Cigarette Smoking among Economically Disadvantaged African-American Older Adults in South Los Angeles: Gender Differences
Abstract
1. Introduction
Aims
2. Materials and Methods
2.1. Design and Setting
2.2. Institutional Review Board (IRB)
2.3. Participants
2.4. Measures
2.4.1. Demographic Characteristics
2.4.2. Educational Attainment
2.4.3. Financial Difficulty
2.4.4. Health Insurance
2.4.5. Comorbid Medical Conditions (CMC)
2.4.6. Depressive Symptoms
2.4.7. Current Alcohol Drinking
2.4.8. Current Cigarette Smoking
2.5. Statistical Analysis
2.6. Missing Data
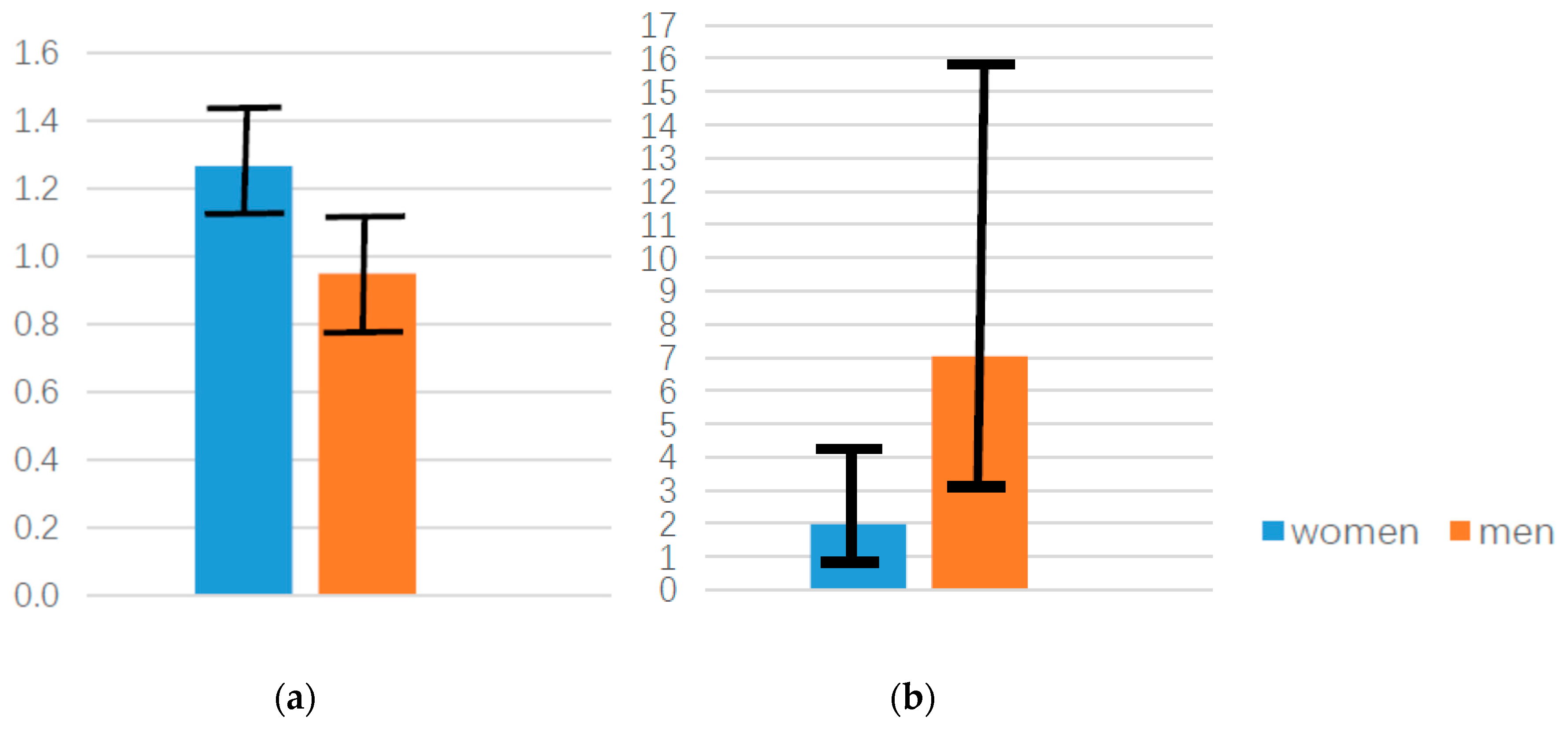
3. Results
3.1. Descriptive Statistics
3.2. Bivariate Analysis
3.3. Multivariable Analysis
4. Discussion
4.1. Limitations
4.2. Implications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novick, L.F. Smoking is the leading preventable cause of death and disability in the United States. J. Public Health Manag. Pract. 2000, 6, vi. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafo, M.R. The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. 2017, 19, 3–13. [Google Scholar] [CrossRef]
- Quezada, S.M.; Langenberg, P.; Cross, R.K. Cigarette smoking adversely affects disease activity and disease-specific quality of life in patients with Crohn’s disease at a tertiary referral center. Clin. Exp. Gastroenterol. 2016, 9, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.R. Tobacco smoking and hypertension. J. Indian Med. Assoc. 1999, 97, 367–369. [Google Scholar] [PubMed]
- Fagard, R.H. Smoking amplifies cardiovascular risk in patients with hypertension and diabetes. Diabetes Care 2009, 32 (Suppl. 2), S429–S431. [Google Scholar] [CrossRef]
- Pan, A.; Wang, Y.; Talaei, M.; Hu, F.B. Relation of Smoking With Total Mortality and Cardiovascular Events Among Patients With Diabetes Mellitus: A Meta-Analysis and Systematic Review. Circulation 2015, 132, 1795–1804. [Google Scholar] [CrossRef]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef]
- Chang, C.M.; Corey, C.G.; Rostron, B.L.; Apelberg, B.J. Systematic review of cigar smoking and all cause and smoking related mortality. BMC Public Health 2015, 15, 390. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.B.; Liu, X.O.; Gao, Y.; Dai, H.J.; Song, F.J.; Li, W.Q.; Wang, J.; Yan, Y.; Wang, P.S.; et al. Active and passive smoking with breast cancer risk for Chinese females: A systematic review and meta-analysis. Chin. J. Cancer 2014, 33, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Tsuji, I.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; Kitamura, Y.; et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn. J. Clin. Oncol. 2019, 49, 77–86. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Boeing, H.; Wahrendorf, J.; Popiela, T.; Tobiasz-Adamczyk, B.; Kulig, J. Vodka consumption, tobacco smoking and risk of gastric cancer in Poland. Int. J. Epidemiol. 1993, 22, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Hessler, J.B.; Bronner, M.; Etgen, T.; Gotzler, O.; Forstl, H.; Poppert, H.; Sander, D.; Bickel, H. Smoking increases the risk of delirium for older inpatients: A prospective population-based study. Gen. Hosp. Psychiatry 2015, 37, 360–364. [Google Scholar] [CrossRef]
- Adjemian, M.K.; Volpe, R.J.; Adjemian, J. Relationships between Diet, Alcohol Preference, and Heart Disease and Type 2 Diabetes among Americans. PLoS ONE 2015, 10, e0124351. [Google Scholar] [CrossRef]
- Ajani, U.A.; Hennekens, C.H.; Spelsberg, A.; Manson, J.E. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch. Intern. Med. 2000, 160, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Babor, T.; Rehm, J.; Jernigan, D.; Vaeth, P.; Monteiro, M.; Lehman, H. Alcohol, diabetes, and public health in the Americas. Revista Panamericana de Salud Pública 2012, 32, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; van der Schouw, Y.T.; Bergmann, M.M.; Rohrmann, S.; Schulze, M.B.; Buijsse, B.; Grobbee, D.E.; Arriola, L.; Cauchi, S.; Tormo, M.J.; et al. Alcohol consumption and risk of type 2 diabetes in European men and women: Influence of beverage type and body size The EPIC-InterAct study. J. Intern. Med. 2012, 272, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Arase, Y.; Saito, K.; Tsuji, H.; Fujihara, K.; Hsieh, S.D.; Kodama, S.; Shimano, H.; Yamada, N.; Hara, S.; et al. Role of alcohol drinking pattern in type 2 diabetes in Japanese men: The Toranomon Hospital Health Management Center Study 11 (TOPICS 11). Am. J. Clin. Nutr. 2013, 97, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Keenan, P.S. Smoking and weight change after new health diagnoses in older adults. Arch. Intern. Med. 2009, 169, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Newsom, J.T.; Huguet, N.; McCarthy, M.J.; Ramage-Morin, P.; Kaplan, M.S.; Bernier, J.; McFarland, B.H.; Oderkirk, J. Health behavior change following chronic illness in middle and later life. J. Gerontol. B Psychol. Sci. Soc. Sci. 2012, 67, 279–288. [Google Scholar] [CrossRef]
- Alvanzo, A.A.; Storr, C.L.; La Flair, L.; Green, K.M.; Wagner, F.A.; Crum, R.M. Race/ethnicity and sex differences in progression from drinking initiation to the development of alcohol dependence. Drug Alcohol Depend. 2011, 118, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Billimek, J.; Malik, S.; Sorkin, D.H.; Schmalbach, P.; Ngo-Metzger, Q.; Greenfield, S.; Kaplan, S.H. Understanding disparities in lipid management among patients with type 2 diabetes: Gender differences in medication nonadherence after treatment intensification. Womens Health Issues 2015, 25, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Keyes, K.M.; Martins, S.S.; Blanco, C.; Hasin, D.S. Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. Am. J. Psychiatry 2010, 167, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Secades-Villa, R.; Okuda, M.; Wang, S.; Perez-Fuentes, G.; Kerridge, B.T.; Blanco, C. Gender differences in cannabis use disorders: Results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug Alcohol Depend. 2013, 130, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Ackermann, K.; Croissant, B.; Mundle, G.; Nakovics, H.; Diehl, A. Neuroimaging of gender differences in alcohol dependence: Are women more vulnerable? Alcohol. Clin. Exp. Res. 2005, 29, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.C.; Yeager, T.N.; Lebsack, M.E.; Panter, S.S. Variations in alcohol metabolism: Influence of sex and age. Pharmacol. Biochem. Behav. 1975, 3, 973–978. [Google Scholar] [CrossRef]
- Mezey, E. Influence of sex hormones on alcohol metabolism. Alcohol. Clin. Exp. Res. 2000, 24, 421. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Shindo, M.; Shimada, T. Sex differences in the metabolism of (+)- and (−)-limonene enantiomers to carveol and perillyl alcohol derivatives by cytochrome p450 enzymes in rat liver microsomes. Chem. Res. Toxicol. 2002, 15, 15–20. [Google Scholar] [CrossRef]
- Oshima, S.; Haseba, T.; Masuda, C.; Kakimi, E.; Kitagawa, Y.; Ohno, Y. [Dose effect of alcohol on sex differences in blood alcohol metabolism—Cases where healthy subjects with ALDH2*1/1 genotype drunk beer with meal]. Nihon Arukoru Yakubutsu Igakkai Zasshi 2013, 48, 187–197. [Google Scholar]
- Rachamin, G.; MacDonald, J.A.; Wahid, S.; Clapp, J.J.; Khanna, J.M.; Israel, Y. Modulation of alcohol dehydrogenase and ethanol metabolism by sex hormones in the spontaneously hypertensive rat. Effect of chronic ethanol administration. Biochem. J. 1980, 186, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.M. Commentary on Kuntsche et al. (2011): Mothers and bottles—The role of gender norms in shaping drinking. Addiction 2011, 106, 1933–1934. [Google Scholar] [CrossRef]
- Bacharach, S.B.; Bamberger, P.A.; McKinney, V.M. Harassing under the influence: The prevalence of male heavy drinking, the embeddedness of permissive workplace drinking norms, and the gender harassment of female coworkers. J. Occup. Health Psychol. 2007, 12, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.L.; Martin, J.; Foxcroft, D.R. Age differences in alcohol prototype perceptions and willingness to drink in U.K. adolescents. Psychol. Health Med. 2016, 21, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Goode, T.D.; Jack, L., Jr. The alliance to reduce disparities in diabetes: Infusing policy and system change with local experience. Health Promot. Pract. 2014, 15, 6S–10S. [Google Scholar] [CrossRef]
