Compliance with Multiple Health Behaviour Recommendations: A Cross-Sectional Comparison between Female Cancer Survivors and Those with no Cancer History
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
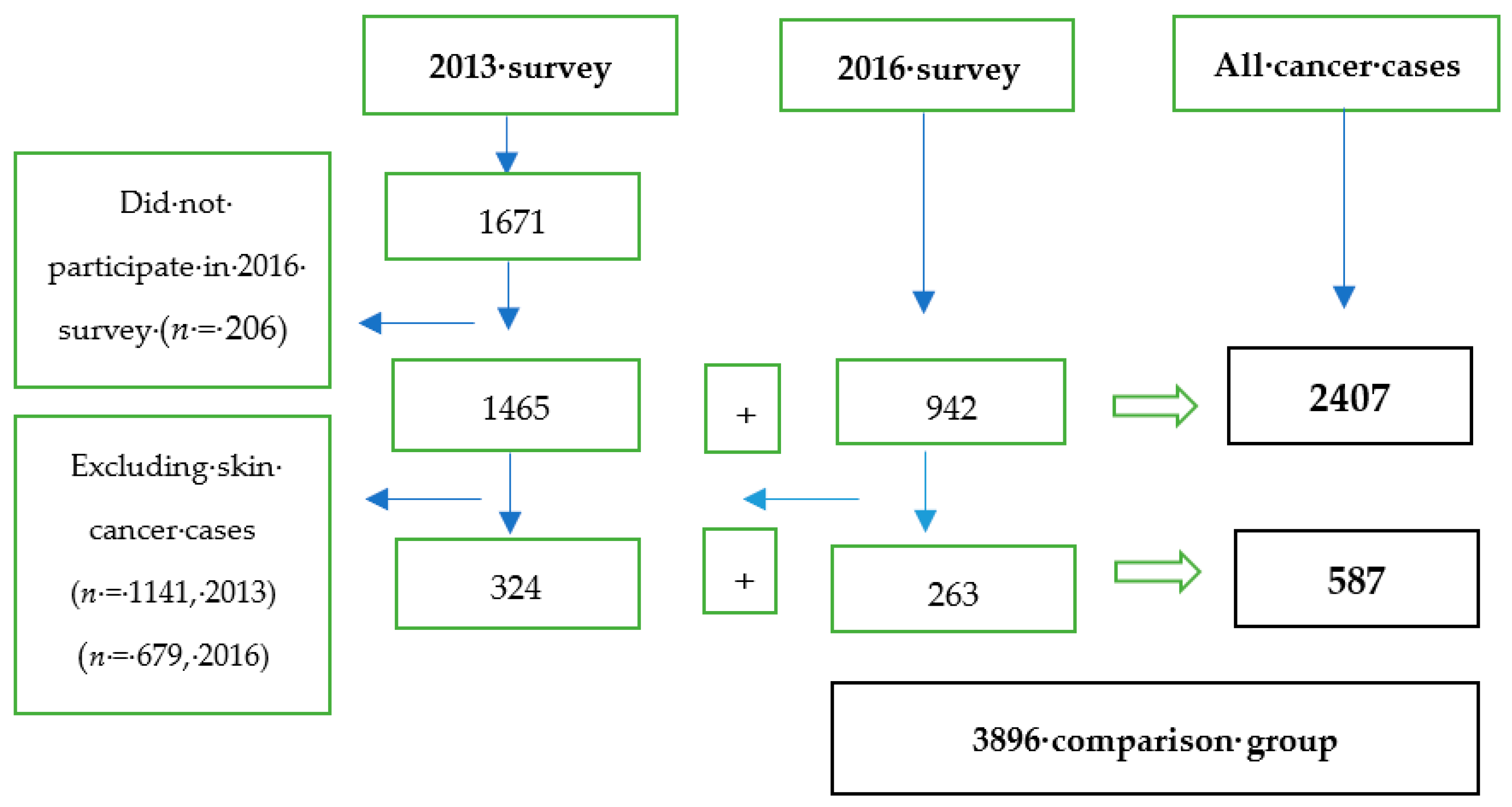
2.2. Study Participants
2.3. Measures
Covariates
2.4. Health Behaviours
2.4.1. Smoking
2.4.2. Physical Activity
2.4.3. Fruit and Vegetable Intake
2.4.4. Alcohol Consumption
2.4.5. Body Mass Index (BMI)
2.4.6. Sugary Drinks
2.4.7. Multiple Health Behaviours
2.5. Data Analysis
3. Results
3.1. Characteristics of Cancer Survivors and Controls
3.2. Adherence to Individual Health Behaviours
3.3. Adherence to Multiple Health Behaviours and Associated Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- WCRF/AICR. Recommendations and Public Health and Policy Implications. Continious Update Projects. 2018. Available online: https://www.wcrf.org/dietandcancer/recommendations/policy-public-health-implications (accessed on 20 October 2018).
