Diversity in the Factors Associated with ADL-Related Disability among Older People in Six Middle-Income Countries: A Cross-Country Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Outcome Variable
2.2. Covariates
2.2.1. Sociodemographic Factors
2.2.2. Health Behaviours and Chronic Conditions
2.3. Statistical Analysis
2.4. Ethical Consideration
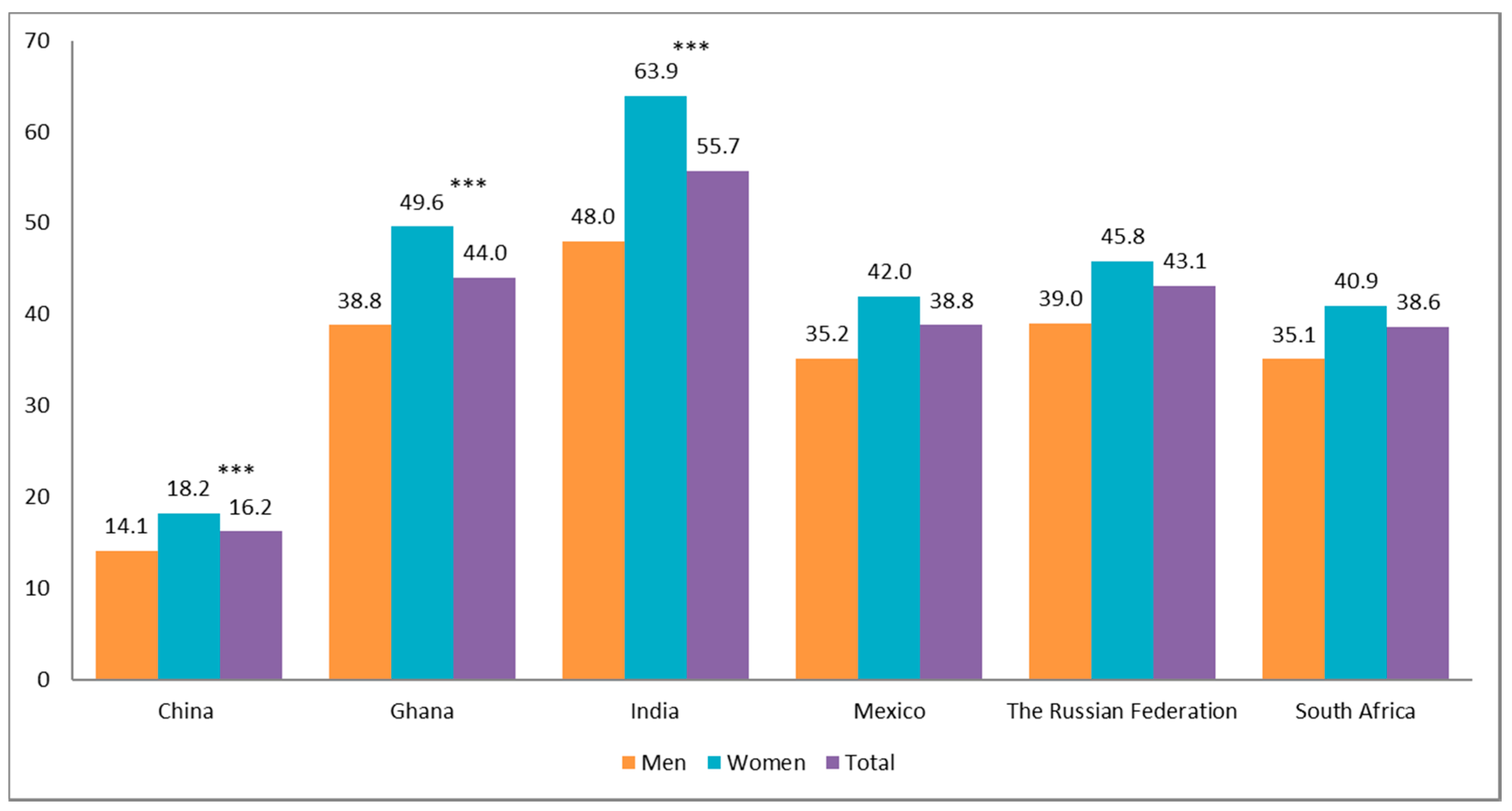
3. Results
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Variables | China | Ghana | India | Mexico | Russian Federation | South Africa | All countries |
|---|---|---|---|---|---|---|---|
| Angina (%) | 8.0 | 12.2 | 17 | 14 | 36.4 | 7.9 | 16.7 |
| Stroke (%) | 1.9 | 1.5 | 1.0 | 1.9 | 3.1 | 2.7 | 1.8 |
| Asthma (%) | 3.9 | 3.7 | 10.8 | 3.7 | 5.9 | 6.6 | 7.0 |
| Chronic lung disease (%) | 4.9 | 0.3 | 2.2 | 0.6 | 6.8 | 1.6 | 4.1 |
| Diabetes (%) | 6.5 | 3.8 | 6.8 | 17.5 | 6.9 | 9.4 | 6.7 |
| Hypertension (%) | 25.2 | 13.1 | 14.1 | 25.4 | 51.3 | 30.0 | 25.8 |
| Arthritis (%) | 20.5 | 23.5 | 23.7 | 12.3 | 33.5 | 26.3 | 24.3 |
| Depression (%) | 2.0 | 9.1 | 19.1 | 14.3 | 7.0 | 5.5 | 9.6 |
References
- World Health Organization (WHO). US National Institute of Aging; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- United Nations. World Population Ageing 2015; United Nations: New York, NY, USA, 2015. [Google Scholar]
- United Nations DESA. World Population Ageing 2009; United Nations Publications: New York, NY, USA, 2010; Volume 295. [Google Scholar]
- World Health Organization (WHO). World Report on Disability; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4 068521-5. [Google Scholar]
- World Health Organization (WHO). World Health Day 2012: Ageing and Health: Toolkit for Event Organizers; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Wunderlich, G.S. Improving the Measurement of Late-life Disability in Population Surveys: Beyond ADLs and IADLs: Summary of a Workshop; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged—The Index of ADL—A Standardized Measure of Biological and Psychosocial Function. Jama 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Mor, V.; Wilcox, V.; Rakowski, W.; Hiris, J. Functional transitions among the elderly—patterns, predictors, and related hospital use. Am. J. Public Health 1994, 84, 1274–1280. [Google Scholar] [CrossRef]
- Covinsky, K. Aging, arthritis, and disability. Arthritis Rheum. 2006, 55, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Taş, Ü.; Verhagen, A.P.; Bierma-Zeinstra, S.; Hofman, A.; Odding, E.; Pols, H.A.; Koes, B.W. Incidence and risk factors of disability in the elderly: The Rotterdam Study. Prev. Med. 2007, 44, 272–278. [Google Scholar] [CrossRef]