- Grossbard, J.R.; Mastroleo, N.R.; Geisner, I.M.; Atkins, D.; Ray, A.E.; Kilmer, J.R.; Mallett, K.; Larimer, M.E.; Turrisi, R. Drinking norms, readiness to change, and gender as moderators of a combined alcohol intervention for first-year college students. Addict. Behav. 2016, 52, 75–82. [Google Scholar] [CrossRef]
- Lewis, M.A.; Neighbors, C. Gender-specific misperceptions of college student drinking norms. Psychol. Addict. Behav. 2004, 18, 334–339. [Google Scholar] [CrossRef]
- Deutsch, A.R.; Steinley, D.; Slutske, W.S. The role of gender and friends’ gender on peer socialization of adolescent drinking: A prospective multilevel social network analysis. J. Youth Adolesc. 2014, 43, 1421–1435. [Google Scholar] [CrossRef]
- Bonin, M.F.; McCreary, D.R.; Sadava, S.W. Problem drinking behavior in two community-based samples of adults: Influence of gender, coping, loneliness, and depression. Psychol. Addict. Behav. 2000, 14, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Manley, D. Dual diagnosis: Co-existence of drug, alcohol and mental health problems. Br. J. Nurs. 2005, 14, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Phillips, K.; Dorsey, C.J. Alcohol and other drug disorders, comorbidity, and violence: Comparison of rural African American and Caucasian women. Arch. Psychiatr. Nurs. 2003, 17, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, B.W.; de Goeij, A.F.; van den Brandt, P.A.; Weijenberg, M.P. Alcohol and the risk of colon and rectal cancer with mutations in the K-ras gene. Alcohol 2006, 38, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Mistry, R. Educational Attainment and Smoking Status in a National Sample of American Adults; Evidence for the Blacks’ Diminished Return. Int. J. Environ. Res. Public Health 2018, 15, 763. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Jacobson, K.C. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. J. Adolesc. Health 2012, 50, 154–163. [Google Scholar] [CrossRef]
- Anderson, C.; Burns, D.M. Patterns of adolescent smoking initiation rates by ethnicity and sex. Tob. Control 2000, 9 (Suppl. 2), II4–II8. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Separate and Combined Effects of Anxiety, Depression and Problem Drinking on Subjective Health among Black, Hispanic and Non-Hispanic White Men. Int. J. Prev. Med. 2014, 5, 269–279. [Google Scholar]
- Avalos, L.A.; Mulia, N. Formal and informal substance use treatment utilization and alcohol abstinence over seven years: Is the relationship different for blacks and whites? Drug Alcohol Depend. 2012, 121, 73–80. [Google Scholar] [CrossRef]
- Caetano, R. Alcohol-related health disparities and treatment-related epidemiological findings among whites, blacks, and Hispanics in the United States. Alcohol. Clin. Exp. Res. 2003, 27, 1337–1339. [Google Scholar] [CrossRef]
- Odvina, C.V.; Safi, I.; Wojtowicz, C.H.; Barengolts, E.I.; Lathon, P.; Skapars, A.; Desai, P.N.; Kukreja, S.C. Effect of heavy alcohol intake in the absence of liver disease on bone mass in black and white men. J. Clin. Endocrinol. Metab. 1995, 80, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Ramisetty-Mikler, S.; Caetano, R.; McGrath, C. Sexual aggression among White, Black, and Hispanic couples in the U.S.: Alcohol use, physical assault and psychological aggression as its correlates. Am. J. Drug Alcohol Abuse 2007, 33, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Robyak, J.E.; Byers, P.H.; Prange, M.E. Patterns of alcohol abuse among black and white alcoholics. Int. J. Addict. 1989, 24, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Rothman, E.F.; Wise, L.A.; Bernstein, E.; Bernstein, J. The timing of alcohol use and sexual initiation among a sample of Black, Hispanic, and White adolescents. J. Ethn. Subst. Abuse 2009, 8, 129–145. [Google Scholar] [CrossRef]
- Watson, D.W.; Sobell, M.B. Social influences on alcohol consumption by black and white males. Addict. Behav. 1982, 7, 87–91. [Google Scholar] [CrossRef]
