Melkersson–Rosenthal Syndrome in Childhood: Report of Three Paediatric Cases and a Review of the Literature
Abstract
1. Introduction
2. Methods
Ethical Statement
3. Case Report
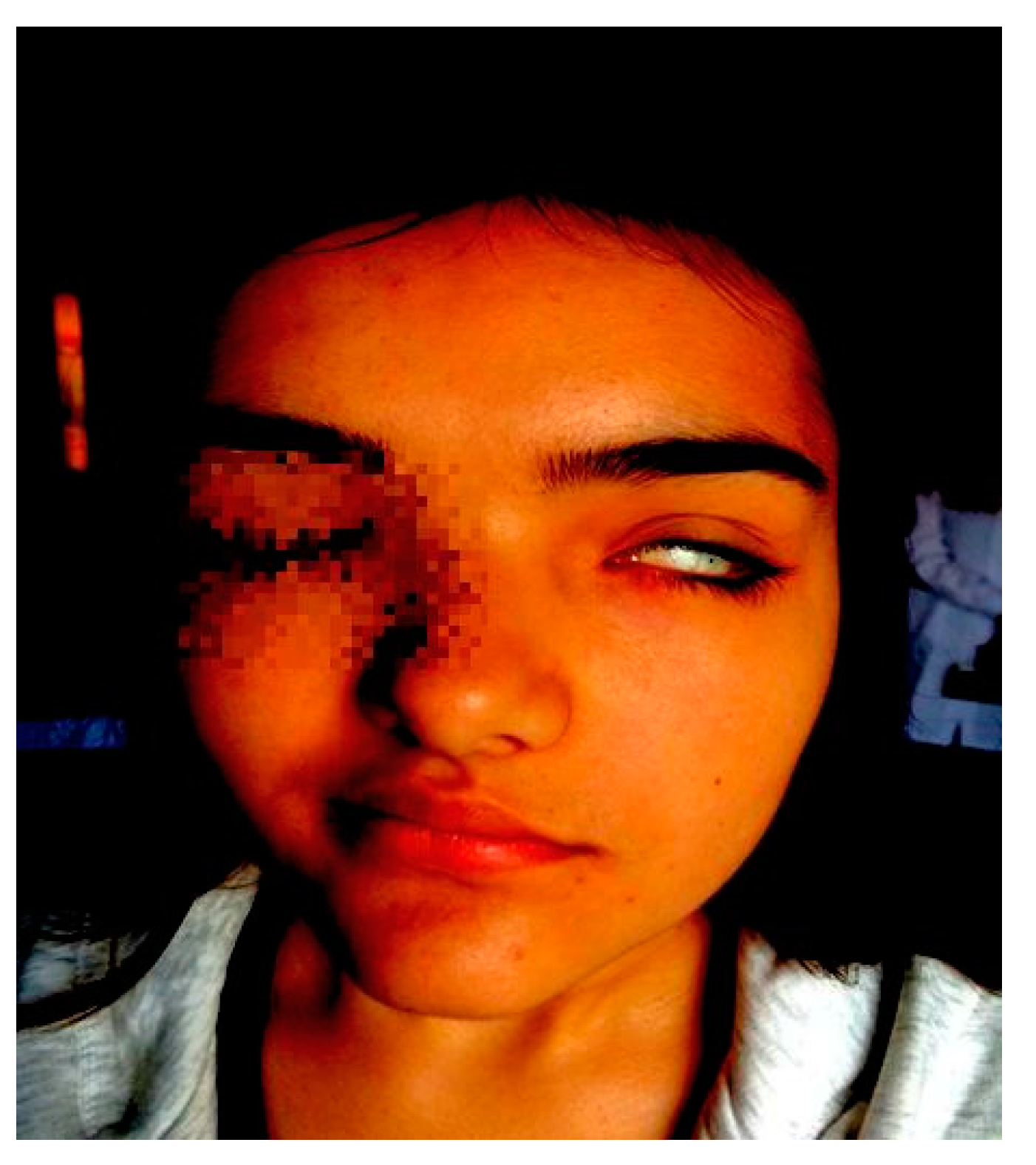
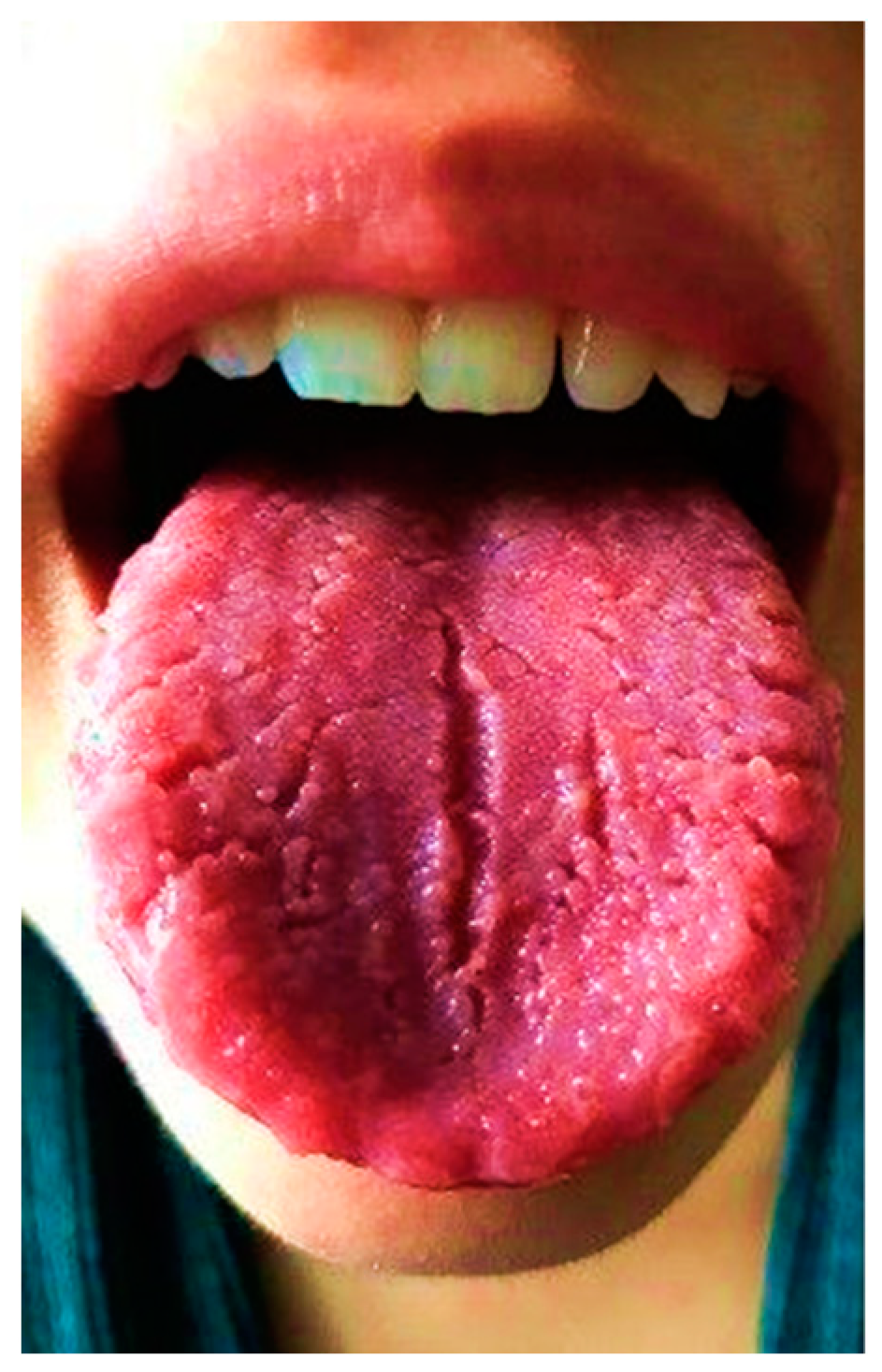
2.1. Patient 1
2.2. Patient 2
2.3. Patient 3
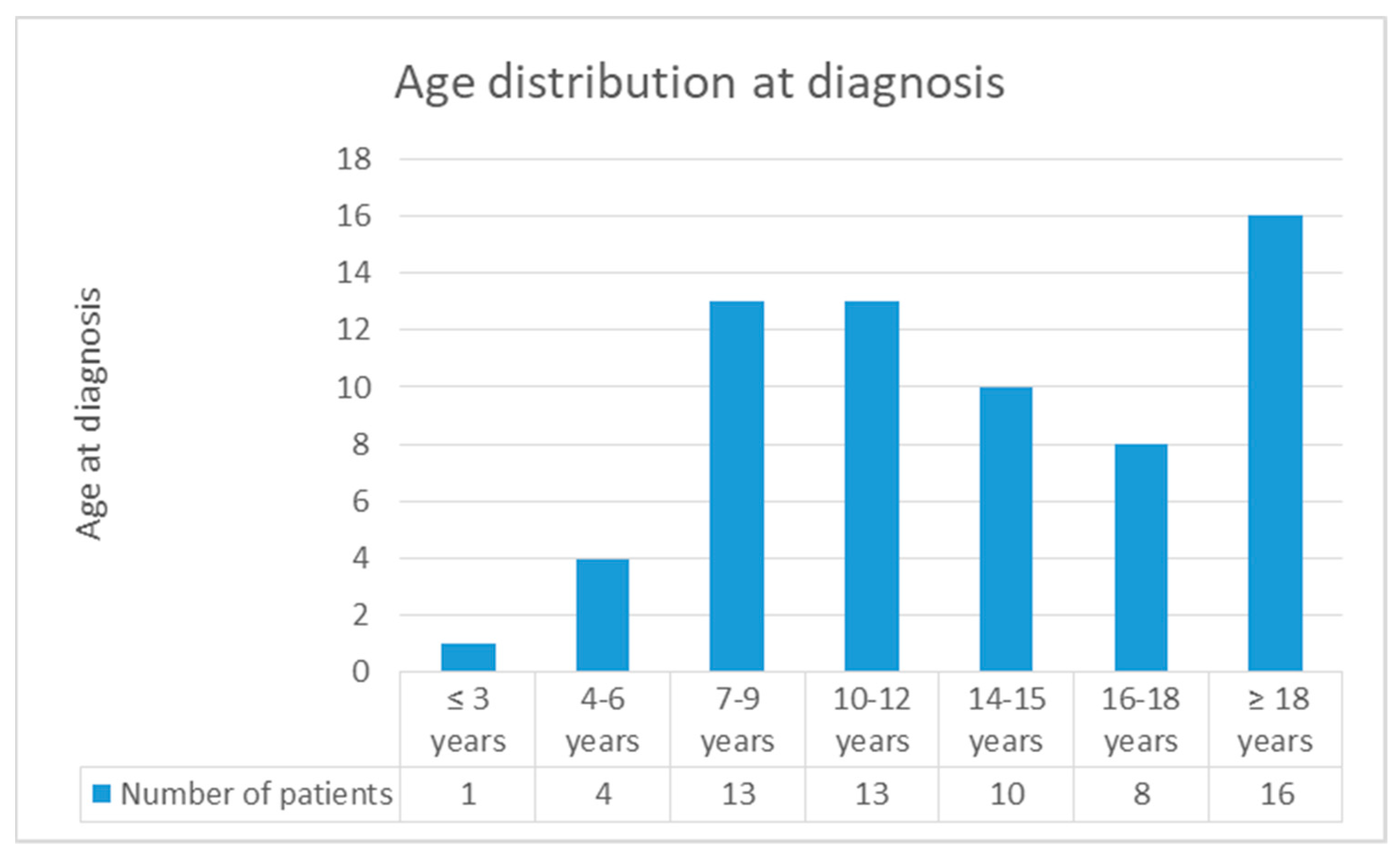
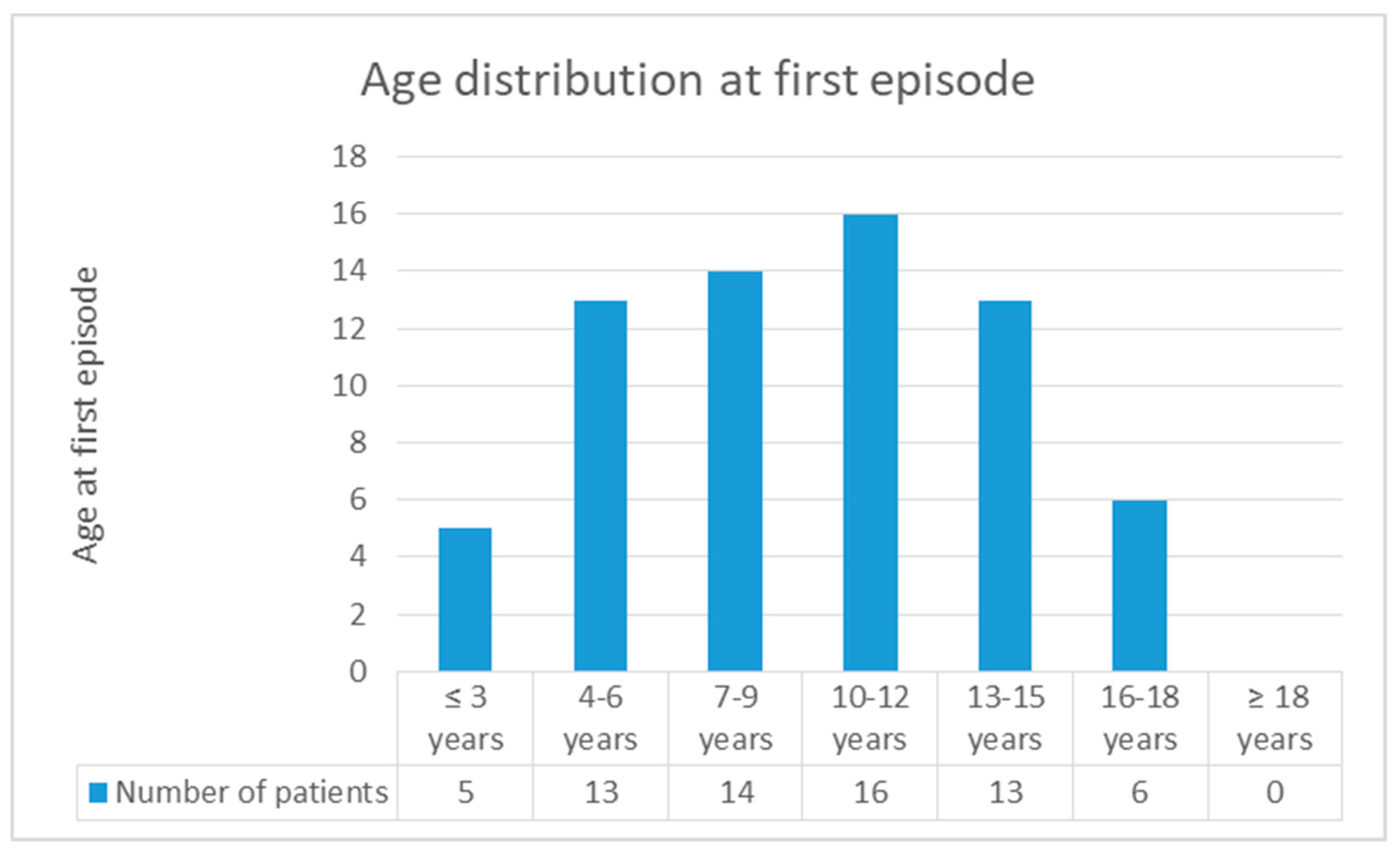
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, T.-Y.; Chiang, C.-H.; Cheng, P.-S. Melkersson-Rosenthal syndrome. J. Formos. Med. Assoc. 2016, 115, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Serrano, C.M.; Man, L.X.; Klein, S.; Schaitkin, B.M. Melkersson-Rosenthal syndrome: A facial nerve center perspective. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Kayhan, F.; Ilik, F.; Kayhan, A. Obsessive-compulsive disorder concurrent with Melkersson-Rosenthal Syndrome: A case report. Gen. Hosp. Psychiatry 2015, 37, 497.e7–497.e9. [Google Scholar] [CrossRef] [PubMed]
- Bohra, S.; Kariya, P.B.; Bargale, S.D.; Kiran, S. Clinicopathological significance of Melkersson-Rosenthal syndrome. BMJ Case Rep. 2015, 2015, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Lalosevic, J.; Gajic-Veljic, M.; Nikolic, M. Orofacial granulomatosis in a 12-year-old girl successfully treated with intravenous pulse corticosteroid therapy and chloroquine. Pediatr. Dermatol. 2017, 34, e324–e327. [Google Scholar] [CrossRef]
- Liu, R.; Yu, S. Melkersson-Rosenthal syndrome: A review of seven patients. J. Clin. Neurosci. 2013, 20, 993–995. [Google Scholar] [CrossRef]
- Ozgursoy, O.B.; Karatayli Ozgursoy, S.; Tulunay, O.; Kemal, O.; Akyol, A.; Dursun, G. Melkersson-Rosenthal syndrome revisited as a misdiagnosed disease. Am. J. Otolaryngol. Head Neck Med. Surg. 2009, 30, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.S.; Tsay, Y.L. Cheilitis granulomatosa associated with allergic contact dermatitis to betel quid. Contact Dermat. 2008, 58, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Dummer, W.; Lurz, C.; Jeschke, R.; Meissner, N.; Rose, C.; Bröcker, E.B. Granulomatous cheilitis and Crohn’s disease in a 3-year-old boy. Pediatr. Dermatol. 1999, 16, 39–42. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cheon, C.K.; Yeon, G.M.; Kim, Y.M.; Nam, S.O. Melkersson-rosenthal syndrome with hashimoto thyroiditis in a 9-year-old girl: An autoimmune disorder. Pediatr. Neurol. 2014, 50, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Basman, A.; Gumusok, M.; Degerli, S.; Kaya, M. Melkersson-rosenthal syndrome: A case report. J. Istanb. Univ. Fac. Dent. 2017, 51, 42–45. [Google Scholar]