- ACS. Cancer Treatment & Survivorship Facts & Figures 2016–2017. Available online: https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html (accessed on 15 October 2018).
- Demark-Wahnefried, W.; Rogers, L.Q.; Alfano, C.M.; Thomson, C.A.; Courneya, K.S.; Meyerhardt, J.A.; Stout, N.L.; Kvale, E.; Ganzer, H.; Ligibel, J.A. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. Ca Cancer J. Clin. 2015, 65, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Doyle, C.; Demark-Wahnefried, W.; Meyerhardt, J.; Courneya, K.S.; Schwartz, A.L.; Bandera, E.V.; Hamilton, K.K.; Grant, B.; McCullough, M.; et al. Nutrition and physical activity guidelines for cancer survivors. Ca Cancer J. Clin. 2012, 62, 243–274. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Survivorship Initiative. Living with and beyond Cancer; Taking Action to Improve Outcomes; Department of Health and Scoial Care: London, UK, 2013. [Google Scholar]
- Demark-Wahnefried, W.; Jones, L.W. Promoting a healthy lifestyle among cancer survivors. Hematol. Oncol. Clin. North Am. 2008, 22, 319–342. [Google Scholar] [CrossRef] [PubMed]
- Minian, N.; deRuiter, W.K.; Lingam, M.; Corrin, T.; Dragonetti, R.; Manson, H.; Taylor, V.H.; Zawertailo, L.; Ebnahmady, A.; Melamed, O.C.; et al. The effects of interventions targeting multiple health behaviors on smoking cessation outcomes: A rapid realist review protocol. Syst. Rev. 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.J.; Spring, B.; Nigg, C.R. Multiple health behavior change research: An introduction and overview. Prev. Med. 2008, 46, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Williams, K.; Steptoe, A.; Wardle, J. The impact of a cancer diagnosis on weight change: Findings from prospective, population-based cohorts in the UK and the US. BMC Cancer 2014, 14, 926. [Google Scholar] [CrossRef]
- Satia, J.A.; Campbell, M.K.; Galanko, J.A.; James, A.; Carr, C.; Sandler, R.S. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol. Biomark. Prev. A 2004, 13, 1022–1031. [Google Scholar]
- Sprague, B.L.; Trentham-Dietz, A.; Nichols, H.B.; Hampton, J.M.; Newcomb, P.A. Change in lifestyle behaviors and medication use after a diagnosis of ductal carcinoma in situ. Breast Cancer Res. Treat. 2010, 124, 487–495. [Google Scholar] [CrossRef]
- Steinhilper, L.; Geyer, S.; Sperlich, S. Health behavior change among breast cancer patients. Int. J. Public Health 2013, 58, 603–613. [Google Scholar] [CrossRef]
- Hawkins, M.L.; Buys, S.S.; Gren, L.H.; Simonsen, S.E.; Kirchhoff, A.C.; Hashibe, M. Do cancer survivors develop healthier lifestyle behaviors than the cancer-free population in the PLCO study? J. Cancer Surviv. Res. Pract. 2017, 11, 233–245. [Google Scholar] [CrossRef]
- Humpel, N.; Magee, C.; Jones, S.C. The impact of a cancer diagnosis on the health behaviors of cancer survivors and their family and friends. Support Care Cancer 2007, 15, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Choi, K.S.; Suh, M.; Jun, J.K.; Chuck, K.W.; Park, B. Risky Lifestyle Behaviors Among Gastric Cancer Survivors Compared with Matched Non-cancer Controls: Results from Baseline Result of Community Based Cohort Study. Cancer Res 2017, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Bluethmann, S.M.; Basen-Engquist, K.; Vernon, S.W.; Cox, M.; Gabriel, K.P.; Stansberry, S.A.; Carmack, C.L.; Blalock, J.A.; Demark-Wahnefried, W. Grasping the ‘teachable moment’: Time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology 2015. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Aziz, N.M.; Rowland, J.H.; Pinto, B.M. Riding the crest of the teachable moment: Promoting long-term health after the diagnosis of cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 5814–5830. [Google Scholar] [CrossRef] [PubMed]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. A 2013, 22, 792–802. [Google Scholar] [CrossRef]
- Kanera, I.M.; Bolman, C.A.; Mesters, I.; Willems, R.A.; Beaulen, A.A.; Lechner, L. Prevalence and correlates of healthy lifestyle behaviors among early cancer survivors. BMC Cancer 2016, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kong, S.Y.; Kim, J.; Kim, Y.; Park, I.H.; Jung, S.Y.; Lee, E.S. Health Behaviors of Cancer Survivors in Nationwide Cross-Sectional Survey in Korea: Higher Alcohol Drinking, Lower Smoking, and Physical Inactivity Pattern in Survivors with Higher Household Income. Medicine 2015, 94, e1214. [Google Scholar] [CrossRef]
- Brown, W.; Bryson, L.; Byles, J.; Dobson, A.; Manderson, L.; Schofield, M.; Williams, G. Women’s health Australia: Establishment of the Australian longitudinal study on women’s health. J. Women’s Health 1996, 5. [Google Scholar] [CrossRef]
- Loxton, D.; Powers, J.; Anderson, A.E.; Townsend, N.; Harris, M.L.; Tuckerman, R.; Pease, S.; Mishra, G.; Byles, J. Online and Offline Recruitment of Young Women for a Longitudinal Health Survey: Findings From the Australian Longitudinal Study on Women’s Health 1989–95 Cohort. J. Med. Internet Res. 2015, 17, e109. [Google Scholar] [CrossRef]
- Stavrou, E.; Vajdic, C.M.; Loxton, D.; Pearson, S.A. The validity of self-reported cancer diagnoses and factors associated with accurate reporting in a cohort of older Australian women. Cancer Epidemiol. 2011, 35, e75–e80. [Google Scholar] [CrossRef]
- Heesch, K.C.; Hill, R.L.; van Uffelen, J.G.Z.; Brown, W.J. Are Active Australia physical activity questions valid for older adults? J. Sci. Med. Sport 2011, 14, 233–237. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Bruno, E.; Gargano, G.; Villarini, A.; Traina, A.; Johansson, H.; Mano, M.P.; Santucci De Magistris, M.; Simeoni, M.; Consolaro, E.; Mercandino, A.; et al. Adherence to WCRF/AICR cancer prevention recommendations and metabolic syndrome in breast cancer patients. Int. J. Cancer 2016, 138, 237–244. [Google Scholar] [CrossRef]
- Song, F.; Qureshi, A.A.; Giovannucci, E.L.; Fuchs, C.S.; Chen, W.Y.; Stampfer, M.J.; Han, J. Risk of a second primary cancer after non-melanoma skin cancer in white men and women: A prospective cohort study. PLoS Med. 2013, 10, e1001433. [Google Scholar] [CrossRef]
- Eakin, E.G.; Youlden, D.R.; Baade, P.D.; Lawler, S.P.; Reeves, M.M.; Heyworth, J.S.; Fritschi, L. Health behaviors of cancer survivors: Data from an Australian population-based survey. Cancer Causes Control 2007, 18, 881–894. [Google Scholar] [CrossRef]
- LeMasters, T.J.; Madhavan, S.S.; Sambamoorthi, U.; Kurian, S. Health behaviors among breast, prostate, and colorectal cancer survivors: A US population-based case-control study, with comparisons by cancer type and gender. J. Cancer Surviv. Res. Pract. 2014, 8, 336–348. [Google Scholar] [CrossRef]
