Optimization of Polyaluminum Chloride-Chitosan Flocculant for Treating Pig Biogas Slurry Using the Box–Behnken Response Surface Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Biogas Slurry
2.2. Test Equipment and Reagents
2.3. Preparation of the CF
2.4. Experimental Design
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Analysis
3.1. Development of the Regression Model Equation
3.2. Verification Model
3.2.1. Analysis of Variance (ANOVA)
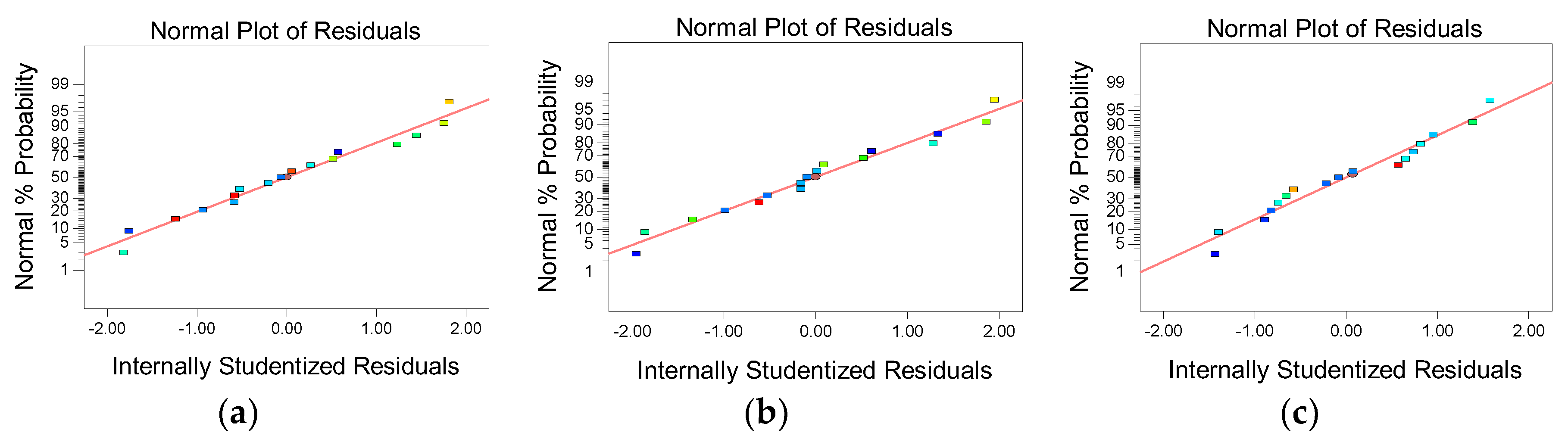
3.2.2. Normal Analysis
3.3. Response Surface Rendering and Parameter Evaluation
3.3.1. Absorbance Response Surface Analysis and Parameter Optimization
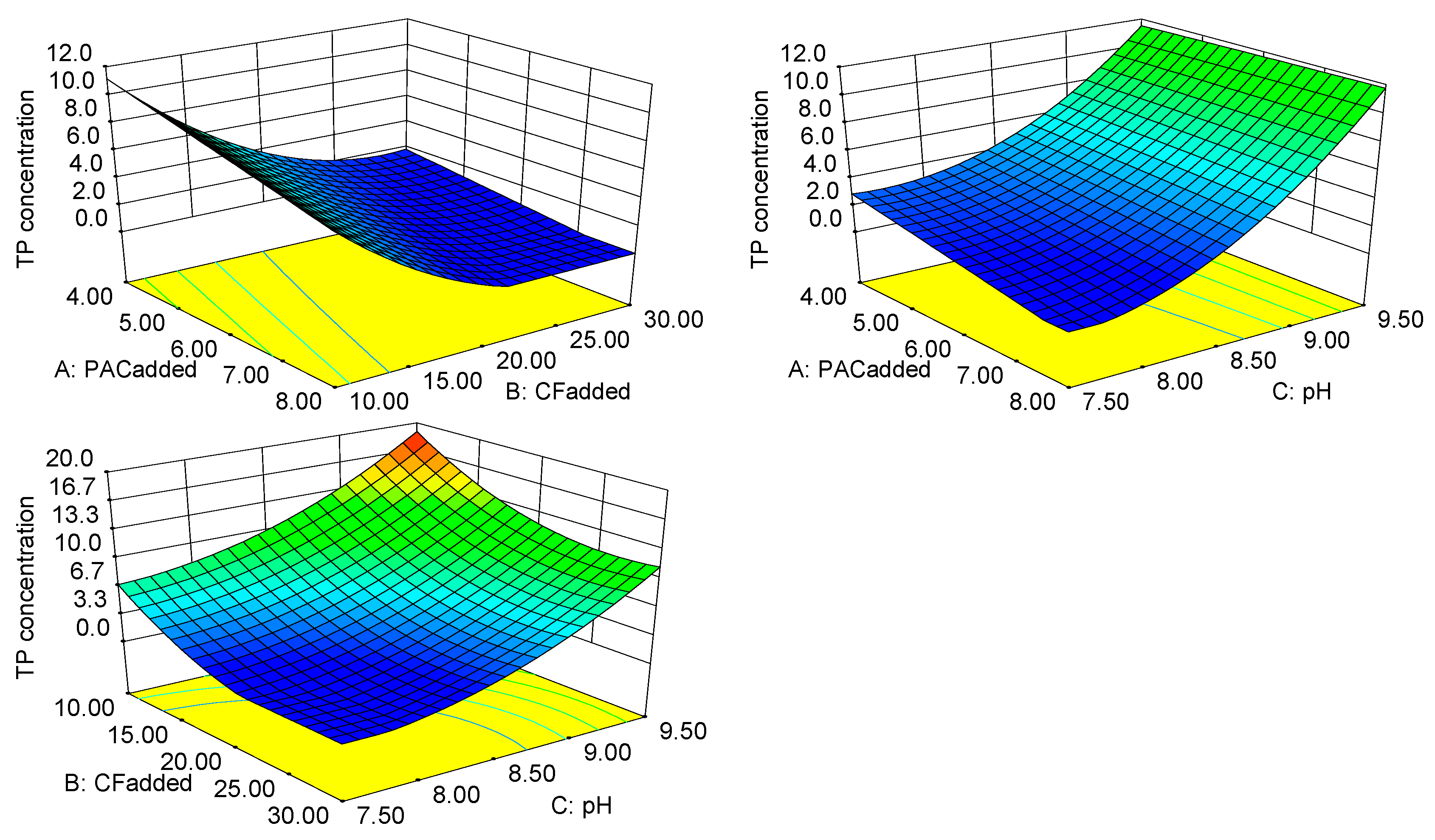
3.3.2. Response Surface Analysis and Optimization of the TP Concentration
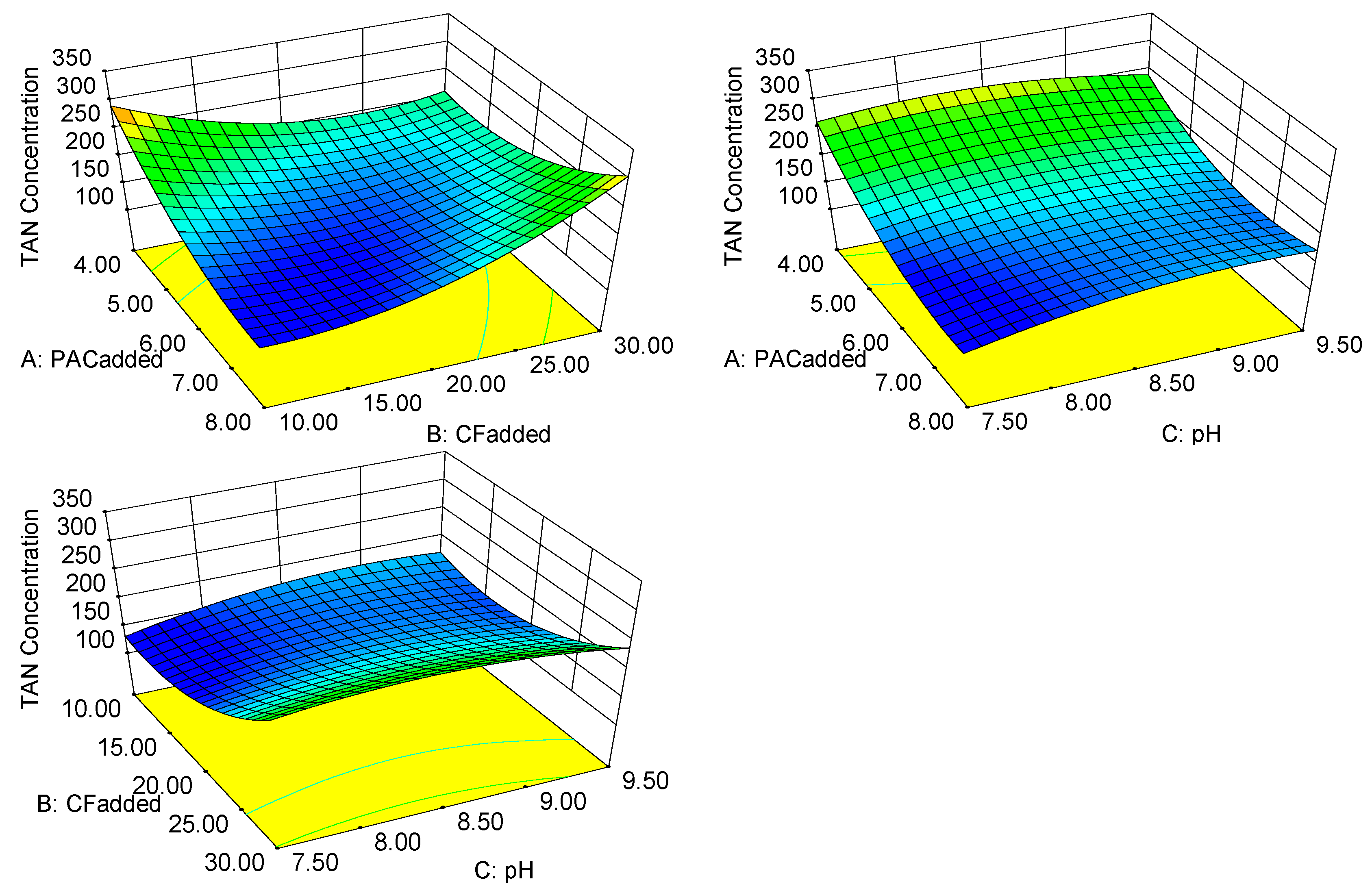
3.3.3. Response Surface Analysis and Optimization of the TAN Concentration
3.4. Optimization of the Flocculation Optimal Conditions
3.5. Data Validation and Flocculation Observation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTS | chitosan |
| PAC | polyaluminum chloride |
| CF | the composite flocculant |
| TP | total phosphorus |
| TAN | total ammonia nitrogen |
References
- Naushad, M. Surfactant assisted nano-composite cation exchanger: development, characterization and applications for the removal of toxic pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Naushad, M.; Ahamad, T.; Sharma, G.; Al-Muhtaseb, H.A.; Albadarin, A.B.; Alam, M.M.; et al. Synthesis and characterization of a new starch/sno2 nanocomposite for efficient adsorption of toxic hg2+ metal ion. Chem. Eng. J. 2016, 300, 306–316. [Google Scholar] [CrossRef]
- Folens, K.; Huysman, S.; Van Hulle, S.; Du Laing, G. Chemical and economic optimization of the coagulation-flocculation process for silver removal and recovery from industrial wastewater. Sep. Purif. Technol. 2017, 179, 145–151. [Google Scholar] [CrossRef]
- Šulc, R.; Ditl, P. The effect of process conditions on the flocculation process occurring in an agitated vessel. Pol. J. Chem. Technol. 2012, 14, 88–96. [Google Scholar] [CrossRef]