- New, G.B.; Kirch, W.A. Permanent enlargement of the lips and face secondary to recurring swellings and asso- ciated with facial paralysis: A clinical entity. J. Am. Med. Assoc. 1933, 100, 1230–1233. [Google Scholar] [CrossRef]
- Ekbom, K.A. Plicated Tongue in Melkersson’s Syndrome and in Paralysis of the Facial Nerve. Acta Med. Scand. 1950, 138, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, B.; Stickl, H. Recurrent Facial Nerve Paresis and Facial Swelling. Mschr. Kinderheilk. 1962, 110, 541–543. [Google Scholar]
- Kunstadter, R.H. Melkersson’s Syndrome. Am. J. Dis. Child. 1965, 110, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Belf, M.D. Observations on Melkersson’s syndrome. Postgrad. Med. J. 1968, 44, 447–452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ziem, P.E.; Pfrommer, C.; Goerdt, S.; Orfanos, C.E. Melkersson—Rosenthal syndrome in childhood: A challenge in differential diagnosis and treatment. Br. J. Dermatol. 2000, 143, 860–863. [Google Scholar] [CrossRef]
- Mukherjee, P.; Dongree, R. A case of acquired facial diplegia, macular oedema and lingua plicata (‘Melkersson-Rosenthal syndrome’). Indian J. Ophtalmol. 1973, 21, 36–39. [Google Scholar]
- Lygidakis, C.; Tsakanikas, C.; Ilias, A.; Vassilopoulos, D. Melkersson—Rosenthal’s syndrome in four generations. Clin. Genet. 1979, 15, 189–192. [Google Scholar] [CrossRef]
- May, M.; Fria, T.J.; Blumenthal, F. Facial paralysis in children: Differential diagnosis. Otolaryngol. Head Neck Surg. 1981, 89, 841–848. [Google Scholar] [CrossRef]
- Grundfast, K.M.; Guarisco, J.L.; Thomsen, J.R.; Koch, B. Diverse etiologies of facial paralysis in children. Int. J. Pediatr. Otorhinolaryngol. 1990, 19, 223–239. [Google Scholar] [CrossRef]
- Stein, S.L.; Mancini, A.J. Melkersson-Rosenthal syndrome in childhood: Successful management with combination steroid and minocycline therapy. J. Am. Acad. Dermatol. 1999, 41, 746–748. [Google Scholar] [CrossRef]
- Zimmer, W.A.L.; Iii, R.S.R.; Reeve, C.M.; Sheridan, P.J. Orofacial manifestation of Melkersson-Rosenthal syndrome. A study of 42 patients and review of 220 cases from the literature. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 610–619. [Google Scholar] [CrossRef]