- Caetano, R.; Baruah, J.; Ramisetty-Mikler, S.; Ebama, M.S. Sociodemographic predictors of pattern and volume of alcohol consumption across Hispanics, Blacks, and Whites: 10-year trend (1992–2002). Alcohol. Clin. Exp. Res. 2010, 34, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Caetano, R.; Schafer, J. DSM-IV alcohol dependence and drug abuse/dependence in a treatment sample of whites, blacks and Mexican Americans. Drug Alcohol Depend. 1996, 43, 93–101. [Google Scholar] [CrossRef]
- Caetano, R.; Schafer, J. DSM-IV alcohol dependence in a treatment sample of white, black, and Mexican-American men. Alcohol. Clin. Exp. Res. 1996, 20, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Caetano, R.; Schafer, J.; Cunradi, C.B. Alcohol-related intimate partner violence among white, black, and Hispanic couples in the United States. Alcohol Res. Health 2001, 25, 58–65. [Google Scholar] [PubMed]
- Dawkins, R.L.; Dawkins, M.P. Alcohol use and delinquency among black, white and hispanic adolescent offenders. Adolescence 1983, 18, 799–809. [Google Scholar] [PubMed]
- Jones-Webb, R.; Snowden, L.; Herd, D.; Short, B.; Hannan, P. Alcohol-related problems among black, Hispanic and white men: The contribution of neighborhood poverty. J. Stud. Alcohol 1997, 58, 539–545. [Google Scholar] [CrossRef]
- Kerr, W.C.; Patterson, D.; Greenfield, T.K. Differences in the measured alcohol content of drinks between black, white and Hispanic men and women in a US national sample. Addiction 2009, 104, 1503–1511. [Google Scholar] [CrossRef]
- Klatsky, A.L.; Friedman, G.D.; Siegelaub, A.B.; Gerard, M.J. Alcohol consumption among white, black, or oriental men and women: Kaiser-Permanente multiphasic health examination data. Am. J. Epidemiol. 1977, 105, 311–323. [Google Scholar] [CrossRef]
- Lillie-Blanton, M.; MacKenzie, E.; Anthony, J.C. Black-white differences in alcohol use by women: Baltimore survey findings. Public Health Rep. 1991, 106, 124–133. [Google Scholar] [PubMed]
- McCarthy, D.M.; Miller, T.L.; Smith, G.T.; Smith, J.A. Disinhibition and expectancy in risk for alcohol use: Comparing black and white college samples. J. Stud. Alcohol 2001, 62, 313–321. [Google Scholar] [CrossRef]
- Mulia, N.; Ye, Y.; Greenfield, T.K.; Zemore, S.E. Disparities in alcohol-related problems among white, black, and Hispanic Americans. Alcohol. Clin. Exp. Res. 2009, 33, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Mulia, N.; Ye, Y.; Zemore, S.E.; Greenfield, T.K. Social disadvantage, stress, and alcohol use among black, Hispanic, and white Americans: Findings from the 2005 U.S. National Alcohol Survey. J. Stud. Alcohol Drugs 2008, 69, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Nyaronga, D.; Greenfield, T.K.; McDaniel, P.A. Drinking context and drinking problems among black, white, and Hispanic men and women in the 1984, 1995, and 2005 U.S. National Alcohol Surveys. J. Stud. Alcohol Drugs 2009, 70, 16–26. [Google Scholar] [CrossRef]
- Berman, B.A.; Jones, L.; Jones, F.; Jones, A.; Pacheco, B.A.; McCarthy, W.J. How can we help African American substance users stop smoking? client and agency perspectives. J. Ethn. Subst. Abuse 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Smith, J.; Movassaghi, M.; Martins, D.; Yazdanshenas, H.; Salehe Mortazavi, S.; Orum, G. Polypharmacy among Underserved Older African American Adults. J. Aging Res. 2017, 2017, 6026358. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Smith, J.; Yazdanshenas, H.; Movassaghi, M.; Martins, D.; Orum, G. Non-adherence to medication regimens among older African-American adults. BMC Geriatr. 2017, 17, 163. [Google Scholar] [CrossRef] [PubMed]
- Bazargan, M.; Smith, J.L.; King, E.O. Potentially inappropriate medication use among hypertensive older African-American adults. BMC Geriatr. 2018, 18, 238. [Google Scholar] [CrossRef]
- Bazargan, M.; Yazdanshenas, H.; Gordon, D.; Orum, G. Pain in Community-Dwelling Elderly African Americans. J. Aging Health 2016, 28, 403–425. [Google Scholar] [CrossRef]
- Assari, S. Number of Chronic Medical Conditions Fully Mediates the Effects of Race on Mortality; 25-Year Follow-Up of a Nationally Representative Sample of Americans. J. Racial Ethn. Health Disparities 2017, 4, 623–631. [Google Scholar] [CrossRef]