- Karbassi, A.; Marefat, A. The impact of increased oxygen conditions on heavy metal flocculation in the Sefidrud estuary. Mar. Pollut. Bull. 2017, 121, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.U.; Kim, D.J. Phosphorus extraction and sludge dissolution by acid and alkali treatments of PAC (PAC) treated wastewater sludge. Bioresour. Technol. 2016, 217, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation·flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manage. 2012, 93, 154–168. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, X.; Gao, B.; Wang, Y.; Yue, Q.; Chen, T. Fabrication and characterization of poly(ferric chloride)-polyamine flocculant and its application to the decolorization of reactive dyes. J. Mater. Sci. 2014, 49, 4962–4972. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Seo, S.-J.; Moon, H.-S.; Yoo, M.-K.; Park, I.-Y.; Kim, B.-C.; Cho, C.-S. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Szyguła, A.; Guibal, E.; Palacín, M.A.; Ruiz, M.; Sastre, A.M. Removal of an anionic dye (Acid Blue 92) by coagulation-flocculation using chitosan. J. Environ. Manage. 2009, 90, 2979–2986. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation·flocculation processes – An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Khawar, A.; Aslam, Z.; Javed, S.; Abbas, A. Pb(II) biosorption using DAP·EDTA-modified biopolymer (chitosan). Chem. Eng. Commun. 2018, 205, 1555–1567. [Google Scholar] [CrossRef]
- Zeng, D.; Wu, J.; Kennedy, J.F. Application of a chitosan flocculant to water treatment. Carbohydr. Polym. 2008, 71, 135–139. [Google Scholar] [CrossRef]
- Zainol, N.A.; Aziz, H.B.A.; Yusoff, M.S. Coagulation and flocculation process of landfill lachate in removing COD, color and ammonia using PAC (PACl). Res. J. Chem. Sci. 2011, 1, 34–39. [Google Scholar]
- Daud, Z.; Nasir, N.; Awang, H. Treatment of biodiesel wastewater by coagulation and flocculation using PAC. Aust. J. Basic & Appl. Sci. 2013, 7, 258–262. [Google Scholar]