- Ollberding, N.J.; Maskarinec, G.; Wilkens, L.R.; Henderson, B.E.; Kolonel, L.N. Comparison of modifiable health behaviours between persons with and without cancer: The Multiethnic Cohort. Public Health Nutr. 2011, 14, 1796–1804. [Google Scholar] [CrossRef]
- James, E.; Freund, M.; Booth, A.; Duncan, M.J.; Johnson, N.; Short, C.E.; Wolfenden, L.; Stacey, F.G.; Kay-Lambkin, F.; Vandelanotte, C. Comparative efficacy of simultaneous versus sequential multiple health behavior change interventions among adults: A systematic review of randomised trials. Prev. Med. 2016, 89, 211–223. [Google Scholar] [CrossRef]
- Berdan, C.A.; Tangney, C.C.; Scala, C.; Stolley, M. Childhood cancer survivors and adherence to the American Cancer Society Guidelines on Nutrition and Physical Activity. J. Cancer Surviv. Res. Pract. 2014, 8, 671–679. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Lazovich, D.; Prizment, A.E.; Robien, K. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations for cancer prevention is associated with better health-related quality of life among elderly female cancer survivors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 1758–1766. [Google Scholar] [CrossRef]
- Smith, W.A.; Li, C.; Nottage, K.A.; Mulrooney, D.A.; Armstrong, G.T.; Lanctot, J.Q.; Chemaitilly, W.; Laver, J.H.; Srivastava, D.K.; Robison, L.L.; et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer 2014, 120, 2742–2750. [Google Scholar] [CrossRef]
- Song, S.; Hwang, E.; Moon, H.-G.; Noh, D.-Y.; Lee, J.E. Adherence to Guidelines for Cancer Survivors and Health-Related Quality of Life among Korean Breast Cancer Survivors. Nutrients 2015, 7, 10307–10319. [Google Scholar] [CrossRef]
- Spector, D.J.; Noonan, D.; Mayer, D.K.; Benecha, H.; Zimmerman, S.; Smith, S.K. Are lifestyle behavioral factors associated with health-related quality of life in long-term survivors of non-Hodgkin lymphoma? Cancer 2015, 121, 3343–3351. [Google Scholar] [CrossRef]
- Von Gruenigen, V.E.; Waggoner, S.E.; Frasure, H.E.; Kavanagh, M.B.; Janata, J.W.; Rose, P.G.; Courneya, K.S.; Lerner, E. Lifestyle challenges in endometrial cancer survivorship. Obstet. Gynecol. 2011, 117, 93–100. [Google Scholar] [CrossRef]
- Winkels, R.M.; van Lee, L.; Beijer, S.; Bours, M.J.; van Duijnhoven, F.J.; Geelen, A.; Hoedjes, M.; Mols, F.; de Vries, J.; Weijenberg, M.P.; et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research lifestyle recommendations in colorectal cancer survivors: Results of the PROFILES registry. Cancer Med. 2016, 5, 2587–2595. [Google Scholar] [CrossRef]
- Pinto, B.M.; Trunzo, J.J. Health behaviors during and after a cancer diagnosis. Cancer 2005, 104, 2614–2623. [Google Scholar] [CrossRef]
- Lee, L.; Cheung, W.Y.; Atkinson, E.; Krzyzanowska, M.K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 106–117. [Google Scholar] [CrossRef]
- Drake, B.F.; Quintiliani, L.M.; Sapp, A.L.; Li, Y.; Harley, A.E.; Emmons, K.M.; Sorensen, G. Comparing strategies to assess multiple behavior change in behavioral intervention studies. Transl. Behav. Med. 2013, 3, 114–121. [Google Scholar] [CrossRef]
| Variable | WCRF/AICR Recommendation | Measurement Scale | Operationalize | Scoring |
|---|---|---|---|---|
| Body weight | Be as lean as possible without becoming underweight and maintain body weight within the normal range | Body Mass Index (BMI) | BMI (kg/m2) < 18.5 | 0 |