- Quiñones, A.R.; Nagel, C.L.; Newsom, J.T.; Huguet, N.; Sheridan, P.; Thielke, S.M. Racial and ethnic differences in smoking changes after chronic disease diagnosis among middle-aged and older adults in the United States. BMC Geriatr. 2017, 17, 48. [Google Scholar] [CrossRef]
- Laaksonen, E.; Lallukka, T.; Lahelma, E.; Ferrie, J.E.; Rahkonen, O.; Head, J.; Marmot, M.G.; Martikainen, P. Economic difficulties and physical functioning in Finnish and British employees: Contribution of social and behavioural factors. Eur. J. Public Health 2011, 21, 456–462. [Google Scholar] [CrossRef]
- Heliovaara, M.; Aromaa, A.; Klaukka, T.; Knekt, P.; Joukamaa, M.; Impivaara, O. Reliability and validity of interview data on chronic diseases. The Mini-Finland Health Survey. J. Clin. Epidemiol. 1993, 46, 181–191. [Google Scholar] [CrossRef]
- Martin, L.M.; Leff, M.; Calonge, N.; Garrett, C.; Nelson, D.E. Validation of self-reported chronic conditions and health services in a managed care population. Am. J. Prev. Med. 2000, 18, 215–218. [Google Scholar] [CrossRef]
- Bae, J.N.; Cho, M.J. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J. Psychosom. Res. 2004, 57, 297–305. [Google Scholar] [CrossRef]
- Assari, S.; Smith, J.; Bazargan, M. Financial Strain not Education Attainment Impacts Various Health Domains in an Urban Sample of African American Older Adults in Los Angeles. 2019; in press. [Google Scholar]
- Burke, W.J.; Roccaforte, W.H.; Wengel, S.P. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. J. Geriatr. Psychiatry Neurol. 1991, 4, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.S.; Green, K.E.; Cox, E.O. Rasch analysis of the Geriatric Depression Scale-Short Form. Gerontologist 2009, 49, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, B.; Soysal, P.; Ellidokuz, H.; Isik, A.T. Validity and reliability of geriatric depression scale-15 (short form) in Turkish older adults. North. Clin. Istanb. 2018, 5, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.R.; Chelminski, I. Preliminary normative data on the Geriatric Depression Scale-Short Form (GDS-SF) in a young adult sample. J. Clin. Psychol. 1996, 52, 443–447. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’Hara, R.; Kazis, A.; Ierodiakonou, C. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging 1999, 11, 367–372. [Google Scholar] [CrossRef]
- Greenberg, S.A. How to try this: The Geriatric Depression Scale: Short Form. Am. J. Nurs. 2007, 107, 60–69; quiz 69–70. [Google Scholar] [CrossRef]
- Lesher, E.L.; Berryhill, J.S. Validation of the Geriatric Depression Scale—Short Form among inpatients. J. Clin. Psychol. 1994, 50, 256–260. [Google Scholar] [CrossRef]
- Pedraza, O.; Dotson, V.M.; Willis, F.B.; Graff-Radford, N.R.; Lucas, J.A. Internal Consistency and Test-Retest Stability of the Geriatric Depression Scale-Short Form in African American Older Adults. J. Psychopathol. Behav. Assess. 2009, 31, 412–416. [Google Scholar] [CrossRef]
- Zalavadiya, D.D.; Banerjee, A.; Sheth, A.M.; Rangoonwala, M.; Mitra, A.; Kadri, A.M. A Comparative Study of Depression and Associated Risk Factors among Elderly Inmates of Old Age Homes and Community of Rajkot: A Gujarati Version of the Geriatric Depression Scale-Short Form (GDS-G). Indian J. Community Med. 2017, 42, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Alaja, R.; Seppa, K.; Sillanaukee, P.; Tienari, P.; Huyse, F.J.; Herzog, T.; Malt, U.F.; Lobo, A. Psychiatric referrals associated with substance use disorders: Prevalence and gender differences. European Consultation-Liaison Workgroup. Alcohol. Clin. Exp. Res. 1997, 21, 620–626. [Google Scholar] [PubMed]
- Beasley, G.M.; Ostbye, T.; Muhlbaier, L.H.; Foley, C.; Scarborough, J.; Turley, R.S.; Shapiro, M.L. Age and gender differences in substance screening may underestimate injury severity: A study of 9793 patients at level 1 trauma center from 2006 to 2010. J. Surg. Res. 2014, 188, 190–197. [Google Scholar] [CrossRef]
- Boyd, C.J.; Blow, F.; Orgain, L.S. Gender differences among African-American substance abusers. J. Psychoact. Drugs 1993, 25, 301–305. [Google Scholar] [CrossRef]
- Brady, K.T.; Grice, D.E.; Dustan, L.; Randall, C. Gender differences in substance use disorders. Am. J. Psychiatry 1993, 150, 1707–1711. [Google Scholar] [CrossRef]