- Peng, T.; Li, W.; Gui-Ying, F.; Hong-Xia, D. Study on the species distribution and transformation kinetics of pac-cts. J. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2006, 9, 183–186. [Google Scholar]
- Zheng, W.; Migui, Z.; Lei, H.; Qiao, X.; Zizeng, L.; Lei, C. Research on pac-cts composite flocculant for the decolorization of reactive blue 19. Ind. Water Treatment. 2017, 9, 21–25. [Google Scholar]
- Hongxia, M.A.; Yaocang, L.I. The preparation and application of chitosan compound flocculant. Meteorol. Environ. Res. 2012, 478, 22–24. [Google Scholar]
- Dawei, Y. Preparation and flocculation of composite flocculant(pac-cts). New Chem. Mater. 2011, 39, 94–95. [Google Scholar]
- Shuyun, S.; Xiaozhi, G.U.; Qichao, Z.; Kaining, C. Research on an emergency treatment technology for black-odor water caused by macrophytes decaying. J. Lake Sci. 2016, 28, 485–493. [Google Scholar] [CrossRef]
- Heviánková, S.; Souček, R.; Kyncl, M.; Surovcová, N. Contribution to the study of flocculation of digestate. Geos. Eng. 2015, 61, 37–44. [Google Scholar] [CrossRef]
- Huang, M.F.; Song, Q.-J.; Mao, Z.-H.; Xing, X.-F.; Bai, Z.-A.; Gu, P.; Zhao, Z.-L. The retrieval model for COD in waters using optical absorption properties of CDOMa case study at the Shuangtaizi River and the Liaodong Gulf. Acta Oceanol. Sin. 2011, 3, 47–54. [Google Scholar]
- Ferreira-Pinto, L.; Feihrmann, A.C.; Tavares, C.R.G.; dos Reis Coimbra, J.S.; Saldaña, M.D.A.; Vedoy, D.R.L.; Cardozo-Filho, L. Leachate treatment using supercritical water. Can. J. Chem. Eng. 2017, 95, 1442–1448. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, M.-G.; Lei, H.; Xue, Q.; Lin, Z.-Z.; Chen, L. Study on flocculation characteristics of humic acid by poly aluminum chloride-chitosan composite flocculant. Environ. Eng. 2017, 10, 61–65. [Google Scholar]
- Şirin, S.; Trobajo, R.; Ibanez, C.; Salvadó, J. Harvesting the microalgae Phaeodactylum tricornutum with PAC, aluminium sulphate, chitosan and alkalinity-induced flocculation. J. Appl. Phycol. 2012, 24, 1067–1080. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Zheng, Z.-X.; Han, Y.-F.; Zhan, M.-F. Mechanisms of nitrogen and phosphorus adsorption from biogas slurry of piggery by modified zeolite. J. Agro-Environ. Sci. 2014, 33, 1837–1842. [Google Scholar]