- Bourgeois-Droin, C.; Havard, S.; Granier, F.; Vesse, M.; Salomon, J.L.; Furioli, J.; Grossin, M. Granulomatous cheilitis in two children with sarcoidosis. J. Am. Acad. Dermatol. 1993, 822–824. [Google Scholar] [CrossRef]
- Marques, C.; Machado, A.; Baptista, A.P. Macroqueilites e sìndrome de Melkersson-Rosenthal. Revisao de 19 casos. Acta Med. Port. 1994, 7, 533–540. [Google Scholar]
- Salgado Alves Vilela, D.; Oto Baliero, F. Melkersson-Rosenthal syndrome: Cases report and literature review. Rev. Bras. Otorrinolaringol. 2002, 68, 755–760. [Google Scholar] [CrossRef]
- Dodi, I.; Verri, R.; Brevi, B. A monosymptomatic Melkersson-Rosenthal syndrome in an 8-year old boy. Acta Biomed. 2006, 77, 20–23. [Google Scholar]
- Hentati, H.; Hammami, S.; Oualha, L.; Selmi, J. Syndrome de Melkersson-Rosenthal à caractère familial: À propos d ’ un cas Melkersson-Rosenthal syndrome with family characters: A case report. Médecine Buccale Chir. Buccale 2007, 14, 29–34. [Google Scholar] [CrossRef]
- Lourenaço, S.V.; Boggio, P.; Suguyama, K.; Nico, M.M.S. Severe and relapsing upper lip enlargement in a 10-year-old boy (Case Presentation). Acta Paediatr. Int. J. Paediatr. 2010, 99, 5253. [Google Scholar] [CrossRef]
- Elias, M.K.; Mateen, F.J.; Weiler, C.R. The Melkersson-Rosenthal syndrome: A retrospective study of biopsied cases. J. Neurol. 2013, 260, 138–143. [Google Scholar] [CrossRef]
- Psillas, G.; Antoniades, E.; Ieridou, F.; Constantinidis, J. Facial nerve palsy in children: A retrospective study of 124 cases. J. Paediatr. Child Health 2018, 55. [Google Scholar] [CrossRef]
- Feng, S.; Yin, J.; Li, J.; Song, Z.; Zhao, G. Melkersson-Rosenthal syndrome: A retrospective study of 44 patients. Acta Otolaryngol. 2014, 134, 977–981. [Google Scholar] [CrossRef]
- Kayabaşoğlu, G.; Fuat Varlı, A.; Güven, M.; Sinan Yılmaz, M. Melkersson-Rosenthal Syndrome in pediatric age group. Eur. J. Gen. Med. 2015, 12, 78–81. [Google Scholar] [CrossRef]
- Saini, A.G.; Sankhyan, N.; Padmanabh, H.; Das, A.; Singhi, P. Recurrent Facial Palsy and Electrophysiological Findings in Oligosymptomatic Melkersson Rosenthal Syndrome. Indian J. Pediatr. 2016, 83, 1188–1190. [Google Scholar] [CrossRef]
- Bakshi, S.S. Melkersson-Rosenthal Syndrome. J. Allergy Clin. Immunol. Pract. 2017, 5, 471–472. [Google Scholar] [CrossRef]