- Bahat, G.; Tufan, F.; Saka, B.; Akin, S.; Ozkaya, H.; Yucel, N.; Erten, N.; Karan, M.A. Which body mass index (BMI) is better in the elderly for functional status? Arch. Gerontol. Geriatr. 2012, 54, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Tak, E.; Kuiper, R.; Chorus, A.; Hopman-Rock, M. Prevention of onset and progression of basic ADL disability by physical activity in community dwelling older adults: A meta-analysis. Ageing Res. Rev. 2013, 12, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Cena, D.; Jimenez-Garcia, R.; Hernandez-Barrera, V.; Alonso-Blanco, C.; Carrasco-Garrido, P.; Fernandez-de-las-Penas, C. Has the Prevalence of Disability Increased Over the Past Decade (2000—2007) in Elderly People? A Spanish Population-based Survey. J. Am. Med. Dir. Assoc. 2012, 13, 136–142. [Google Scholar] [CrossRef]
- Balzi, D.; Lauretani, F.; Barchielli, A.; Ferrucci, L.; Bandinelli, S.; Buiatti, E.; Milaneschi, Y.; Guralnik, J.M. Risk factors for disability in older persons over 3-year follow-up. Age Ageing 2010, 39, 92–98. [Google Scholar] [CrossRef]
- Taş, Ü.; Steyerberg, E.W.; Bierma-Zeinstra, S.M.A.; Hofman, A.; Koes, B.W.; Verhagen, A.P. Age, gender and disability predict future disability in older people: The Rotterdam Study. BMC Geriatr. 2011, 11, 22. [Google Scholar] [CrossRef]
- Dunlop, D.D.; Manheim, L.M.; Sohn, M.-W.; Liu, X.; Chang, R.W. Incidence of functional limitation in older adults: The impact of gender, race, and chronic conditions. Arch. Phys. Med. Rehabil. 2002, 83, 964–971. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Fried, L.P.; Salive, M.E. Disability as a public health outcome in the aging population. Ann. Rev. Public Health 1996, 17, 25–46. [Google Scholar] [CrossRef]
- Aida, J.; Kondo, K.; Kawachi, I.; Subramanian, S.V.; Ichida, Y.; Hirai, H.; Kondo, N.; Osaka, K.; Sheiham, A.; Tsakos, G.; et al. Does social capital affect the incidence of functional disability in older Japanese? A prospective population-based cohort study. J. Epidemiol. Community Health 2013, 67, 42–47. [Google Scholar] [CrossRef] [PubMed]
- den Ouden, M.E.M.; Schuurmans, M.J.; Mueller-Schotte, S.; Brand, J.S.; van der Schouw, Y.T. Domains Contributing to Disability in Activities of Daily Living. J. Am. Med. Dir. Assoc. 2013, 14, 18–24. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Muenchrath, M.N.; Kowal, P.R. Shades of Gray: A Cross-Country Study of Health and Well-Being of the Older Populations in SAGE Countries, 2007–2010; US Department of Commerce, Economics and Statistics Administration, US Census Bureau: Washington, DC, USA, 2012.