| BMI = 18.5–24.9 | 1 | |||
| BMI = 25–29.9 | 0 | |||
| BMI ≥ 30 | 0 | |||
| Physical activity | Avoid inactivity and return to normal daily activities as soon as possible following cancer diagnosis Aim to exercise at least 30 min per day or at least 150 min/week, which is equivalent to 600 MET/Week | Metabolic equivalent of task (MET) value | Level 1 (sedentary) = 0 −< 40 MET min/week | 0 |
| Level 2 (insufficiently active) = 40 −< 600 MET min/week | 0 | |||
| Level 3 (sufficiently active) = 600 −<1200 MET min/week | 1 | |||
| Level 4 (very active) = ≥1200 MET min/week | 1 | |||
| Fruit intake | Consume at least 2 serves of fruit per day | Number of serves | ≥2 serves of fruit per day | 1 |
| <2 serves of fruit per day | 0 | |||
| Vegetable intake | Consume at least 5 portions/servings (>400 g) of a variety vegetables every day | Portion size (Serving) | ≥5 serving of vegetable per day | 1 |
| <5 serving of fruit and vegetable (F&V) per day | 0 | |||
| Smoking | Entirely avoided tobacco If you currently smoke or use tobacco in any form, ask your health professional about ways to quit | Yes/no | Current smoker | 0 |
| Non-smoker or ex-smoker | 1 | |||
| Alcohol | Avoid alcoholic drinks, otherwise limit intake to two drinks for men and one drink for women a day. | Standard drink (1 SD refers to 10 grams of alcohol) | ≤1–2 standard drink per day | 1 |
| >2 standard drink per day | 0 | |||
| Sugary drinks | Avoid the intake of sugar drinks | Frequency | Not drink sugary drinks | 1 |
| Drink at least once in a month | 0 |
| Scio-Demographic and Health Characteristics | Controls (%) | Cancer Survivors Including Skin Cancer | Pa | Cancer Survivors Excluding Skin Cancer | Pb | All Cancer Cases (Long-Term and Recent Survivors) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Long-Term Freq., (%) | Recent Freq., (%) | Long-Term Freq., (%) | RecentFreq., (%) | With Skin Cancer (%) | Pc | Excl. Skin Cancer (%) | Pd | ||||
| Age | |||||||||||
| Total (Mean, SD) | 3896 (66.9, 1.47) | 1465 (67.2, 1.44) | 942 (67.2, 1.43) | 0.714 | 324 (67.2, 1.47) | 263 (67.2, 1.43) | 0.862 | 2407 (67.8, 1.42) | <0.01 | 587 (67.8, 1.42) | 0.012 |
| Age group | |||||||||||
| 63–67 years 68–70 years | 60.8 39.2 | 57.0 43.0 | 56.9 43.1 | 0.529 | 57.1 42.9 | 58.2 41.8 | 0.658 | 53.4 46.6 | <0.01 | 55.2 44.8 | 0.308 |
| Current residential area (N) | 3810 | 1436 | 919 | 320 | 256 | 2355 | 576 | ||||
| Urban Not Urban | 66.2 33.8 | 63.4 36.6 | 62.5 37.5 | 0.717 | 62.8 37.2 | 61.6 38.4 | 0.805 | 63.1 36.9 | 0.023 | 62.3 37.7 | 0.103 |
| Current marital status (N) | 3859 | 1456 | 932 | 322 | 259 | 2388 | 581 | ||||
| Married Not married | 66.2 33.8 | 64.7 35.3 | 66.3 33.6 | 0.515 | 63.8 36.2 | 62.2 37.8 | 0.742 | 65.3 34.7 | 0.582 | 63.1 36.9 | 0.249 |
| Current occupation (N) | 3767 | 1429 | 908 | 311 | 254 | 2,337 | 565 | ||||
| Un paid job Paid job | 76.1 23.9 | 79.4 20.6 | 82.6 17.4 | 0.129 | 76.4 23.6 | 84.8 15.2 | 0.049 | 80.7 19.3 | <0.01 | 80.2 19.8 | 0.086 |
| Highest qualification (at baseline survey) (N) | 3863 | 1456 | 936 | 321 | 262 | 2597 | 660 | ||||
| No formal education | 13.6 | 10.8 | 12.4 | 0.038 | 13.6 | 15.9 | 0.274 | 11.4 | 0.015 | 14.7 | 0.699 |
| Certificate (Intermediate/high school) | 45.8 | 46.9 | 52.7 | 45.6 | 50.2 | 49.2 | 47.7 | ||||