- Chu, Z.; Liu, Y.; Zhang, H.; Zeng, W.; Geng, S. Melkersson-Rosenthal Syndrome with Genitalia Involved in a 12-Year-Old Boy. Ann. Dermatol. 2016, 28, 232–236. [Google Scholar] [CrossRef]
- Bordino, L.; Juchli, M. Síndrome de Melkersson-Rosenthal. Presentación de dos casos pediátricos. Arch. Argent Pediatr. 2016, 114, 224–227. [Google Scholar]
- Xu, X.G.; Guan, L.P.; Lv, Y.; Wan, Y.S.; Wu, Y.; Qi, R.Q.; Liu, Z.G.; Zhang, J.G.; Chen, Y.L.; Xu, F.P.; et al. Exome sequencing identifies FATP1 mutation in Melkersson–Rosenthal syndrome. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e230–e232. [Google Scholar] [CrossRef]
- Fantacci, C.; Mariotti, P.; Miceli Sopo, S.; Ferrara, P.; Rendeli, C.; Chiaretti, A. Intravenous Immunoglobulins in Melkersson-Rosenthal Syndrome: A clinical and neuroimaging study. Pediatr. Allergy Immunol. 2018, 29, 881–883. [Google Scholar] [CrossRef]
- Gerressen, M.; Ghassemi, A.; Stockbrink, G.; Riediger, D.; Zadeh, M.D. Melkersson-rosenthal syndrome: Case report of a 30-year misdiagnosis. J. Oral Maxillofac. Surg. 2005, 63, 1035–1039. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Bruscoli, S.; Riccardi, C. Glucocorticoids, sex hormones, and immunity. Front. Immunol. 2018, 9, 1332. [Google Scholar] [CrossRef]
- Scagliusi, P.; Sisto, M.; Lisi, S.; Lazzari, A.; D’Amore, M. Hashimoto’s thyroiditis in melkersson-rosenthal syndrome patient: Casual association or related diseases? Panminerva Med. 2008, 50, 255–257. [Google Scholar]
- Sun, B.; Zhou, C.; Han, Z. Facial Palsy in Melkersson-Rosenthal Syndrome and Bell’s Palsy: Familial History and Recurrence Tendency. Ann. Otol. Rhinol. Laryngol. 2015, 124, 107–109. [Google Scholar] [CrossRef]
- Pei, Y.; Beaman, G.M.; Mansfield, D.; Clayton-Smith, J.; Stewart, M.; Newman, W.G. Clinical and genetic heterogeneity in Melkersson-Rosenthal Syndrome. Eur. J. Med. Genet. 2018. [Google Scholar] [CrossRef]
- Greene, R.M.; Rogers, R.S. Melkersson-Rosenthal syndrome: A review of 36 patients. J. Am. Acad. Dermatol. 1989, 21, 1263–1270. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Jin, X.; Xu, Y.; Zhang, X. Outcomes of Recurrent Facial Palsy in Melkersson Rosenthal Syndrome. Ann. Otol. Rhinol. Laryngol. 2015, 124, 232–234. [Google Scholar] [CrossRef]
- Desai, S.; Dumraliya, P.; Mehta, D. Melkersson-Rosenthal syndrome. J. Neurosci. Rural Pract. 2014, 5, 112–114. [Google Scholar] [CrossRef]
