Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey
Abstract
1. Introduction
2. Material and Methods
2.1. Inclusion Criteria
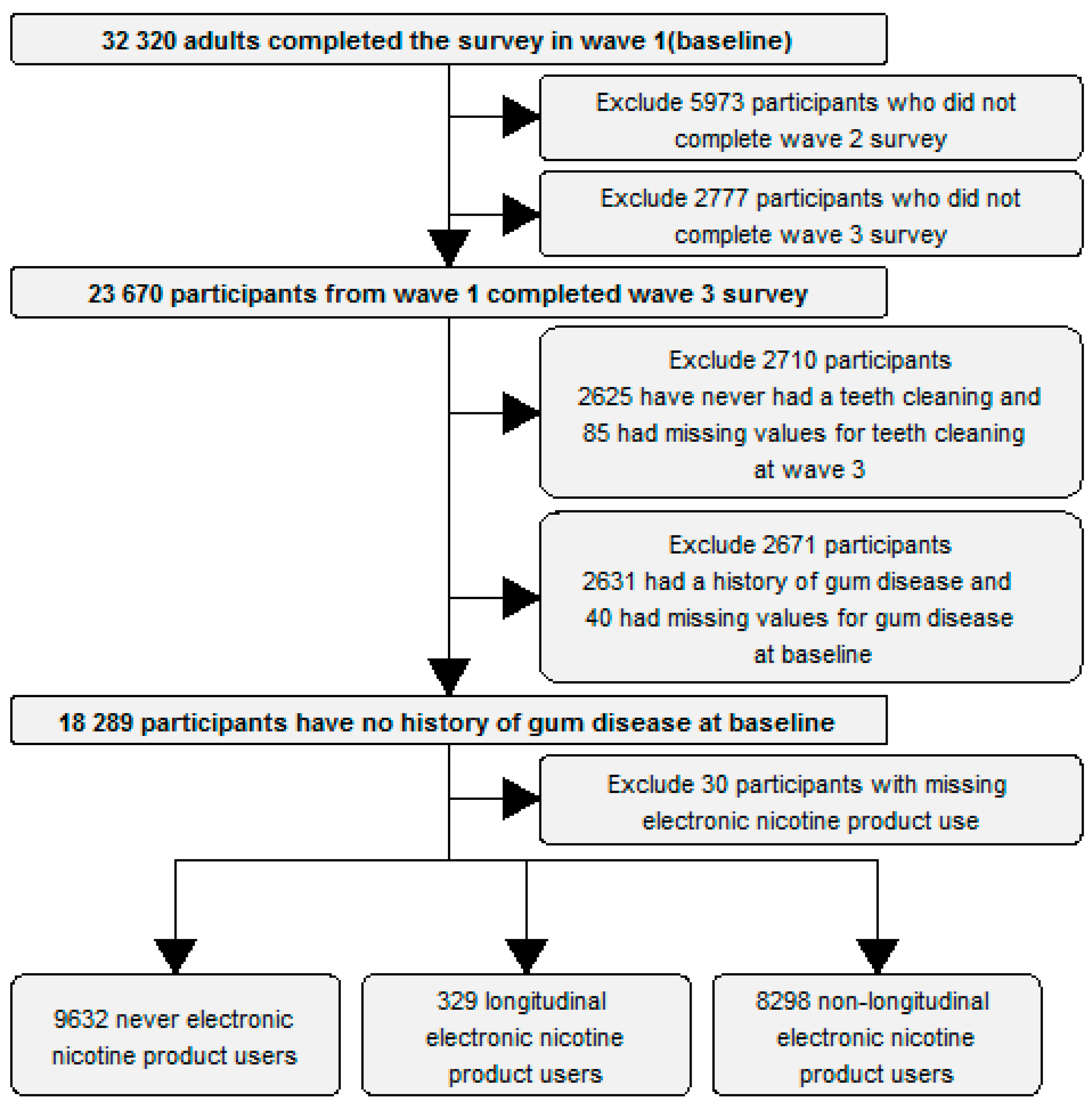
- (1)
- Adults who completed Waves 1, 2 and 3 of the PATH survey.
- (2)
- Participants who reported ever having had their teeth cleaned by a dentist, hygienist, or other health professionals by wave 3.
- (3)
- Participants who reported no history of “gum disease” at baseline (wave 1).