- Gomez-Olive, F.X.; Schröders, J.; Aboderin, I.; Byass, P.; Chatterji, S.; Davies, J.I.; Debpuur, C.; Hirve, S.; Hodgson, A.; Juvekar, S.; et al. Variations in disability and quality of life with age and sex between eight lower income and middle-income countries: Data from the INDEPTH WHO-SAGE collaboration. BMJ Glob. Health. 2017, 2, e000508. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; King, A.C. Disability and chronic disease among older adults in India: Detecting vulnerable populations through the WHO SAGE Study. Am. J. Epidemiol. 2013, 178, 1620–1628. [Google Scholar] [CrossRef]
- Stewart Williams, J.; Norström, F.; Ng, N. Disability and ageing in China and India – decomposing the effects of gender and residence. Results from the WHO study on global AGEing and adult health (SAGE). BMC Geriatr. 2017, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Kowal, P.; Kahn, K.; Ng, N.; Naidoo, N.; Abdullah, S.; Bawah, A.; Binka, F.; Chuc, N.T.K.; Debpuur, C.; Ezeh, A.; et al. Ageing and adult health status in eight lower-income countries: The INDEPTH WHO-SAGE collaboration. Glob. Health Action 2010, 3, 11–22. [Google Scholar] [CrossRef]
- Kowal, P.; Chatterji, S.; Naidoo, N.; Biritwum, R.; Fan, W.; Ridaura, R.L.; Maximova, T.; Arokiasamy, P.; Phaswana-Mafuya, N.; Williams, S. Data resource profile: The World Health Organization Study on global AGEing and adult health (SAGE). Int. J. Epidemiol. 2012, 41, 1639–1649. [Google Scholar] [CrossRef]
- Üstün, T.B.; Organization, W.H.; Kostanjsek, N.; Chatterji, S.; Rehm, J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Harpham, T.; Grant, E.; Thomas, E. Measuring social capital within health surveys: Key issues. Health Policy Plan. 2002, 17, 106–111. [Google Scholar] [CrossRef]
- Ng, N.; Eriksson, M. Social capital and self-rated health in older populations in lower-and upper-middle income countries. In Social Capital as a Health Resource in Later Life: The Relevance of Context; Nyqvist, F., Forsman, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 157–176. ISBN 978-94-017-9614-9. [Google Scholar]
- Bull, F.C.; Maslin, T.S.; Armstrong, T. Global physical activity questionnaire (GPAQ): Nine country reliability and validity study. J. Phys. Act. Health 2009, 6, 790–804. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Physical Activity Questionnaire (GPAQ) Analysis Guide; Department of Chronic Diseases and Health Promotion, Surveillance and Population-Based Prevention, World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization (WHO). The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Crimmins, E.M.; Kim, J.K.; Solé-Auró, A. Gender differences in health: Results from SHARE, ELSA and HRS. Eur. J. Public Health 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Arias-Merino, E.D.; Mendoza-Ruvalcaba, N.M.; Ortiz, G.G.; Velazquez-Brizuela, I.E.; Meda-Lara, R.M.; Cueva-Contreras, J. Physical function and associated factors in community-dwelling elderly people in Jalisco, Mexico. Arch. Gerontol. Geriatr. 2012, 54, E271–E278. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.C.; Mau, L.W.; Tasi, W.L.; Hsieh, Y.H.; Liu, H.W. Chronic medical conditions as predictors of functional disability in an older population in Taiwan. Australas. J. Ageing 2004, 23, 19–24. [Google Scholar] [CrossRef]
- Zuccolo, P.F.; Avila, R.; Nakano, E.Y.; Litvoc, J.; Lopes, M.A.; Bottino, C.M.C. Instrumental activities of daily living performance in healthy and cognitively intact seniors from a Brazilian sample and its relation to age and other socio-demographic variables. Int. Psychogeriatr. 2012, 24, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Painter, P.; Franklin, B.; Morgan, D.; Pitetti, K.; Roberts, S. Physical Activity for the Chronically Ill and Disabled. Sports Med. 2000, 30, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, C.F.; Mambrini, J.V.; Luz, T.C.B.; Loyola Filho, A.I.d. Association between disability and social capital among community-dwelling elderly. Rev. Bras. Epidemiol. 2016, 19, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Nyqvist, F.; Gustavsson, J.; Gustafsson, Y. Social capital and health in the oldest old: The Umeå 85+ study. Int. J. Ageing Later Life 2006, 1, 91–114. [Google Scholar] [CrossRef]
- Berkman, L.F.; Kawachi, I. Social cohesion, social capital, and health. In Social Epidemiology, 2nd ed.; Berkman, L.F., Kawachi, I., Glymour, M.M., Eds.; Oxford University Press: Oxford, UK, 2014; pp. 321–361. ISBN 13: 9780195377903. [Google Scholar]
- Murayama, H.; Fujiwara, Y.; Kawachi, I. Social capital and health: A review of prospective multilevel studies. J. Epidemiol. 2012, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wang, C.; Yang, F.; Cui, D.; Wang, Q.; Mao, Z. Association between social capital and physical activity among community-dwelling elderly in Wuhan, China. Int. J. Gerontol. 2017, 12, 155–159. [Google Scholar] [CrossRef]
- Hosseinpoor, A.R.; Bergen, N.; Kunst, A.; Harper, S.; Guthold, R.; Rekve, D.; d’Espaignet, E.T.; Naidoo, N.; Chatterji, S. Socioeconomic inequalities in risk factors for non communicable diseases in low-income and middle-income countries: Results from the World Health Survey. BMC Public Health 2012, 12, 912. [Google Scholar] [CrossRef]