- Brady, K.T.; Randall, C.L. Gender differences in substance use disorders. Psychiatr. Clin. N. Am. 1999, 22, 241–252. [Google Scholar] [CrossRef]
- Brown, T.G.; Kokin, M.; Seraganian, P.; Shields, N. The role of spouses of substance abusers in treatment: Gender differences. J. Psychoact. Drugs 1995, 27, 223–229. [Google Scholar] [CrossRef]
- Brunette, M.; Drake, R.E. Gender differences in homeless persons with schizophrenia and substance abuse. Community Ment. Health J. 1998, 34, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Brunette, M.F.; Drake, R.E. Gender differences in patients with schizophrenia and substance abuse. Compr. Psychiatry 1997, 38, 109–116. [Google Scholar] [CrossRef]
- Buu, A.; Dabrowska, A.; Heinze, J.E.; Hsieh, H.F.; Zimmerman, M.A. Gender differences in the developmental trajectories of multiple substance use and the effect of nicotine and marijuana use on heavy drinking in a high-risk sample. Addict. Behav. 2015, 50, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, I.; Kang, S. Age and gender differences in health risk perception. Cent. Eur. J. Public Health 2018, 26, 54–59. [Google Scholar] [CrossRef]
- Cohen-Louck, K.; Levy, I. Risk perception of a chronic threat of terrorism: Differences based on coping types, gender and exposure. Int. J. Psychol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Limson, E.C.; D’Onofrio, G.; Daneshvar, M.; Geda, M.; Bueno, H.; Spertus, J.A.; Krumholz, H.M.; Lichtman, J.H. Sex Differences in Cardiac Risk Factors, Perceived Risk, and Health Care Provider Discussion of Risk and Risk Modification Among Young Patients With Acute Myocardial Infarction: The VIRGO Study. J. Am. Coll. Cardiol. 2015, 66, 1949–1957. [Google Scholar] [CrossRef]
- Rhodes, N.; Pivik, K. Age and gender differences in risky driving: The roles of positive affect and risk perception. Accid. Anal. Prev. 2011, 43, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Spigner, C.; Hawkins, W.; Loren, W. Gender differences in perception of risk associated with alcohol and drug use among college students. Women Health 1993, 20, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yawson, A.E.; Appiah, L.K.; Yawson, A.O.; Bonsu, G.; Aluze-Ele, S.; Amanhyia, N.A.; Lartey, M.; Adjei, A.A.; Lawson, A.L.; Beckwith, C.; et al. Sex differences in perceived risk and testing experience of HIV in an urban fishing setting in Ghana. Int. J. Equity Health 2014, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, E.M.; Werntz, A.J.; Magee, J.C.; Lindgren, K.P.; Teachman, B.A. Evaluating age differences in coping motives as a mediator of the link between social anxiety symptoms and alcohol problems. Psychol. Addict. Behav. 2014, 28, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Lahelma, E.; Pietilainen, O.; Ferrie, J.; Kivimaki, M.; Lahti, J.; Marmot, M.; Rahkonen, O.; Sekine, M.; Shipley, M.; Tatsuse, T.; et al. Changes Over Time in Absolute and Relative Socioeconomic Differences in Smoking: A Comparison of Cohort Studies From Britain, Finland, and Japan. Nicotine Tob. Res. 2016, 18, 1697–1704. [Google Scholar] [CrossRef]
- Preston, S.H.; Wang, H. Sex mortality differences in the United States: The role of cohort smoking patterns. Demography 2006, 43, 631–646. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, K.I.; Galanti, M.R.; Gilljam, H. Trajectories of smokeless tobacco use and of cigarette smoking in a cohort of Swedish adolescents: Differences and implications. Nicotine Tob. Res. 2008, 10, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Sellers, T.A.; Bailey-Wilson, J.E.; Potter, J.D.; Rich, S.S.; Rothschild, H.; Elston, R.C. Effect of cohort differences in smoking prevalence on models of lung cancer susceptibility. Genet. Epidemiol. 1992, 9, 261–271. [Google Scholar] [CrossRef]
- Gyamfi, P.; Brooks-Gunn, J.; Jackson, A.P. Associations between employment and financial and parental stress in low-income single black mothers. Women Health 2001, 32, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Moran, K.E.; Ommerborn, M.J.; Blackshear, C.T.; Sims, M.; Clark, C.R. Financial Stress and Risk of Coronary Heart Disease in the Jackson Heart Study. Am. J. Prev. Med. 2019, 56, 224–231. [Google Scholar] [CrossRef]