2.2. Data and Definitions
2.3. Longitudinal Product Use
2.4. Outcomes
2.5. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- King, B.A.; Patel, R.; Nguyen, K.H.; Dube, S.R. Trends in Awareness and Use of Electronic Cigarettes Among US Adults, 2010–2013. Nicot. Tob. Res. 2015, 17, 219–227. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2016.
- Javed, F.; Kellesarian, S.V.; Sundar, I.K.; Romanos, G.E.; Rahman, I. Recent Updates on Electronic Cigarette Aerosol and Inhaled Nicotine Effects on Periodontal and Pulmonary Tissues. Oral Dis. 2017, 23, 1052–1057. [Google Scholar] [CrossRef]
- Brown, L.J.; A Brunelle, J.; Kingman, A. Periodontal status in the United States, 1988–1991: Prevalence, extent, and demographic variation. J. Dent. Res. 1996, 75, 672–683. [Google Scholar] [CrossRef]
- Spinell, T.; DeMayo, F.; Cato, M.; Thai, A.; Lebwohl, B.; Demmer, R.T.; Helmerhorst, E.J.; Green, P.H.R. The association between coeliac disease and periodontitis: Results from NHANES 2009-2012. J. Clin. Periodontol. 2018, 45, 303–310. [Google Scholar] [CrossRef]
- Stoltenberg, J.L.; Osborn, J.B.; Pihlstrom, B.L.; Herzberg, M.C.; Aeppli, D.M.; Wolff, L.F.; Fischer, G.E. Association Between Cigarette Smoking, Bacterial Pathogens, and Periodontal Status. J. Periodontol. 1993, 64, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Asma, S. Smoking-Attributable Periodontitis in the United States: Findings From NHANES III. J. Periodontol. 2000, 71, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Rouabhia, M.; Park, H.J.; Semlali, A.; Zakrzewski, A.; Chmielewski, W.; Chakir, J. E-Cigarette Vapor Induces an Apoptotic Response in Human Gingival Epithelial Cells Through the Caspase-3 Pathway. J. Cell. Physiol. 2017, 232, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Janket, S.-J.; Baird, A.E.; Chuang, S.-K.; Jones, J.A. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 95, 559–569. [Google Scholar] [CrossRef]
- Gurav, A.N. Periodontitis and Insulin Resistance: Casual or Causal Relationship? Metab. J. 2012, 36, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.G.; Han, K.; Kim, H.A.; Pyo, S.W.; Cho, Y.S.; Kim, K.S.; Yim, H.W.; Lee, W.C.; Park, Y.G.; Park, Y.M. Association between insulin resistance and periodontitis in Korean adults. J. Clin. Periodontol. 2014, 41, 121–130. [Google Scholar] [CrossRef]
- Saremi, A.; Nelson, R.G.; Tulloch-Reid, M.; Hanson, R.L.; Sievers, M.L.; Taylor, G.W.; Shlossman, M.; Bennett, P.H.; Genco, R.; Knowler, W.C. Periodontal Disease and Mortality in Type 2 Diabetes. Diabetes Care 2005, 28, 27–32. [Google Scholar] [CrossRef]
- Fisher, M.A.; Taylor, G.W. A Prediction Model for Chronic Kidney Disease Includes Periodontal Disease. J. Periodontol. 2009, 80, 16–23. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Athavale, A.; Chen, J.; Hampole, H.; Garside, D.; Marucha, P.; Lash, J.P. Periodontal disease, chronic kidney disease and mortality: Results from the third national health and nutrition examination survey. BMC Nephrol. 2015, 16, 97. [Google Scholar] [CrossRef]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Fitzpatrick, S.G.; Katz, J. The association between periodontal disease and cancer: A review of the literature. J. Dent. 2010, 38, 83–95. [Google Scholar] [CrossRef]
- Kamer, A.R.; Morse, D.E.; Holm-Pedersen, P.; Mortensen, E.L.; Avlund, K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J. Alzheimers Dis. 2012, 28, 613–624. [Google Scholar] [CrossRef]