- Bruce, M.L. Depression and Disability in Late Life: Directions for Future Research. Am. J. Geriatr. Psychiatry 2001, 9, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ormel, J.; Rijsdijk, F.V.; Sullivan, M.; van Sonderen, E.; Kempen, G.I.J.M. Temporal and Reciprocal Relationship Between IADL/ADL Disability and Depressive Symptoms in Late Life. J. Gerontol. B. Psychol. Sci. Soc. Sci. 2002, 57, P338–P347. [Google Scholar] [CrossRef]
- Bacon, K.L.; Heeren, T.; Keysor, J.J.; Stuver, S.O.; Cauley, J.A.; Fredman, L. Longitudinal and Reciprocal Relationships Between Depression and Disability in Older Women Caregivers and Noncaregivers. Gerontologist 2016, 56, 723–732. [Google Scholar] [CrossRef]
- Kumar, A.; Karmarkar, A.M.; Tan, A.; Graham, J.E.; Arcari, C.M.; Ottenbacher, K.J.; Snih, S.A. The effect of obesity on incidence of disability and mortality in Mexicans aged 50 years and older. Salud Publica Mex. 2015, 57 (Suppl. 1), S31–S38. [Google Scholar]
- Al Snih, S.; Graham, J.E.; Kuo, Y.-F.; Goodwin, J.S.; Markides, K.S.; Ottenbacher, K.J. Obesity and disability: Relation among older adults living in Latin America and the Caribbean. Am. J. Epidemiol. 2010, 171, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Himes, C.L.; Reynolds, S.L. Effect of obesity on falls, injury, and disability. J. Am. Geriatr. Soc. 2012, 60, 124–129. [Google Scholar] [CrossRef]
- Schäfer, I.; Hansen, H.; Schön, G.; Höfels, S.; Altiner, A.; Dahlhaus, A.; Gensichen, J.; Riedel-Heller, S.; Weyerer, S.; Blank, W.A. The influence of age, gender and socio-economic status on multimorbidity patterns in primary care. First results from the multicare cohort study. BMC Health Serv. Res. 2012, 12, 89. [Google Scholar] [CrossRef]
- Green, S.M.; Watson, R. Nutritional screening and assessment tools for older adults: Literature review. J. Adv. Nurs. 2006, 54, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Cook, Z.; Kirk, S.; Lawrenson, S.; Sandford, S. Use of BMI in the assessment of undernutrition in older subjects: Reflecting on practice. Proc. Nutr. Soc. 2005, 64, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Coin, A.; Sergi, G.; Inelmen, E.M.; Enzi, G. Pathophysiology of Body Composition Changes in Elderly People. In Cachexia and Wasting: A Modern Approach; Mantovani, G., Anker, S.D., Inui, A., Morley, J.E., Fanelli, F.R., Scevola, D., Schuster, M.W., Yeh, S.-S., Eds.; Springer: Milan, Italy, 2006; pp. 369–375. ISBN 978-88-470-0552-5. [Google Scholar]