| Certificate (diploma/apprenticeship) | 21.2 | 21.9 | 17.8 | 18.2 | 19.4 | 20.3 | 18.7 | ||||
| University degree | 19.4 | 20.4 | 17.1 | 22.6 | 14.5 | 19.1 | 18.9 | ||||
| Reported comorbid chronic diseases * (N) | 3896 | 1465 | 942 | 324 | 263 | 2407 | 587 | ||||
| None One to two Three or more | 36.3 51.6 12.1 | 30.7 51.1 18.2 | 29.1 53.8 17.1 | 0.602 | 32.2 51.3 16.5 | 31.3 52.9 15.7 | 0.952 | 30.1 52.2 17.7 | <0.01 | 31.8 52.0 16.2 | 0.047 |
| Lifestyle Behaviours | Controls (n = 4415) | Cancer Survivors Including Skin Cancer | Pa | Cancer Survivors Excluding Skin Cancer | Pb | All Cancer Cases (Long-Term and Recent Survivors) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Long-Term Freq., (%) | Recent Freq., (%) | Long-Term Freq., (%) | Recent Freq., (%) | Incl. Skin Cancer Survivors Freq., (%) | Pc | Excl. Skin Cancer Survivors Freq., (%) | Pd | ||||
| Body Mass Index (BMI) | |||||||||||
| <18.5 Kg/m2 8.5–24.9 Kg/m2 25 kg/m2–29.9 Kg/m2 ≥30 kg/m2 Met the recommendation Not met the recommendation | 44 (1.2) 1254 (34.1) 1267 (34.5) 1111 (30.2) | 19 (1.4) 485 (35.0) 461 (33.9) 434 (29.7) | 17 (2.1) 317 (38.7 )281 (30.7) 286 (28.4) | 0.312 | 4 (1.3) 86 (28.1) 97 (31.7) 119 (38.9) | 6 (2.4) 89 (35.0) 71 (28.0) 88 (34.7) | 0.066 | 36 (1.6) 802 (34.9) 742 (32.2) 720 (31.3) | 0.530 | 10 (1.7) 175 (31.3) 168 (30.0) 207 (39.9) | 0.130 |
| 1254 (34.1) 2422 (65.9) | 485 (34.9) 914 (65.1) | 317 (38.7) 584 (61.3) | 0.156 | 86 (28.1) 220 (71.9) | 89 (35.0) 165 (65.0) | 0.027 * | 802 (34.8) 1498 (65.2) | 0.796 | 175 (31.2) 385 (68.8) | 0.544 | |
| Physical activity | |||||||||||
| ≤40 MET/week 40–600 MET /week 600–1200 MET/week ≥1200 MET/week Sufficient (≥600 MET/week) Insufficient (<600 MET/week) | 752 (19.3) 888 (22.8) 754 (19.4) 1502 (38.5) | 284 (18.6) 323 (22.1) 314 (21.2) 544 (38.1) | 191 (17.7) 190 (21.1) 198 (19.7) 363 (41.5) | 0.633 | 78 (24.1) 78 (24.1) 71 (21.9) 97 (29.9) | 62 (23.5) 62 (23.5) 53 (20.2) 86 (32.7) | 0.856 | 475 (19.7) 513 (21.3) 512 (21.3) 907 (37.7) | 0.848 | 140 (23.8) 140 (23.8) 124 (21.1) 183 (31.2) | 0.204 |
| 2256 (57.9) 1640 (42.1) | 858 (59.3) 607 (40.7) | 561 (61.2) 381 (38.8) | 0.463 | 168 (51.8) 156 (48.2) | 139 (52.8) 124 (47.2) | 0.820 | 1,419 (58.9) 988 (41.1) | 0.685 | 307 (52.3) 280 (47.7) | 0.078 | |
| Smoking | |||||||||||
| Not smoke at all (met the recommendation) | 3630 (94.6) | 1381 (94.8) | 888 (95.4) | 0.959 | 302 (94.1) | 243 (93.5) | 0.467 | 2,269 (95.1) | 0.246 | 545 (93.8) | 0.311 |
| Smoke; daily, weekly or monthly (not met the recommendation) | 207 (5.4) | 75 (5.2) | 43 (4.6) | 19 (5.9) | 17 (6.5) | 118 (4.9) | 36 (6.2) | ||||
| Fruit consumption | |||||||||||
| <2 pieces per day≥2 pieces per day | 1356 (35.0) 2517 (65.0) | 505 (34.6) 953 (65.4) | 346 (37.0) 589 (63.0) | 0.181 | 113 (35.0) 210 (65.0) | 101 (38.5) 161 (61.5) | 0.250 | 851 (35.6) 1542 (64.4) | 0.261 | 214 (36.5) 371 (63.4) | 0.231 |
| Vegetable consumption | |||||||||||
| <5 serving per day≥5 serving per day | 3312 (85.6) 558 (14.4) | 1236 (84.7) 223 (15.3) | 800 (85.6) 135 (14.4) | 0.621 | 284 (87.9) 39 (12.1) | 223 (85.1) 39 (14.9) | 0.897 | 2036 (85.1) 358 (14.9) | 0.839 | 507 (86.6) 78 (13.3) | 0.217 |
| Fruit and vegetable | |||||||||||