- Zeng, W.; Geng, S.; Niu, X.; Yuan, J. Complete Melkersson–Rosenthal syndrome with multiple cranialnerve palsies. Clin. Exp. Dermatol. 2010, 35, 272–274. [Google Scholar] [CrossRef]
- Okudo, J.; Oluyide, Y. Melkersson-Rosenthal Syndrome with Orofacial Swelling and Recurrent Lower Motor Neuron Facial Nerve Palsy: A Case Report and Review of the Literature. Case Rep. Otolaryngol. 2015, 2015, 214946. [Google Scholar] [CrossRef][Green Version]
- Li, C.; Jiang, X.Z.; Zhao, Y.F. Connection of trigeminal nerve and facial nerve branches and its clinical significance. Shanghai J. Stomatol. 2009, 18, 545–550. [Google Scholar]
- Ciorba, A.; Corazzi, V.; Conz, V.; Bianchini, C.; Aimoni, C. Facial nerve paralysis in children. World J. Clin. Cases 2015, 3, 973–979. [Google Scholar] [CrossRef]
- Picciolini, O.; Porro, M.; Cattaneo, E.; Castelletti, S.; Masera, G.; Mosca, F.; Bedeschi, M.F. Moebius syndrome: Clinical features, diagnosis, management and early intervention. Ital. J. Pediatr. 2016, 42, 56. [Google Scholar] [CrossRef]
- Berker, N.; Acaroğlu, G.; Soykan, E. Goldenhar’s Syndrome (Oculo-Auriculo-Vertebral Dysplasia) with Congenital Facial Nerve Palsy. Yonsei Med. J. 2004, 45, 157–160. [Google Scholar] [CrossRef]
- Pilon, A.; Rhee, P.; Newman, T.; Messner, L. Bilateral abducens palsies and facial weakness as initial manifestations of a Chiari 1 malformation. Optom. Vis. Sci. 2007, 84, 936–940. [Google Scholar] [CrossRef]
- Pavlou, E.; Gkampeta, A.; Arampatzi, M. Facial nerve palsy in childhood. Brain Dev. 2011, 33, 644–650. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Çubukçu, D.; Yılmaz, T.S.; Akıncı, G.; Özcan, M.; Güzel, O. Peripheral Facial Palsy in Children. J. Child Neurol. 2014, 29, 1473–1478. [Google Scholar] [CrossRef]
- Kanerva, M.; Moilanen, K.; Virolainen, S.; Vaheri, A.; Pitkäranta, A. Melkersson-Rosenthal syndrome. Otolaryngol. Neck Surg. 2008, 138, 246–251. [Google Scholar] [CrossRef]
- Austin, W.; David, C. Cheilitis Granulomatosa: A Review. Head Neck Pathol. 2014, 8, 209–213. [Google Scholar]
- Sudarshan, R.; Sree Vijayabala, G.; Samata, Y.; Ravikiran, A. Newer Classification System for Fissured Tongue: An Epidemiological Approach. J. Trop. Med. 2015, 2015, 262079. [Google Scholar] [CrossRef]