| Sociodemographic variables | China (n = 12,085) | Ghana (n = 4057) | India (n = 6257) | Mexico (n = 3940) | Russian Federation (n = 3422) | South Africa (n = 2806) | |
|---|---|---|---|---|---|---|---|
| Gender (%) | |||||||
| Men | 49.3 | 52.5 | 51.2 | 46.3 | 39.7 | 40.7 | |
| Women | 50.7 | 47.5 | 48.8 | 53.7 | 60.3 | 59.3 | |
| Age group (%) | |||||||
| 50–59 years | 45.2 | 40.5 | 49.2 | 50.7 | 46.8 | 49.3 | |
| 60–69 years | 32.0 | 27.4 | 30.9 | 25.4 | 24.7 | 31.0 | |
| 70–79 years | 18.5 | 22.8 | 15.7 | 17.0 | 21.6 | 14.3 | |
| 80+ years | 4.2 | 9.3 | 4.2 | 6.9 | 6.9 | 5.3 | |
| Marital status (%) | |||||||
| Partnered | 85.3 | 59.4 | 77.5 | 73.9 | 59.4 | 52.3 | |
| Not currently partnered | 14.7 | 40.6 | 22.5 | 26.1 | 40.6 | 47.7 | |
| Education level (%) | |||||||
| High | 3.9 | 3.7 | 5.2 | 7.7 | 17.7 | 6.1 | |
| Medium | 31.9 | 21.6 | 19.2 | 12.6 | 75.2 | 22.5 | |
| Low | 64.1 | 74.7 | 75.6 | 79.7 | 7.1 | 71.4 | |
| Access to social capital (%) | |||||||
| Structural and cognitive | 36.9 | 88.9 | 60.7 | 45.4 | 37.1 | 55.5 | |
| Structural only | 0.5 | 5.3 | 5.5 | 11.1 | 16.9 | 39.3 | |
| Cognitive only | 61.2 | 5.5 | 29.9 | 36.3 | 29.0 | 2.8 | |
| Neither | 1.3 | 0.4 | 3.9 | 7.2 | 17.0 | 2.4 | |
| Wealth status (%) | |||||||
| 5th quintile | 20.6 | 21.3 | 24.0 | 27.1 | 24.5 | 21.5 | |
| 4th quintile | 23.6 | 21.0 | 19.7 | 16.0 | 21.8 | 18.6 | |
| 3rd quintile | 20.6 | 20.4 | 19.0 | 16.3 | 18.8 | 19.8 | |
| 2nd quintile | 18.7 | 19.0 | 19.0 | 25.6 | 19.5 | 20.4 | |
| 1st quintile | 16.5 | 18.3 | 18.3 | 15.0 | 15.5 | 19.7 | |
| Area of Residence (%) | |||||||
| Urban | 46.0 | 40.7 | 25.7 | 78.0 | 72.5 | 65.3 | |
| Rural | 54.0 | 59.3 | 74.3 | 22.0 | 27.5 | 34.7 | |
| Body Mass Index (%) | |||||||
| Underweight | 6.3 | 19.3 | 44.1 | 1.0 | 1.6 | 4.6 | |
| Normal | 58.5 | 51.2 | 43.0 | 20.7 | 23.1 | 23.1 | |
| Overweight | 26.5 | 16.6 | 9.3 | 40.8 | 33.7 | 22.4 | |
| Obese | 8.8 | 12.9 | 3.5 | 37.5 | 41.6 | 49.8 | |
| Physical activity level (%) | |||||||
| High | 45.8 | 63.1 | 53.3 | 40.9 | 60.5 | 29.2 | |
| Moderate | 26.8 | 12.4 | 23.1 | 23.0 | 16.0 | 13.6 | |
| Low | 27.4 | 24.5 | 23.7 | 36.0 | 23.5 | 57.2 | |
| Chronic conditions (%) | |||||||
| None | 51.8 | 54.1 | 44.9 | 44.7 | 27.9 | 46.9 | |
| 1 chronic condition | 30.8 | 29.4 | 29.9 | 31.7 | 26.8 | 29.8 | |
| Multimorbidity | 17.4 | 16.6 | 25.2 | 23.6 | 45.3 | 23.4 | |
| Depression (%) | 2.0 | 9.1 | 19.1 | 14.3 | 7.0 | 5.5 | |
| Odds Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| China | Ghana | India | Mexico | Russian Federation | South Africa | ||
