%) | 37 (80.4%) |
| 1.25–2.50 | Doubtful | 10 (19.6%) | 6 (13.1%) |
| >2.50 | Unsuitable | 28 (54.9%) | 3 (6.5%) |
| PS (meq/L) | Water Quality | No. of Samples (Percent) | |
|---|---|---|---|
| NBWR Groundwater | SBWR Groundwater | ||
| <3.0 | Excellent to good | 41 (80.4%) | 45 (97.8%) |
| 3.0–5.0 | Good to injurious | 3 (5.9%) | - |
| >5.0 | Injurious to unsatisfactory | 7 (13.7%) | 1 (2.2%) |
| Ka | Water Quality | No. of samples (Percent) | |
|---|---|---|---|
| NBWR Groundwater | SBWR Groundwater | ||
| >18 | Excellent | 7 (13.7%) | 33 (71.7%) |
| 6–18 | Permissible | 25 (49.0%) | 13 (28.3%) |
| 1.2–6 | Doubtful | 19 (37.3%) | - |
| <1.2 | Unsuitable | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Feng, W.; Qian, H.; Zhang, Q. Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China. Int. J. Environ. Res. Public Health 2019, 16, 1492. https://doi.org/10.3390/ijerph16091492
Xu P, Feng W, Qian H, Zhang Q. Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China. International Journal of Environmental Research and Public Health. 2019; 16(9):1492. https://doi.org/10.3390/ijerph16091492
Chicago/Turabian StyleXu, Panpan, Wenwen Feng, Hui Qian, and Qiying Zhang. 2019. "Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China" International Journal of Environmental Research and Public Health 16, no. 9: 1492. https://doi.org/10.3390/ijerph16091492
APA StyleXu, P., Feng, W., Qian, H., & Zhang, Q. (2019). Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China. International Journal of Environmental Research and Public Health, 16(9), 1492. https://doi.org/10.3390/ijerph16091492