- Kaye, E.K.; Valencia, A.; Baba, N.; Spiro, A., III; Dietrich, T.; Garcia, R.I. Tooth loss and periodontal disease predict poor cognitive function in older men. J. Am. Geriatr. Soc. 2010, 58, 713–718. [Google Scholar] [CrossRef]
- Kamer, A.R.; Pirraglia, E.; Tsui, W.; Rusinek, H.; Vallabhajosula, S.; Mosconi, L.; Yi, L.; McHugh, P.; Craig, R.G.; Svetcov, S.; et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging 2015, 36, 627–633. [Google Scholar] [CrossRef]
- Javed, F.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Rahman, I.; Romanos, G.E. Comparison of Periodontal Parameters and Self-Perceived Oral Symptoms Among Cigarette Smokers, Individuals Vaping Electronic Cigarettes, and Never-Smokers. J. Periodontol. 2017, 88, 1059–1065. [Google Scholar] [CrossRef]
- Tatullo, M.; Gentile, S.; Paduano, F.; Santacroce, L.; Marrelli, M. Crosstalk between oral and general health status in e-smokers. Medicine 2016, 95, e5589. [Google Scholar] [CrossRef]
- Wadia, R.; Booth, V.; Yap, H.F.; Moyes, D.L. A pilot study of the gingival response when smokers switch from smoking to vaping. BDJ 2016, 221, 722. [Google Scholar] [CrossRef]
- Huilgol, P.; Bhatt, S.P.; Biligowda, N.; Wright, N.C.; Wells, J.M. Association of e-cigarette use with oral health: A population-based cross-sectional questionnaire study. J. Public Health 2018. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services; National Institutes of Health; National Institute on Drug Abuse, and United States Department of Health and Human Services; Food and Drug Administration; Center for Tobacco Products. Population Assessment of Tobacco and Health (PATH) Study [United States] Public-Use Files; Inter-university Consortium for Political and Social Research: Ann Arbor, MI, USA, 2018.
- Hyland, A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Lambert, E.; Carusi, C.; Taylor, K.; Crosse, S.; Fong, G.T.; Cummings, K.M.; et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob. Control 2017, 26, 371–378. [Google Scholar] [CrossRef]
- National Academies of Sciences, E. and Medicine. Public Health Consequences of E-Cigarettes; Stratton, K., Kwan, L.Y., Eaton, D.L., Eds.; The National Academies Press: Washington, DC, USA, 2018; Volume 774.
- Shariff, J.A.; Ahluwalia, K.P.; Papapanou, P.N. Relationship Between Frequent Recreational Cannabis (Marijuana and Hashish) Use and Periodontitis in Adults in the United States: National Health and Nutrition Examination Survey 2011 to 2012. J. Periodontol. 2016, 88, 273–280. [Google Scholar] [CrossRef]
- Saini, G.K.; Gupta, N.D.; Prabhat, K.C. Drug addiction and periodontal diseases. J. Indian Soc. Periodontol. 2013, 17, 587–591. [Google Scholar] [CrossRef]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef]
- Willershausen, I.; Wolf, T.; Weyer, V.; Sader, R.; Ghanaati, S.; Willershausen, B. Influence of E-smoking liquids on human periodontal ligament fibroblasts. Head Face Med. 2014, 10, 39. [Google Scholar] [CrossRef]
- Schroeder, M.J.; Hoffman, A.C. Electronic cigarettes and nicotine clinical pharmacology. Tob. Control 2014, 23 (Suppl. 2), ii30–ii35. [Google Scholar] [CrossRef]
- Tipton, D.; Dabbous, M.K. Effects of Nicotine on Proliferation and Extracellular Matrix Production of Human Gingival Fibroblasts In Vitro. J. Periodontol. 1996, 66, 1056–1064. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.L.; Shin, S.I.; Herr, Y.; Lee, Y.M.; Kim, E.C. Endoplasmic reticulum stress modulates nicotine-induced extracellular matrix degradation in human periodontal ligament cells. J. Periodontal Res. 2012, 47, 299–308. [Google Scholar] [CrossRef]