| Gender | Men | 1 | 1 | 1 | 1 | 1 | 1 |
| Women | 1.14 * (1.01–1.29) | 1.22 * (1.01–1.48) | 1.65 *** (1.37–1.99) | 1.26 (0.75–2.10) | 0.77 (0.46–1.27) | 1.00 (0.76–1.31) | |
| Age group | 50–59 | 1 | 1 | 1 | 1 | 1 | 1 |
| 60–69 | 1.49 *** (1.24–1.79) | 1.40 *** (1.16–1.71) | 1.31 ** (1.08–1.59) | 1.05 (0.63–1.76) | 1.73 * (1.04–2.86) | 1.12 (0.84–1.49) | |
| 70–79 | 2.60 *** (2.06–3.29) | 2.45 *** (1.94–3.08) | 1.87 *** (1.44–2.43) | 1.43 (0.90–2.28) | 3.50 *** (1.74–7.04) | 2.10 *** (1.48–2.97) | |
| 80+ | 4.99 *** (3.66–6.80) | 3.89 *** (2.79–5.44) | 3.24 *** (2.17–4.83) | 3.52 *** (1.71–7.22) | 8.52 *** (4.27–17.0) | 3.63 *** (2.05–6.44) | |
| Marital status | Partnered | 1 | 1 | 1 | 1 | 1 | 1 |
| Not partnered | 0.99 (0.83–1.17) | 1.16 (0.95–1.41) | 1.01 (0.82–1.24) | 0.50 ** (0.30–0.83) | 1.44 (0.97–2.14) | 1.21 (0.89–1.63) | |
| Education level | High | 1 | 1 | 1 | 1 | 1 | 1 |
| Medium | 1.39 (0.90–2.13) | 1.20 (0.76–1.90) | 1.79 ** (1.20–2.67) | 0.94 (0.23–3.76) | 1.80 * (1.15–2.80) | 1.60 (0.76–3.39) | |
| Low | 1.91 ** (1.30–2.79) | 1.58 * (1.01–2.47) | 2.54 *** (1.71–3.79) | 1.28 (0.43–3.78) | 3.54 *** (1.69–7.42) | 1.79 (0.84–3.79) | |
| Access to social capital | Both | 1 | 1 | 1 | 1 | 1 | 1 |
| Structural only | 0.78 (0.25–2.45) | 0.86 (0.46–1.62) | 0.99 (0.67–1.46) | 0.69 (0.30–1.56) | 2.21 * (1.08–4.55) | 1.36 * (1.03–1.81) | |
| Cognitive only | 1.07 (0.83–1.38) | 0.83 (0.51–1.35) | 0.80 * (0.65–1.00) | 2.54 *** (1.54–4.21) | 1.59 * (1.09–2.31) | 1.84 (0.91–3.73) | |
| Neither | 2.57 *** (1.54–4.31) | 1.24 (0.39–3.97) | 1.21 (0.81–1.79) | 1.03 (0.36–2.92) | 1.68 (0.88–3.24) | 4.11 *** (1.79–9.43) | |
| Wealth status | 1st quintile | 2.03 *** (1.39–2.96) | 1.23 (0.88–1.70) | 1.59 ** (1.20–2.11) | 1.96 * (1.01–3.83) | 1.11 (0.73–1.69) | 0.95 (0.57–1.59) |
| 2nd quintile | 1.91 ** (1.31–2.77) | 1.34 (0.99–1.82) | 1.45 ** (1.15–1.83) | 0.80 (0.37–1.72) | 1.13 (0.71–1.81) | 0.93 (0.60–1.45) | |
| 3rd quintile | 2.24 *** (1.63–3.08) | 1.86 *** (1.40–2.47) | 1.50 ** (1.13–1.98) | 1.74 (0.84–3.60) | 0.98 (0.58–1.67) | 0.75 (0.49–1.16) | |
| 4th quintile | 1.65 ** (1.19–2.29) | 1.09 (0.85–1.39) | 1.28 (0.99–1.65) | 0.73 (0.37–1.43) | 0.49 ** (0.29–0.82) | 0.76 (0.54–1.07) | |
| 5th quintile | 1 | 1 | 1 | 1 | 1 | 1 | |
| Residence | Urban | 1 | 1 | 1 | 1 | 1 | 1 |
| Rural | 2.16 *** (1.73–2.69) | 1.12 (0.83–1.50) | 0.94 (0.75–1.19) | 2.39 * (1.16–4.92) | 0.98 (0.63–1.51) | 1.13 (0.81–1.58) | |
| Body mass index (BMI) | Underweight | 0.90 (0.74–1.08) | 1.09 (0.86–1.39) | 1.16 (0.93–1.43) | 1.64 (0.43–6.27) | 1.96 (0.75–5.13) | 0.93 (0.52–1.67) |