- Hartsfield, J.K.; Cameron, A.C. Chapter 3—Acquired and Developmental Disturbances of the Teeth and Associated Oral Structures. In McDonald and Avery’s Dentistry for the Child and Adolescent; Dean, J.A.B.T.-M., Ed.; Mosby: London, UK, 2016; pp. 39–79. [Google Scholar]
- Tan, Z.; Zhang, Y.; Chen, W.; Gong, W.; Zhao, J.; Xu, X. Recurrent facial palsy in Melkersson Rosenthal syndrome: Total facial nerve decompression is effective to prevent further recurrence. Am. J. Otolaryngol. Head Neck Med. Surg. 2015, 36, 334–337. [Google Scholar] [CrossRef]
| Reference | Sex * | Age ** | Episodes *** | FP § | Left FP | Right FP | Orofacial Oedema | Lingua Plicata | Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Onset | |||||||||
| G.B. New, 1933 [12] ¶ | F | 31 | 4 | 5 | Y | ≥1 | ≥1 | Y | NA | P |
| M | 25 | 12 | 3 | Y | 1 | 1 | Y | NA | P | |
| M | 19 | 2 | ≥2 | Y | NA | ≥1 | Y | NA | P | |
| K.A. Ekbom, 1950 [13] | F | 25 | 16 | 6 | Y | 2 | 1 | Y | Y | None |
| NA | NA | 8 | 1 | Y | NA | NA | Y | Y | None | |
| M | 20 | 15 | 4 | Y | 1 | 3 | N | Y | None | |
| Ehmann, 1962 [14] | NA | 22 m | NA | NA | Y | NA | NA | Y | Y | NA |
| NA | 8 | NA | NA | Y | NA | NA | Y | Y | NA | |
| R.H. Kundstadter, 1965 [15] | M | 5 y 6 m | 2 y 6 m | 4 | Y | 2 | 2 | Y | N | C |
| G.A. Scott, 1968 [16] | M | 8 | 6 | 2 | Y | 1 | 1 | N | Y | None |
| F | 16 | 16 | 1 | Y | 0 | 1 | N | Y | None | |
| F | 15 | NA | 8 | Y | ≥1 | ≥1 | Y | Y | None | |
| M | 10 | 10 | 3 | Y | 1 | 2 | Y | Y | None | |
| F | 12 | 12 | 1 | Y | 0 | 1 | N | Y | None | |
| M | 62 | 10 | >1 | Y | >1 | 0 | Y | Y | None | |
| D. Dylewska, 1969 [17] | NA | 9 | NA | NA | NA | NA | NA | NA | NA | NA |
| P.K. Mukherjee, 1973 [18] | F | 8 | ≤8 | 1 | Y | 1 | 1 | Y | Y | None |
| AIT.J. Storrs, 1975 [17] | F | 16 | NA | NA | Y | NA | NA | Y | NA | C, H |
| H. Butenschon, 1976 [17] | M ‡ | 10–13 | C, AB, ND | |||||||
| F †† | 5–14 | |||||||||
| B. Roseman, 1978 [17] | F | 7 | NA | >1 | Y | NA | NA | Y | Y | H |
| C. Lygidakis, 1979 [19] | F | 12 | 7 | 3 | Y | 2 | 1 | Y | N | None |
| M. May, 1981 [20] † | M | NA | ≤4 | 10 | Y | 1–9 | 1–9 | Y | Y | NA |
| J. Neuhofer, 1984 [17] | F | 15 | NA | NA | Y | NA | NA | Y | Y | CF |
| V. Hridinova, 1985 [17] | NA | child | NA | NA | NA | NA | NA | NA | NA | NA |
| S. Yuzuk, 1985 [17] | F | 13 | NA | NA | N | 0 | 0 | Y | Y | P |
| K.M. Grundfast, 1990 [21] | NA | 15 | NA | 3 | Y | 0 – 2 | 1 – 3 | Y | Y | C |
| E. Truy, 1992 [17] | NA | child | NA | NA | Y | NA | NA | Y | Y | NA |