| <5 serving F&V per/day Not met both recommendations Met at least one recommendation ≥5 serving F&V per day | 3460 (89.4) 1207 (34.9) 2253 (65.1) 409 (10.6) | 1292 (88.6) 449 (30.8) 843 (57.8) 166 (11.4) | 830 (88.7) 316 (33.8) 514 (55.0) 105 (11.3) | 0.569 | 296 (91.6) 101 (34.1) 195 (65.9) 27 (8.4) | 229 (87.4) 95 (41.5) 134 (58.5) 33 (12.6) | 0.582 | 2,122 (88.7) 765 (36.1) 1357 (63.9) 271 (11.3) | 0.935 | 525 (89.7) 196 (37.3) 329 (62.7) 60 (10.3) | 0.360 |
| Alcohol | |||||||||||
| Never drink (current and ever) 1–2 SD per day >2 SD per day Met the recommendation Not met the recommendation | 629 (16.7) 2710 (71.8) 434 (11.5) | 249 (17.5) 1012 (71.0) 164 (11.5) | 169 (18.5) 649 (71.1) 95 (10.4) | 0.237 | 64 (20.5) 215 (68.7) 34 (10.9) | 42 (16.5) 190 (74.5) 23 (9.1) | 0.733 | 418 (17.9) 1661 (71.0) 259 (11.1) | 0.699 | 106 (18.6) 405 (71.3) 57 (10.0) | 0.701 |
| 3339 (88.5) 434 (11.5) | 1261 (88.5) 164 (11.5) | 818 (89.6) 95 (10.4) | 0.402 | 279 (89.1) 34 (10.9) | 232 (90.9) 23 (9.1) | 0.480 | 2079 (88.9) 259 (11.1) | 0.427 | 511 (89.9) 57 (10.0) | 0.719 | |
| Sugar drinks | |||||||||||
| Never drink Drink at least once in a month | 2947 (76.7) 896 (23.3) | 1124 (77.3) 331 (22.7) | 688 (74.3) 238 (25.7) | 0.083 | 233 (72.2) 90 (27.8) | 181 (69.1) 81 (30.9) | 0.380 | 1812 (76.1) 569 (23.9) | 0.197 | 414 (70.7) 171 (29.3) | 0.001 * |
| Multiple behaviours | |||||||||||
| ≤1 behaviour 2–3 behaviours 4 behaviours 5–6 behaviours | 134 (3.3) 1713 (44.0) 1254 (32.3) 795 (20.4) | 41 (2.8) 619 (42.3) 484 (33.0) 321 (21.9) | 28 (2.9) 405 (42.9) 305 (32.4) 204 (21.6) | 0.640 | 10 (3.1) 165 (50.9) 103 (31.8) 46 (14.2) | 8 (3.0) 131 (49.8) 76 (28.9) 48 (18.2) | 0.395 | 69 (2.8) 1024 (42.5) 789 (32.8) 525 (21.8) | 0.571 | 18 (3.1) 296 (50.4) 179 (30.5) 94 (16.0) | 0.031 * |
| Adherence to Health Behaviours (Coded “1” for Those Who Adhered and “0” for Those Who did not) | Controls n = 3896 | Cancer Survivors Including Skin Cancer (n = 2407) | Cancer Survivors Excluding Skin Cancer (n = 587) | All Survivors (Long and Recent Survivors) Together | |||
|---|---|---|---|---|---|---|---|
| Long-Term AOR (95% CI) | Recent AOR (95% CI) | Long-Term AOR (95% CI) | Recent AOR (95% CI) | With Skin Cancer AOR (95% CI) | Excl. Skin Cancer AOR (95% CI) | ||
| Body Mass Index (BMI) | Ref. | 1.04 (0.87, 1.23) | 1.25 (1.02, 1.53) * | 0.79 (0.56, 1.11) | 1.33 (0.95, 1.85) | 1.11 (0.96, 1.29) | 1.01 (0.78, 1.29) |
| Physical activity | Ref. | 1.04 (0.89, 1.22) | 1.15 (0.94, 1.39) | 0.83 (0.62, 1.12) | 0.85 (0.61, 1.19) | 1.08 (0.94, 1.24) | 0.84 (0.67, 1.06) |
| Smoking | Ref. | 1.33 (0.94, 1.89) | 1.31 (0.84, 2.04) | 1.23 (0.63, 2.42) | 0.66 (0.35, 1.24) | 1.33 (0.98, 1.79) | 0.89 (0.55, 1.44) |
| Fruit consumption | Ref. | 0.94 (0.80, 1.11) | 0.80 (0.66, 0.97) * | 0.95 (0.70, 1.31) | 0.70 (0.50, 0.98) | 0.89 (0.78, 1.01) | 0.83 (0.65, 1.05) |
| Vegetable consumption | Ref. | 0.96 (0.80, 1.20) | 0.92 (0.70, 1.21) | 0.80 (0.51, 1.25) | 0.82 (0.51, 1.32) | 0.94 (0.81, 1.14) | 0.80 (0.57, 1.13) |
| Fruit and vegetable consumption | Ref. | 0.98 (0.76,1.31) | 0.92 (0.70, 1.24) | 0.77 (0.45, 1.31) | 0.94 (0.60, 1.56) | 0.95 (0.77, 1.22) | 0.86 (0.59, 1.25) |
| Alcohol intake | Ref. | 1.05 (0.82, 1.35) | 1.26 (0.92, 1.72) | 0.97 (0.61, 1.54) | 1.27 (0.74, 2.25) | 1.12 (0.90, 1.38) | 1.08 (0.76, 1.59) |
| Sugar drinks | Ref. | 1.02 (0.84, 1.21) | 0.82 (0.65, 1.02) | 0.74 (0.52, 1.02) | 0.62 (0.42, 0.87) | 0.93 (0.78, 1.09) | 0.68 (0.53, 0.87) * |