- Malhotra, R.; Kapoor, A.; Grover, V.; Kaushal, S. Nicotine and periodontal tissues. J. Indian Soc. Periodontol. 2010, 14, 72–79. [Google Scholar] [CrossRef]
- Pearson, J.L.; Richardson, A.; Niaura, R.S.; Vallone, D.M.; Abrams, D.B. e-Cigarette Awareness, Use, and Harm Perceptions in US Adults. Am. J. Public Health 2012, 102, 1758–1766. [Google Scholar] [CrossRef]
- Polosa, R.; Cibella, F.; Caponnetto, P.; Maglia, M.; Prosperini, U.; Russo, C.; Tashkin, D. Health impact of E-cigarettes: A prospective 3.5-year study of regular daily users who have never smoked. Sci. Rep. 2017, 7, 13825. [Google Scholar] [CrossRef]
- Goniewicz, M.L.; Smith, D.M.; Edwards, K.C.; Blount, B.C.; Caldwell, K.L.; Feng, J.; Wang, L.; Christensen, C.; Ambrose, B.; Borek, N.; et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open 2018, 1, e185937. [Google Scholar] [CrossRef]
| Never Electronic Nicotine Product User N = 9632 % (95% CI) | Longitudinal Electronic Nicotine Product User N = 329 % (95% CI) | Non Longitudinal Electronic Product User N = 8298 % (95% CI) | |
|---|---|---|---|
| Age group | |||
| 18 to 24 years old | 9.6 (9.2–10) | 23.8 (19.5–28.2) | 30.8 (29.8–31.8) |
| 25 to 34 years old | 15.7 (14.8–16.6) | 30.8 (24.4–37.1) | 29 (27.8–30.3) |
| 35 to 44 years old | 17.4 (16.5–18.3) | 15.9 (10.5–21.3) | 16.6 (15.6–17.6) |
| 45 to 54 years old | 19.3 (18.5–20.1) | 14.4 (9.5–19.3) | 12.4 (11.6–13.3) |
| 55 years old or older | 38 (37–39) | 15.1 (11.6–18.5) | 11.1 (10.2–12) |
| Gender | |||
| Female | 55.6 (54.9–56.3) | 46.8 (40.3–53.3) | 47.7 (46.6–48.8) |
| Male | 44.4 (43.7–45.1) | 53.2 (46.7–59.7) | 52.3 (51.2–53.4) |
| Race | |||
| White | 79.9 (79.1–80.7) | 86 (82–90) | 76.7 (75.3–78.1) |
| Black | 11 (10.5–11.5) | 5.3 (3–7.5) | 13.3 (12.3–14.3) |
| Other | 9.1 (8.5–9.7) | 8.8 (5.4–12.1) | 10 (9.1–10.9) |
| Hispanic | 13.8 (13.2–14.3) | 8.6 (5.6–11.6) | 15.7 (14.7–16.7) |
| Highest Grade of Education | |||
| Less than High School | 9 (8.5–9.6) | 5.4 (3.1–7.6) | 9.9 (9.3–10.5) |
| General Education Diploma (GED) | 3.6 (3.1–4) | 5.9 (3.2–8.6) | 7.6 (6.8–8.3) |
| High school graduate | 23.1 (22.4–23.9) | 20.3 (15.7–24.9) | 25 (23.8–26.3) |
| Some college or associates degree | 29.5 (28.8–30.1) | 47.3 (40.8–53.8) | 40.6 (39.3–41.9) |
| Bachelor’s degree or higher | 34.8 (34.2–35.4) | 21.1 (15.9–26.4) | 16.9 (15.7–18) |
| Body Mass Index (mean) | 28.0 (27.8–28.2) | 27.8 (27.0–28.5) | 27.5 (27.3–27.7) |
| Income | |||
| Less than $10,000 | 9.8 (9–10.6) | 13.5 (9.2–17.7) | 18.8 (17.8–19.8) |
| $10,000 to $24,999 | 16.9 (15.8–18) | 23 (18.1–28) | 23.5 (22.2–24.7) |
| $25,000 to $49,999 | 22.2 (20.9–23.5) | 26.9 (20.7–33.1) | 24.1 (23–25.2) |
| $50,000 to $99,999 | 29.2 (27.7–30.7) | 25.6 (20.1–31.1) | 21.6 (20.4–22.9) |
| $100,000 or more | 21.9 (20.5–23.3) | 11 (7.2–14.9) | 12 (10.9–13.1) |
| Baseline visit to the dentist 1 | 69.4 (68.2–70.6) | 56.9 (51.3–62.4) | 54.3 (52.8–55.8) |
| Longitudinal visit to dentist 2 | 76.4 (75.2–77.6) | 67.3 (61–73.6) | 63.5 (62.1–64.8) |
| History of prescription drug abuse | 11.5 (10.7–12.3) | 34.6 (28.4–40.7) | 28.5 (27–29.9) |
| History of stomach, duodenal or peptic ulcer | 7 (6.2–7.9) | 9.4 (5.8–13) | 6.7 (6.1–7.4) |
| History of respiratory disease 3 | 13.6 (12.7–14.5) | 20.1 (16–24.2) | 16.9 (16.1–17.7) |
| History of diabetes | 13.7 (12.6–14.7) | 6.7 (3.9–9.4) | 8.4 (7.6–9.2) |