| L.T. Glickman, 1992 [22] | F | 14 | NA | NA | Y | NA | NA | N | Y | None |
| M | 15 | NA | NA | NA | NA | NA | Y | NA | C | |
| W.M. Zimmer, 1992 [23] | M | NA | 6 | NA | NA | NA | NA | NA | NA | C |
| M | NA | 0–10 | NA | NA | NA | NA | NA | NA | ||
| C. Bourgeois-Droin, 1993 [24] | F | 10 | 10 | 2 | N | 0 | 0 | Y | Y | C, CF |
| M. Pellegrino, 1993 [17] | M | 13 | NA | NA | Y | NA | NA | Y | Y | NA |
| C. Marques, 1994 [25] ¶¶ | M | 15 | 15 | 3 | Y | NA | NA | Y | Y | C, CF |
| NA | 6 | 6 | 1 | N | 0 | 0 | Y | Y | ||
| H.A. Cohen, 1994 [10] | M | 10 | NA | >1 | Y | 0 | 1 | Y | Y | NA |
| F | 10 | NA | >1 | Y | NA | NA | Y | Y | NA | |
| M | 8 | NA | >1 | Y | NA | NA | Y | N | NA | |
| M | 5 | NA | NA | Y | 1 | 0 | Y | N | NA | |
| E. Ruza Paz-Curbera, 1998 [17] | M | 15 | NA | NA | N | 0 | 0 | Y | N | C, H |
| S.L. Stein, 1999 [22] | F | 12 | 12 | NA | N | 0 | 0 | Y | Y | C, AB |
| M | 10 | 10 | NA | N | 0 | 0 | Y | N | ||
| P.E. Ziem, 2000 [17] | F | 9 | 4 | 3 | Y | 3 | 0 | Y | N | C, AB |
| D. Salgado Alves Vilela, 2002 [26] | M | 17 | ≤16 | 4 | Y | 1 | 3 | N | Y | C, ND |
| F | 23 | 14 | 6 | Y | 3 | 3 | N | Y | ||
| F | 16 | 4 | 3 | Y | 3 | 0 | Y | Y | None | |
| F | 20 | 2 | 3 | Y | 1 | 2 | Y | Y | ||
| H. Caksen, 2002 [10] | NA | child | NA | >1 | Y | NA | NA | Y | Y | NA |
| I. Dodi, 2006 [27] | M | 8 | NA | NA | N | 0 | 0 | Y | N | C |
| H. Hentati, 2008 [28] | M | 18 | 17/18 | 2 | Y | 1 | 0 | Y | Y | C |
| H. Alp, 2009 [10] | M | 10 | NA | >1 | Y | NA | NA | Y | N | NA |
| O. Ozgursoy, 2009 [7] | M | 32 | 16 | 6 | Y | 2 | 3 | Y | Y | C |
| M | 21 | 12 | >1 | N | 0 | 0 | Y | Y | C | |
| S. Lourenaço, 2010 [29] | M | 10 | 7 | P | N | 0 | 0 | Y | Y | NA |
| R. Liu, 2013 [6] | M | 45 | 6 | >1 | Y | >1 | >1 | N | Y | C |
| M | 16 | 4 | NA | N | 0 | 0 | Y | Y | C | |
| M.K. Elias, 2013 [30] | F | 16 | 10 | NA | Y | NA | NA | Y | Y | C |
| Ü. Yilmaz, 2014 [31] | NA | NA | 13 | 2 | Y | NA | NA | NA | Y | NA |
| Y. Lee, 2014 [10] | F | 9 | 8 | 2 | Y | 0 | 2 | Y | Y | C |
| S. Feng, 2014 [32] ‡† | ||||||||||
| C.M. Rivera-Serrano, 2014 [2] | M | 22 | 2 | 8 | Y | 4 | 4 | Y | N | NA |
| M | 32 | 10 | 3 | Y | 1 | 2 | Y | N | ND | |
| F | 40 | 15 | 6 | Y | 0 | 6 | Y | Y | NA | |
| F | 31 | 7 | 6 | Y | 5 | 1 | N | Y | ND | |
| S. Bohra, 2015 [4] | F | 16 | 16 | 1 | Y | 1 | 0 | Y | Y | C, S |
| F. Kayhan, 2015 [3] | F | 13 | 5 | 6 | Y | 0 | 6 | Y | Y | C, S |