- Andrade, F.C.D.; Kramer, K.Z.; Monk, J.K.; Greenlee, A.J.; Mendenhall, R. Financial stress and depressive symptoms: The impact of an intervention of the Chicago Earned Income Tax Periodic Payment. Public Health 2017, 153, 99–102. [Google Scholar] [CrossRef]
- Francoeur, R.B. Cumulative financial stress and strain in palliative radiation outpatients: The role of age and disability. Acta Oncol. 2005, 44, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Hanratty, B.; Holland, P.; Jacoby, A.; Whitehead, M. Financial stress and strain associated with terminal cancer—A review of the evidence. Palliat. Med. 2007, 21, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Logue, B.J. Women at risk: Predictors of financial stress for retired women workers. Gerontologist 1991, 31, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Medical Electronics Buyers Guide 1984, Part 6. CAT scanners, electrical safety & test equipment, financial management, neonatal & pediatric equipment, pulmonary/respiratory equipment, simulators, stress test systems/ergometers. Med. Electron. 1984, 15, 116–192. [Google Scholar]
- Guillaumier, A.; Twyman, L.; Paul, C.; Siahpush, M.; Palazzi, K.; Bonevski, B. Financial Stress and Smoking within a Large Sample of Socially Disadvantaged Australians. Int. J. Environ. Res. Public Health 2017, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Blacks’ Diminished Return of Education Attainment on Subjective Health; Mediating Effect of Income. Brain Sci. 2018, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. The Benefits of Higher Income in Protecting against Chronic Medical Conditions Are Smaller for African Americans than Whites. Healthcare 2018, 6, 2. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H. Family Income at Birth and Risk of Attention Deficit Hyperactivity Disorder at Age 15: Racial Differences. Children 2019, 6, 10. [Google Scholar] [CrossRef]
- Assari, S.; Caldwell, C.H.; Mincy, R. Family Socioeconomic Status at Birth and Youth Impulsivity at Age 15; Blacks’ Diminished Return. Children 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Hani, N. Household Income and Children’s Unmet Dental Care Need; Blacks’ Diminished Return. Dent. J. 2018, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Thomas, A.; Caldwell, C.H.; Mincy, R.B. Blacks’ Diminished Health Return of Family Structure and Socioeconomic Status; 15 Years of Follow-up of a National Urban Sample of Youth. J. Urban Health 2018, 95, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Assari, S.; Caldwell, C.H.; Zimmerman, M.A. Family Structure and Subsequent Anxiety Symptoms; Minorities’ Diminished Return. Brain Sci. 2018, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Assari, S. Educational Attainment Better Protects African American Women than African American Men Against Depressive Symptoms and Psychological Distress. Brain Sci. 2018, 8, 182. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In Clinical Gerontology: A Guide to Assessment and Intervention; Brink, T.L., Ed.; The Haworth Press: New York, NY, USA, 1986; pp. 165–173. [Google Scholar]
| Characteristics | All | Women | Men |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (Years) | 74.06 (6.92) | 74.46 (6.91) | 73.30 (6.89) |
| Educational Attainment (Years) * | 12.70 (2.33) | 12.95 (2.02) | 12.23 (2.76) |
| Financial Strain | 8.21 (4.92) | 8.11 (4.67) | 8.39 (5.38) |
| Comorbid Medical Conditions | 2.97 (1.83) | 2.08 (1.83) | 2.74 (1.80) |
| Depressive Symptoms | 2.10 (2.44) | 2.08 (2.45) | 2.16 (2.43) |
| n (%) | n (%) | n (%) | |
| Gender | |||
| Women | 378 (65.6) | 378 (100.0) | - |
| Men | 198 (34.4) | - | 198 (100.0) |
| Current Drinking Status * | |||
| No | 406 (70.5) | 277 (73.3) | 129 (65.2) |
| Yes | 170 (29.5) | 101 (26.7) | 69 (34.8) |
| Current Smoking Status * | |||
| No | 491 (85.2) | 343 (90.7) | 148 (74.7) |
| Yes | 85 (14.8) | 35 (9.3) | 50 (25.3) |
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| All | ||||||||
| 1 Gender (Male) | 1.00 | −0.08 | −0.15 ** | 0.03 | −0.09 * | 0.02 | 0.09 * | 0.21 ** |
| 2 Age (Years) | 1.00 | −0.19 ** | −0.10 * | 0.01 | −0.10 * | −0.14 ** | −0.26 ** | |