| Associated Factors | Adherence to Multiple Behaviours β; with Skin Cancer Survivors | Adherence to multiple behaviours β; without Skin Cancer Survivors | ||
|---|---|---|---|---|
| COR (95% CI) | AOR (95% CI) | COR (95% CI) | AOR (95% CI) | |
| Cancer diagnosis history | ||||
| Controls (no cancer history) | 1.00 | 1.00 | 1.00 | 1.00 |
| Recent survivors | 1.07 (0.93, 1.22) | 1.19 (1.04, 1.37) ** | 0.83 (0.66, 1.05) | 0.95 (0.74, 1.21) |
| Long-term survivors | 1.10 (0.98, 1.23) | 1.14 (1.02, 1.29) ** | 0.75 (0.61, 0.93) ** | 0.80 (0.64, 1.03) |
| Age group | ||||
| 63–68 years | 1.00 | 1.00 | 1.00 | 1.00 |
| 69–73 years | 1.08 (0.98, 1.18) | 1.16 (1.05, 1.27) * | 1.07 (0.93, 1.18) | 1.10 (0.98, 1.23) |
| Residential area/regionality | ||||
| Not urban | 1.00 | 1.00 | 1.00 | 1.00 |
| Urban | 1.21(1.10,1.33) * | 1.12 (1.02, 1.24) ** | 1.21 (1.10, 1.33) * | 1.12 (0.99, 1.26) |
| Current Occupation | ||||
| Not paid job | 1.00 | 1.00 | 1.00 | 1.00 |
| Paid job | 1.16 (1.04, 1.31) * | 1.04 (0.93, 1.17) | 1.16 (1.04, 1.30) | 1.06 (0.92, 1.21) |
| Education status | ||||
| No formal education | 1.00 | 1.00 | 1.00 | 1.00 |
| Certificate (intermediate/high school) | 1.72 (1.49, 1.99) * | 1.49 (1.28, 1.74) ** | 1.72 (1.49, 1.99) * | 1.51 (1.26, 1.80) * |
| Certificate (diploma/apprenticeship) | 2.31 (1.96, 2.71) * | 2.05 (1.73, 2.43) * | 2.30 (1.96, 2.71) * | 2.11 (1.73, 2.58) * |
| University degree | 3.10 (2.61, 3.68) * | 2.74 (2.29, 3.28) * | 3.10 (2.61, 3.68) * | 2.77 (2.24, 3.42) * |
| Marital status | ||||
| Not married | 1.00 | 1.00 | 1.00 | 1.00 |
| Married | 1.26 (1.14, 1.40) * | 1.28 (1.15, 1.42) * | 1.26 (1.14, 1.40) * | 1.25 (1.11, 1.42) * |
| Other chronic diseases | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| 1–2 | 0.65 (0.58, 0.72) * | 0.66 (0.59, 0.73) * | 0.65 (0.58, 0.71) * | 0.64 (0.57, 0.73) * |
| ≥3 | 0.34 (0.29, 0.39) * | 0.36 (0.30, 0.41) * | 0.34 (0.29, 0.39) * | 0.36 (0.30, 0.44) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tollosa, D.N.; Tavener, M.; Hure, A.; James, E.L. Compliance with Multiple Health Behaviour Recommendations: A Cross-Sectional Comparison between Female Cancer Survivors and Those with no Cancer History. Int. J. Environ. Res. Public Health 2019, 16, 1345. https://doi.org/10.3390/ijerph16081345
Tollosa DN, Tavener M, Hure A, James EL. Compliance with Multiple Health Behaviour Recommendations: A Cross-Sectional Comparison between Female Cancer Survivors and Those with no Cancer History. International Journal of Environmental Research and Public Health. 2019; 16(8):1345. https://doi.org/10.3390/ijerph16081345
Chicago/Turabian StyleTollosa, Daniel N, Meredith Tavener, Alexis Hure, and Erica L James. 2019. "Compliance with Multiple Health Behaviour Recommendations: A Cross-Sectional Comparison between Female Cancer Survivors and Those with no Cancer History" International Journal of Environmental Research and Public Health 16, no. 8: 1345. https://doi.org/10.3390/ijerph16081345
APA StyleTollosa, D. N., Tavener, M., Hure, A., & James, E. L. (2019). Compliance with Multiple Health Behaviour Recommendations: A Cross-Sectional Comparison between Female Cancer Survivors and Those with no Cancer History. International Journal of Environmental Research and Public Health, 16(8), 1345. https://doi.org/10.3390/ijerph16081345