- Yan, M.; Wang, D.; Qu, J.; He, W.; Chow, C.W. Relative importance of hydrolyzed Al(III) species (Al(a), Al(b), and Al(c)) during coagulation with PAC: A case study with the typical micro-polluted source waters. J. Colloid Interface Sci. 2007, 316, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guan, J.; Santiwong, S.R.; Waite, T.D. Characterization of floc size and structure under different monomer and polymer coagulants on microfiltration membrane fouling. J. Membr. Sci. 2008, 321, 132–138. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Qingqing, C.; Jianwei, L.; Shibing, H.; Jiaojiao, W.; Na, N.; Liqiong, W. Application of PAC-CTS in high concentration pig wastewater. Acta Agric. Boreali-Occident. Sin. 2016, 25, 1890–1897. [Google Scholar]
- Wenyi, Z.; Peicheng, F.; Qiuyan, L.; Xing, L.; Mingyuan, L. Polyaluminum chloride-chitosan synthetic composite flocculant and the application in pretreatment of cyanobacteria biogas slurry. Environ. Chem. 2012, 31, 7. [Google Scholar]


| TAN Concentration (mg·L−1) | TP Concentration (mg·L−1) | pH | |
|---|---|---|---|
| Concentration | 1568.25 | 214.10 | 8.47 |
| No. | PACadded (g·100 mL−1CF) | CFadded (mL·L−1biogas slurry) | pH | Absorbance | TP Concentration (mg·L−1) | TAN Concentration (mg·L−1) |
|---|---|---|---|---|---|---|
| 1 | 6.00 | 20.00 | 8.50 | 0.459 | 5.35 | 160.30 |
| 2 | 8.00 | 20.00 | 7.50 | 0.227 | 1.39 | 166.80 |
| 3 | 6.00 | 20.00 | 8.50 | 0.308 | 5.35 | 150.51 |
| 4 | 6.00 | 10.00 | 7.50 | 0.176 | 9.47 | 158.63 |
| 5 | 6.00 | 20.00 | 8.50 | 0.241 | 8.48 | 177.23 |
| 6 | 6.00 | 30.00 | 7.50 | 0.132 | 1.21 | 210.90 |
| 7 | 8.00 | 10.00 | 8.50 | 0.808 | 14.66 | 177.23 |
| 8 | 4.00 | 20.00 | 7.50 | 0.501 | 4.63 | 216.01 |
| 9 | 4.00 | 10.00 | 8.50 | 0.692 | 18.56 | 294.46 |
| 10 | 6.00 | 10.00 | 9.50 | 0.972 | 23.92 | 190.31 |
| 11 | 8.00 | 20.00 | 9.50 | 0.960 | 16.44 | 183.10 |
| 12 | 4.00 | 20.00 | 9.50 | 0.910 | 15.22 | 161.80 |