| G. Kayabasoglu, 2015 [33] | F | 17 | 10 | 6 | Y | 3 | 3 | Y | Y | C |
| A.G. Saini, 2016 [34] | F | 8 | 2y6m | 6 | Y | 3 | 3 | Y | Y | C, ACV |
| S.S. Bakshi, 2016 [35] | F | 9 | 7 | 2 | Y | 2 | 0 | Y | Y | C |
| Z. Chu, 2016 [36] | M | 12 | 10 | >1 | Y | 0 | 1 | Y | Y | C |
| L. Bordino, 2016 [37] | M | 7 | 7 | 2 | Y | 1 | 1 | Y | Y | NA |
| F | 11 | 9 | 2 | Y | 1 | 1 | N | Y | C, ACV, S | |
| J. Lalosevic, 2017 [5] | F | 12 | 9 | P | N | 0 | 0 | Y | N | C, AB |
| X.G. Xu, 2017 [38] | F | NA | 12 | 2 | Y | 0 | 2 | Y | Y | NA |
| C. Fantacci, 2018 [39] | F | 8 | 5 | 2 | Y | 0 | 2 | Y | Y | C, ACV, IVIG, S |
| G. Psillas, 2018 [31] | F | 5 | NA | N | Y | 1 | 0 | Y | Y | C |
| S. Savasta, 2018 | F | 14 | 11 | 2 | Y | 1 | 1 | Y | Y | C, ACV, S |
| F | 7 | 3 y 1 m | 4 | Y | ≥ 3 | NA | N | Y | C, S | |
| F | 9 | 18 m | 2 | Y | 0 | 2 | Y | NA | C, ACV, S | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savasta, S.; Rossi, A.; Foiadelli, T.; Licari, A.; Elena Perini, A.M.; Farello, G.; Verrotti, A.; Marseglia, G.L. Melkersson–Rosenthal Syndrome in Childhood: Report of Three Paediatric Cases and a Review of the Literature. Int. J. Environ. Res. Public Health 2019, 16, 1289. https://doi.org/10.3390/ijerph16071289
Savasta S, Rossi A, Foiadelli T, Licari A, Elena Perini AM, Farello G, Verrotti A, Marseglia GL. Melkersson–Rosenthal Syndrome in Childhood: Report of Three Paediatric Cases and a Review of the Literature. International Journal of Environmental Research and Public Health. 2019; 16(7):1289. https://doi.org/10.3390/ijerph16071289
Chicago/Turabian StyleSavasta, Salvatore, Alessandra Rossi, Thomas Foiadelli, Amelia Licari, Anna Maria Elena Perini, Giovanni Farello, Alberto Verrotti, and Gian Luigi Marseglia. 2019. "Melkersson–Rosenthal Syndrome in Childhood: Report of Three Paediatric Cases and a Review of the Literature" International Journal of Environmental Research and Public Health 16, no. 7: 1289. https://doi.org/10.3390/ijerph16071289
APA StyleSavasta, S., Rossi, A., Foiadelli, T., Licari, A., Elena Perini, A. M., Farello, G., Verrotti, A., & Marseglia, G. L. (2019). Melkersson–Rosenthal Syndrome in Childhood: Report of Three Paediatric Cases and a Review of the Literature. International Journal of Environmental Research and Public Health, 16(7), 1289. https://doi.org/10.3390/ijerph16071289