| Normal | 1 | 1 | 1 | 1 | 1 | 1 | |
| Overweight | 0.86 (0.74–1.00) | 0.97 (0.77–1.21) | 1.12 (0.87–1.45) | 3.72 *** (1.93–7.17) | 0.95 (0.56–1.59) | 0.81 (0.57–1.15) | |
| Obese | 1.00 (0.81–1.23) | 1.06 (0.83–1.34) | 1.12 (0.68–1.85) | 2.33 ** (1.27–4.27) | 1.82 * (1.09–3.04) | 0.95 (0.69–1.31) | |
| Physical activity | High | 1 | 1 | 1 | 1 | 1 | 1 |
| Moderate | 1.38 *** (1.17–1.63) | 1.37 * (1.02–1.82) | 1.20 (0.99–1.45) | 1.82 (0.91–3.64) | 1.09 (0.75–1.59) | 0.64 * (0.43–0.96) | |
| Low | 2.13 *** (1.74–2.61) | 1.17 (0.87–1.59) | 1.70 *** (1.38–2.10) | 1.94 * (1.11–3.38) | 2.20 ** (1.64–2.96) | 1.03 (0.75–1.43) | |
| Mental health | No Depression | 1 | 1 | 1 | 1 | 1 | 1 |
| Depression | 2.54 *** (1.82–3.54) | 1.51 * (1.05–2.15) | 2.49 *** (1.99–3.11) | 2.28 * (1.20–4.33) | 1.05 (0.58–1.87) | 1.90 ** (1.20–3.00) | |
| Presence of chronic disease | None | 1 | 1 | 1 | 1 | 1 | 1 |
| One chronic disease | 1.59 *** (1.28–1.96) | 1.57 *** (1.30–1.89) | 1.72 *** (1.46–2.03) | 1.72 (0.98–3.02) | 1.82 * (1.13–2.93) | 1.69 *** (1.25–2.30) | |
| Multimorbidity | 2.91 *** (2.43–3.47) | 3.18 *** (2.42–4.18) | 2.64 *** (2.08–3.36) | 3.44 *** (1.92–6.17) | 4.72 *** (2.54–8.79) | 2.83 *** (1.91–4.18) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestari, S.K.; Ng, N.; Kowal, P.; Santosa, A. Diversity in the Factors Associated with ADL-Related Disability among Older People in Six Middle-Income Countries: A Cross-Country Comparison. Int. J. Environ. Res. Public Health 2019, 16, 1341. https://doi.org/10.3390/ijerph16081341
Lestari SK, Ng N, Kowal P, Santosa A. Diversity in the Factors Associated with ADL-Related Disability among Older People in Six Middle-Income Countries: A Cross-Country Comparison. International Journal of Environmental Research and Public Health. 2019; 16(8):1341. https://doi.org/10.3390/ijerph16081341
Chicago/Turabian StyleLestari, Septi Kurnia, Nawi Ng, Paul Kowal, and Ailiana Santosa. 2019. "Diversity in the Factors Associated with ADL-Related Disability among Older People in Six Middle-Income Countries: A Cross-Country Comparison" International Journal of Environmental Research and Public Health 16, no. 8: 1341. https://doi.org/10.3390/ijerph16081341
APA StyleLestari, S. K., Ng, N., Kowal, P., & Santosa, A. (2019). Diversity in the Factors Associated with ADL-Related Disability among Older People in Six Middle-Income Countries: A Cross-Country Comparison. International Journal of Environmental Research and Public Health, 16(8), 1341. https://doi.org/10.3390/ijerph16081341