| 13 | 8.00 | 30.00 | 8.50 | 0.325 | 1.29 | 324.41 |
| 14 | 6.00 | 20.00 | 8.50 | 0.351 | 4.13 | 142.72 |
| 15 | 6.00 | 20.00 | 8.50 | 0.273 | 5.61 | 186.20 |
| 16 | 4.00 | 30.00 | 8.50 | 0.400 | 4.11 | 192.71 |
| 17 | 6.00 | 30.00 | 9.50 | 0.719 | 16.51 | 188.90 |
| Source | Absorbance | TP Concentration | TAN Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | df | p-Value Prob > F | Sum of Squares | df | p-Value Prob > F | Sum of Squares | df | p-Value Prob > F | |
| Model | 1.24 | 9 | 0.0013 | 745.36 | 9 | 0.0006 | 35,484.40 | 9 | 0.0008 |
| Residual | 0.07 | 7 | 33.73 | 7 | 1827.56 | 7 | |||
| Lack of fit | 0.05 | 3 | 0.2464 | 23.35 | 3 | 0.1581 | 511.94 | 3 | 0.6916 |
| Pure error | 0.03 | 4 | 10.38 | 4 | 1315.62 | 4 | |||
| Cor Total | 1.32 | 16 | 779.09 | 16 | 37,311.96 | 16 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, L.; Yasser Farouk, R.; Wang, Y. Optimization of Polyaluminum Chloride-Chitosan Flocculant for Treating Pig Biogas Slurry Using the Box–Behnken Response Surface Method. Int. J. Environ. Res. Public Health 2019, 16, 996. https://doi.org/10.3390/ijerph16060996
Li Y, Li L, Yasser Farouk R, Wang Y. Optimization of Polyaluminum Chloride-Chitosan Flocculant for Treating Pig Biogas Slurry Using the Box–Behnken Response Surface Method. International Journal of Environmental Research and Public Health. 2019; 16(6):996. https://doi.org/10.3390/ijerph16060996
Chicago/Turabian StyleLi, Yu, Leigang Li, Reham Yasser Farouk, and Yuanyuan Wang. 2019. "Optimization of Polyaluminum Chloride-Chitosan Flocculant for Treating Pig Biogas Slurry Using the Box–Behnken Response Surface Method" International Journal of Environmental Research and Public Health 16, no. 6: 996. https://doi.org/10.3390/ijerph16060996
APA StyleLi, Y., Li, L., Yasser Farouk, R., & Wang, Y. (2019). Optimization of Polyaluminum Chloride-Chitosan Flocculant for Treating Pig Biogas Slurry Using the Box–Behnken Response Surface Method. International Journal of Environmental Research and Public Health, 16(6), 996. https://doi.org/10.3390/ijerph16060996