| History of high blood pressure | 28.1 (27–29.2) | 21.8 (17.2–26.4) | 18.2 (17.1–19.3) |
| History of high cholesterol | 24.4 (23.3–25.5) | 13.8 (10.1–17.5) | 12.8 (11.8–13.9) |
| Longitudinal conventional cigarette user | 4.3 (4.0–4.6) | 38.6 (32.1–45.1) | 40.1 (38.7–41.4) |
| Former conventional cigarette user 4 | 20.5 (19.2–21.9) | 38.6 (32.5–44.7) | 14.5 (13.5–15.5) |
| History of marijuana use | 28.5 (27–29.9) | 70.2 (64.3–76.1) | 66.3 (64.8–67.8) |
| History of alcohol use | 80 (77.9–82.1) | 91.4 (87.8–95) | 90.6 (89.9–91.4) |
| History of other tobacco or tobacco product replacement therapy use 5 | 42.3 (40.7–43.8) | 89.3 (85.3–93.3) | 85.5 (84.3–86.7) |
| History of illicit drug use | 9.2 (8.3–10.1) | 29.8 (24.3–35.2) | 25.7 (24.4–27) |
| History of current second hand exposure to tobacco smoke 6 | 14.8 (13.8–15.8) | 39.3 (33–45.7) | 41.4 (40–42.8) |
| Outcomes | |||
| New cases of gum disease 7 | 5.1 (4.5–5.6) | 9.8 (6.4–13.3) | 6.2 (5.6–6.7) |
| Bone loss around teeth 8 | 8.4 (7.6–9.2) | 11.2 (7.6–14.8) | 7.3 (6.6–8.1) |
| Any periodontal disease 9 | 11.7 (10.8–12.6) | 16.7 (12.2–21.2) | 11.4 (10.6–12.2) |
| Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| New Cases of Gum Disease | Bone Loss around Teeth | Any Periodontal Disease 1 | |
| Entire cohort (N = 18,259) | |||
| Never electronic nicotine product user | Reference | Reference | Reference |
| Longitudinal electronic nicotine product user | 1.76 (1.12–2.76) 2 | 1.67 (1.06–2.63) 2 | 1.58 (1.06–2.34) 2 |
| Non longitudinal electronic product user | 1.09 (0.87–1.35) | 1.10 (0.91–1.33) | 1.09 (0.93–1.29) |
| History of marijuana use (N = 9325) | |||
| Never electronic nicotine product user | Reference | Reference | Reference |
| Longitudinal electronic nicotine product user | 1.95 (1.14–3.34) 2 | 1.91 (1.15–3.19) 2 | 1.91 (1.22–2.99) 2 |
| Non longitudinal electronic product user | 0.91 (0.69–1.21) | 1.16 (0.90–1.50) | 1.06 (0.86–1.30) |
| History of illicit or non-prescribed drug use (N = 5410) | |||
| Never electronic nicotine product user | Reference | Reference | Reference |
| Longitudinal electronic nicotine product user | 2.38 (1.33–4.26) 2 | 1.88 (1.01–3.48) 2 | 2.24 (1.40–3.60) 2 |
| Non longitudinal electronic product user | 1.00 (0.71–1.40) | 1.37 (0.99–1.89) | 1.25 (0.96–1.62) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atuegwu, N.C.; Perez, M.F.; Oncken, C.; Thacker, S.; Mead, E.L.; Mortensen, E.M. Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey. Int. J. Environ. Res. Public Health 2019, 16, 1263. https://doi.org/10.3390/ijerph16071263
Atuegwu NC, Perez MF, Oncken C, Thacker S, Mead EL, Mortensen EM. Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey. International Journal of Environmental Research and Public Health. 2019; 16(7):1263. https://doi.org/10.3390/ijerph16071263
Chicago/Turabian StyleAtuegwu, Nkiruka C., Mario F. Perez, Cheryl Oncken, Sejal Thacker, Erin L. Mead, and Eric M. Mortensen. 2019. "Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey" International Journal of Environmental Research and Public Health 16, no. 7: 1263. https://doi.org/10.3390/ijerph16071263
APA StyleAtuegwu, N. C., Perez, M. F., Oncken, C., Thacker, S., Mead, E. L., & Mortensen, E. M. (2019). Association between Regular Electronic Nicotine Product Use and Self-Reported Periodontal Disease Status: Population Assessment of Tobacco and Health Survey. International Journal of Environmental Research and Public Health, 16(7), 1263. https://doi.org/10.3390/ijerph16071263