| 3 Educational Attainment (Years) | 1.00 | −0.14 ** | −0.08 * | −0.07 | 0.05 | −0.03 | ||
| 4 Financial Difficulty | 1.00 | 0.23 ** | 0.31 ** | 0.16 ** | 0.16 ** | |||
| 5 Comorbid Medical Conditions | 1.00 | 0.33 ** | 0.00 | 0.05 | ||||
| 6 Depressive Symptoms | 1.00 | 0.07 | 0.17 ** | |||||
| 7 Current Drinking | 1.00 | 0.27 ** | ||||||
| 8 Current Smoking | 1.00 | |||||||
| Women | ||||||||
| 2 Age (Years) | 1.00 | −0.22 ** | −0.11 * | 0.02 | −0.12 * | −0.08 | −0.16 ** | |
| 3 Educational Attainment (Years) | 1.00 | −0.05 | −0.12 * | −0.06 | 0.07 | 0.04 | ||
| 4 Financial Difficulty | 1.00 | 0.25 ** | 0.32 ** | 0.14 ** | 0.16 ** | |||
| 5 Comorbid Medical Conditions | 1.00 | 0.31 ** | −0.03 | 0.06 | ||||
| 6 Depressive Symptoms | 1.00 | 0.04 | 0.26 ** | |||||
| 7 Current Drinking | 1.00 | 0.12 * | ||||||
| 8 Current Smoking | 1.00 | |||||||
| Men | ||||||||
| 2 Age (Years) | 1.00 | −0.20 ** | −0.08 | −0.04 | −0.06 | −0.24 ** | −0.39 ** | |
| 3 Educational Attainment (Years) | 1.00 | −0.23 ** | −0.07 | −0.09 | 0.05 | −0.03 | ||
| 4 Financial Difficulty | 1.00 | 0.21 ** | 0.28 ** | 0.20 ** | 0.15 * | |||
| 5 Comorbid Medical Conditions | 1.00 | 0.38 ** | 0.07 | 0.09 | ||||
| 6 Depressive Symptoms | 1.00 | 0.13 | 0.06 | |||||
| 7 Current Drinking | 1.00 | 0.43 ** | ||||||
| 8 Current Smoking | 1.00 |
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Gender (Men) | 2.85 *** | 1.67–4.86 | 1.69 | 0.80–3.58 | 5.60 *** | 2.61–12.00 | 3.90 ** | 1.41–10.80 |
| Age (Years) | 0.87 *** | 0.82–0.92 | 0.87 *** | 0.82–0.92 | 0.87 *** | 0.82–0.92 | 0.87 *** | 0.82–0.92 |
| Educational Attainment | 0.96 | 0.86–1.07 | 0.96 | 0.85–1.07 | 0.96 | 0.86–1.07 | 0.95 | 0.85–1.07 |
| Financial Difficulty | 1.03 | 0.98–1.08 | 1.03 | 0.98–1.08 | 1.03 | 0.98–1.08 | 1.05 | 0.98–1.12 |
| Comorbid Medical Conditions | 1.02 | 0.88–1.19 | 1.02 | 0.87–1.18 | 1.03 | 0.89–1.20 | 1.02 | 0.87–1.19 |
| Depressive Symptoms | 1.14 * | 1.03–1.26 | 1.14 * | 1.03–1.26 | 1.25 *** | 1.11–1.42 | 1.13 * | 1.02–1.26 |
| Current Drinking (Yes) | 3.61 *** | 2.13–6.13 | 2.13 * | 1.00–4.51 | 3.72 *** | 2.18–6.36 | 3.63 *** | 2.14–6.16 |
| Gender (Men) × Current Drinking | - | - | 2.89 * | 1.00–8.41 | - | - | - | - |
| Gender (Men) × Depressive Symptoms | - | - | - | - | 0.79 * | 0.65–0.95 | - | - |
| Gender (Men) × Financial Difficulty | - | - | - | - | - | - | 0.97 | 0.88–1.06 |
| OR | 95% CI | OR | 95% CI | |
|---|---|---|---|---|
| Women (Model 5) | Men (Model 6) | |||
| Age (Years) | 0.92 * | 0.86–0.99 | 0.81 *** | 0.74–0.89 |
| Educational Attainment | 1.07 | 0.86–1.35 | 0.88 # | 0.76–1.02 |
| Financial Difficulty | 1.05 | 0.98–1.13 | 1.01 | 0.93–1.09 |
| Comorbid Medical Conditions | 0.97 | 0.78–1.21 | 1.14 | 0.91–1.44 |
| Depressive Symptoms | 1.27 *** | 1.12–1.43 | 0.95 | 0.79–1.14 |
| Current Drinking (Yes) | 1.97 # | 0.91–4.23 | 7.03 *** | 3.10–15.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assari, S.; Smith, J.L.; Zimmerman, M.A.; Bazargan, M. Cigarette Smoking among Economically Disadvantaged African-American Older Adults in South Los Angeles: Gender Differences. Int. J. Environ. Res. Public Health 2019, 16, 1208. https://doi.org/10.3390/ijerph16071208
Assari S, Smith JL, Zimmerman MA, Bazargan M. Cigarette Smoking among Economically Disadvantaged African-American Older Adults in South Los Angeles: Gender Differences. International Journal of Environmental Research and Public Health. 2019; 16(7):1208. https://doi.org/10.3390/ijerph16071208
Chicago/Turabian StyleAssari, Shervin, James L. Smith, Marc A. Zimmerman, and Mohsen Bazargan. 2019. "Cigarette Smoking among Economically Disadvantaged African-American Older Adults in South Los Angeles: Gender Differences" International Journal of Environmental Research and Public Health 16, no. 7: 1208. https://doi.org/10.3390/ijerph16071208
APA StyleAssari, S., Smith, J. L., Zimmerman, M. A., & Bazargan, M. (2019). Cigarette Smoking among Economically Disadvantaged African-American Older Adults in South Los Angeles: Gender Differences. International Journal of Environmental Research and Public Health, 16(7), 1208. https://doi.org/10.3390/ijerph16071208






